Abstract
Structure and distribution of afferent nerve fibres in the rat bladder were studied by fluorescence microscopy after selective staining with antibodies against neuropeptide CGRP. Afferent fibres are very abundant (by comparison with other viscera) and interconnected in all bladder parts: muscle, urothelium, connective tissue, blood vessels, serosa. Their highest concentration is beneath the urothelium in equatorial and caudal regions, where they form a plexus, while individually maintaining a tree-like structure with innumerable branches running without preferential orientation. In cranial regions, mucosal afferent fibres become rare or absent. Abundant fibres are found in the detrusor, within each muscle bundle, with long strings of varicosities parallel to muscle cells. Afferent fibres, invariably varicose over hundreds of micrometres of their terminal parts and while still branching, comprise chains of hundreds of varicosities. Varicosities are irregular in size, frequency and separation, without specialised terminal structures around them, or within or around the fibre’s ending. The possibility that varicosities are transduction points for sensory inputs is discussed, with the implication of a process taking place over considerable length in each branch of each fibre. Interconnectedness of afferent nerves of various bladder tissues, distribution of varicosities over hundreds of micrometres along axonal branches, absence of clear target structures for the fibres, apparent irregularity in the size and sequence of varicosities suggest an innervation that is not rigidly wired with distinct sensory pathways. In fact, the structural evidence suggests extensive afferent integration at the periphery, with wide distribution of source points and broad range of physical detectors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The afferent innervation of the urinary bladder, which gives rise to the local sensations and to other more restricted reflex processes, is provided by nerve fibres that issue from dorsal root ganglia (Applebaum et al. 1980; Vera and Nadelhaft 1990), pass in the majority through the pelvic ganglion and along the urinary branches of the pelvic nerve, where they are mixed with efferent (motor) post-ganglionic fibres, and terminate in all layers of the bladder wall (in the rat: Mattiasson et al. 1985; Su et al. 1986; Yokokawa et al. 1986; Gabella and Davis 1998; Shea et al. 2000, among others; reviewed in de Groat and Yoshimura 2009; Birder 2014). A recent study on the bladder of mice extended the observations with the use of computer-assisted 3-D two-photon microscopy (Schueth et al. 2017).
In line with a dominant role of the urothelium in bladder physiology (Birder and Andersson 2013) and because of the abundance of afferent nerves in the mucosa (in its tunica propria, to be precise), there has been much interest in the functional links between urothelium and adjacent nerves, including the possibility of reciprocal interactions (Kanai and Andersson 2010).
Possible interactions between urothelium and sub-urothelial nerves are much discussed (Apodaca 2004), under the concept of urothelial signalling (Birder and Andersson 2013) and many of the modern studies are focused on the role of the mucosa in the afferent processes of the bladder (Andersson and McCloskey 2014).
There is afferent innervation in the detrusor muscle too, served by A/delta fibres with relatively high conduction velocity (Sengupta and Gebhart 1994; Kanai and Anderson 2010).
Several classes of bladder afferent fibres are identified physiologically, for example at least four distinct types in the guinea pig (Zagorodnyuk et al. 2007) and several types in the rat (Shea et al. 2000).
Afferent fibres are involved in the mechano-sensitive processes in the bladder, especially the detection of organ distension that produces a strong sensation of fullness and can activate voiding.
In addition to those transmitting impulses from mechanical stimuli there are fibres conveying pain and thermal and chemical stimuli.
Even the sensation of fullness is regarded by many as a form of chemo-reception, where ATP (Ferguson et al. 1997) or acetylcholine (Yoshida et al. 2006) or nitric oxide and other substances (Masunaga et al. 2006) are released from epithelial cells of the urothelium when it is stretched, and may activate the adjacent nerve terminals (Nakagomi et al. 2016).
In addition, mechano-sensitive channels may be present on various cells but also on the sensory nerve terminals themselves (Araki et al. 2008).
In the publications on visceral afferent fibres, the term ‘nerve terminals’ is commonly used, in spite of the absence of a clear anatomical referent; the term is associated with that of ‘target’, even if it is only to indicate that those afferent fibres do not show an obvious target. Indeed, studies by electron microscopy (Dixon and Gilpin 1987), by immunocytochemistry (Wakabayashi et al. 1993) and by immunohistochemistry (as quoted here) have not detected targets or special structures at the anatomical end of these afferent fibres; in fact, the anatomical end of a fibre is hardly ever recognised.
The afferent fibres (or, more strictly, the afferent axons) in the bladder, as in all viscera, are difficult to distinguish ultrastructurally from other types of fibre (Gosling and Dixon 1974). However, they can be identified with some confidence by their specific content of certain neuropeptides such as substance P and CGRP (calcitonin gene–related peptide), which are readily detected histochemically.
That line of investigation has produced many detailed accounts of bladder afferent innervation (Hökfelt et al. 1978; Alm et al. 1978; Mattiasson et al. 1985; Yokokawa et al. 1985, 1986; Gabella and Davis 1998).
The specificity of CGRP for urinary afferent neurons is well documented in the rat (Su et al. 1986) and confirmed by ultrastructural (Gulbenkian et al. 1986; Papka and McNeill 1992, 1993), autoradiographic (Burcher et al. 2000) and physiological and pharmacological (Franco-Cereceda et al. 1987; Maggi et al. 1987; Lundberg et al. 1992) studies.
There are crucial aspects of bladder innervation that are not understood or documented, and an advance in that direction is the purpose of this study, particularly for structural features that bear strongly on bladder functions while raising difficult questions on their morphogenesis.
Material and methods
Adult female rats (Sprague-Dawley) aged 7–10 weeks and weighing 180–200 g were used.
All the procedures involving materials from animals complied fully with the UK Home Office Regulations under a Personal and a Project License to the Author.
Rats, immediately after being killed with an overdose of anaesthetic (pentobarbitone 100 mg/kg i.m.), were vascularly perfused from the heart with Krebs solution for about 3 min and the blood drained through the cut right atrium.
The bladder was dissected out, by cutting the ureters and urethra near their point of entry; it was slit open with a cut from the cranial to the caudal pole; and it was spread out with the mucosa up and pinned down on a petri dish with a base of Sylgard (Dow Corning, Wiesbaden, Germany). By moving and repositioning the pins at the edge of the preparation and making small peripheral cuts, the bladder wall was stretched out into a flat lamina. When a maximal distension was obtained (approximating the size of the organ in situ when fully distended), the mucosa was separated from the muscle as a thin lamina by manual microdissection under a dissecting microscope and was pinned down on a separate base of Sylgard. The remaining of the wall, consisting mainly of musculature (detrusor muscle), was cut into three to four smaller laminae, also pinned down of slabs of Sylgard. All the laminae were then immersed in fixative (2% formaldehyde and 0.2% picric acid in PBS, with 0.2% Triton X-100 and 5% donkey normal serum) for about an hour. Then, while still pinned on Sylgard, the laminae were incubated with a polyclonal primary antibody anti-CGRP raised in rabbit against synthetic rat alpha calcitonin gene–related peptide (from Affiniti, Exeter, UK), at a dilution of 1:1000 for 18–24 h, at room temperature in a moist chamber in the dark. At the end of the incubation, the lamina was unpinned, washed in several changes of PBS, mounted in Citifluor (Canterbury, Kent, UK) and examined in a fluorescence microscope. Experiments to control the specificity of the staining were carried out by replacing the primary antibody with PBS or with the primary antibody pre-absorbed with the antigen.
For frozen sections, a cannula was inserted into the bladder in situ, any urine was drained and then Krebs solution was injected into the lumen to obtain a controlled distension. Fixative was then injected by vascular perfusion and was also slowly injected into the bladder to replace the Krebs solution.
The bladder, with the cannula in its lumen, was dissected out and immersed in fixative for 2–4 h, then divided into segments of a few millimetre size and stored in a 7% sucrose solution with 0.1% Na azide. Cryostat section at 10 μm was cut with a cryostat, collected on poly-l-lysine-coated slides and processed for immune-staining and mounted as described above.
Results
The rat bladder mucosa (that is the tissue extending from the lumenal surface to the border with the detrusor muscle, consisting of an epithelium, known as the urothelium, and a layer of connective tissue, known as the tunica propria) is richly supplied with afferent fibres (CGRP-immuno-positive), as evidenced both in whole-mount preparations (Fig. 1) and in frozen microtomic sections (Fig. 2).
a Transverse section of the bladder wall on a cranial-caudal plane with the lumen on the right. In the lower region, near the caudal pole of the bladder, several nerves (white streaks) are seen, just beneath the urothelium (light grey streak), being intersections of fibres of the sub-urothelial plexus. In the topmost part of the section, a sub-urothelial plexus is absent, while at the bottom, that plexus is very well developed; an intermediate spatial density of fibres occurs in the middle part of the section, indicating a caudal to cranial gradient in the density of the plexus. Elsewhere in the bladder, wall gradients are not seen. Bar 200 μm. b Section through the mucosa (left) and the ureter (right) at its point of entry into the bladder. Abundant immune-fluorescent fibres are gathered immediately beneath the urothelium and around the ureteric epithelium. Bar 100 μm. c Transverse section through the mucosa, with the lumen at the top (part of a photographic montage). The fluorescent fibres are gathered in a region immediately beneath the urothelium, but some penetrate deeper into the connective tissue of the mucosa. Near the bottom left corner is a blood vessel surrounded by several fluorescent fibres. Bar 100 μm. d Transverse section of the mucosa, with folded urothelium and a few immuno-positive fibres immediately beneath it, on the left-hand side. In contrast, on the right-hand side, the entire mucosa is devoid of fibres. At the bottom are elements of the detrusor muscle, with several nerve fibres, transversely sectioned. Bar 100 μm. e The mottled streak across this microscopic field is the urothelium and varicose fibres penetrate in it and extend for some distance among the epithelial cells. Bar 20 μm
In the laminar whole-mount preparations of the mucosa, there is a gradient in the spatial density of afferent nerve fibres, with a minimum (or an absence) in the cranial region of the bladder and a maximum near the caudal region. The gradient is also visible in transverse sections of the wall cut along the cranio-caudal axis (Fig. 2a).
The spatial density of afferent innervation, thus, is highest in the region of trigone and around the opening of the ureters and urethra (Fig. 2b, c). A similar, very high density of afferent innervation is found in the part of the ureters and urethra nearest to the bladder (Fig. 2b).
In contrast, large areas of the cranial part of the bladder mucosa have no nerves, except those around blood vessels (Fig. 2d).
The mucosal nerve fibres run close to and predominantly parallel to the urothelium and form a kind of thin-layered plexus. In the full (distended) bladder, the plexus is quite flat; it becomes corrugated in the contracted (emptied) bladder when it follows the prominent folds formed by the entire urothelium.
Where the innervation is densest, that is caudal to the equator plane of the bladder, the fibres are so numerous and intermingled as to form a plexus (a sub-urothelial plexus) so dense that it is difficult to identify individual axons and to follow them for any distance.
In contrast, in the areas of the mucosa where the innervation is less dense, it is possible, in whole-mount preparations, to recognise and to follow individual nerve fibres along all or most of their extent, including their branching and their terminal segments. However, intricate the appearance, the sub-urothelial plexus has a tree-like structure rather than the structure of a meshed network.
Characteristically, the fibres within the plexus run in all directions without an apparent preferential orientation and can even criss-cross each other.
From the sub-urothelial plexus, short branches emerge at right angle that penetrate into the urothelium, and travel up to close to the lumen (without actually ever reaching it); these intra-epithelial fibres are best seen in the microtomic sections (Fig. 2e). Although they are found in all preparations, they are a rather component of the afferent innervation.
They are limited to the regions caudal to the equatorial plane, that is they are only seen in the presence of a sub-urothelial plexus.
Intra-epithelial fibres are not seen in the short segment of the ureter and the urethra examined here.
From the plexus, some fibres, moving deeper into the wall (or coming from deeper into the wall), become perivascular or penetrate into the muscle, interconnecting with the nerve elements of every part of the bladder. There is no evidence of a distinct compartmentalisation of afferent fibres within separate district, e.g. in the mucosa, but rather an extensive connectivity of all the nerves of the organ.
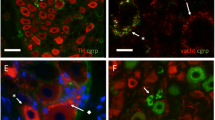
All the afferent fibres of the sub-urothelial plexus and of the other regions of the bladder (including intra-epithelial, intramuscular and perivascular fibres) are varicose, that is they have a beaded appearance that corresponds to a linear sequence of much expanded and much restricted regions of the fibre (Fig. 3).
a Whole-mount preparation of the mucosa with a varicose fibre that divides repeatedly, spreading in various directions. The varicose pattern is distinct, throughout the extent visible in the micrograph. The three branching sites shown are all at the level of a varicosity. The size and the separation of the varicosities appear quite irregular. Bar 30 μm. b A long fibre and its irregular string of varicosities in the mucosa. Two blood vessels (out of focus) cross vertically this microscopic field. Bar 30 μm. c A wavy varicose fibre in the mucosa seen over a distance of some 300 μm up to its end (at the right). Over the terminal part of the fibre (roughly the right half of its length), varicosities are irregular and do not show a gradient related to the proximity to the termination. In the left half of the fibre (which could be caller pre-terminal), the varicosities are initially small and faint and then they increase progressively in a distal direction. Bar 30 μm
The varicose pattern is readily recognised, but its details are so variable even along any single fibre that they defy a rigorous description.
It is common to find strings of more than one hundred varicosities, while the short intra-epithelial branches comprise up to about 20 varicosities.
There are roughly 30 varicosities every 100 μm; however, this value is quite variable, without a recognisable pattern. In some axonal stretches, there are only about 20 varicosities per 100 μm, while elsewhere almost twice as many are found. This variability affects the size and spatial density of varicosities and the length of the individual intervaricose segments.
The size of varicosities cannot be measured in fluorescence microscopy, but it varies markedly along a string, from a barely visible varicosity to some that must be over a micrometre in diameter.
Large and small varicosities follow each other in the sequence without an apparent order. There is not an increase in the size of the varicosities nearer the end of a fibre (the opposite is sometimes the case), except occasionally in the short intra-epithelial branches.
It is also not uncommon that the largest varicosities are some distance away from the end of the fibre.
Within the plexus, the branching is usually dichotomic (that is with branches of similar size), often at a right angle. When it can be detected with confidence, the branching occurs invariably at the level of a varicosity, and repeated branching is seen along most axons (Fig. 3a).
A common, although most unexpected, occurrence is varicose fibres in the mucosa that clearly appear to branch, but then, a short distance away, the two branches merge forming again a single fibre (Fig. 4). Various configurations are observed, such as a splitting of a fibre over a short or a long distance (Fig. 4a), or a closed loop with which the fibre terminates (Fig. 4b).
a A varicose fibre in the lamina propria of themucosa does branch (left of centre) and one of the two branches divides again, but then the two secondary branches become rejoined into a single fibre (to the right) before the termination. Bar 20 μm. b This isolated fibre, varicose through the entire visible length, seems to be forming a closed loop issuing from a large varicosity of the parent fibre. Bar 20 μm
There are afferent fibres in all other areas of the bladder wall, especially around blood vessels and within bundles of the detrusor muscle (Fig. 5). The efferent innervation of the muscle is modest by comparison with the high density of efferent (motor) fibres, but still quite extensive; even the smallest muscle bundles show at least one varicose fibre (Fig. 5a), generally running parallel to the muscle cells (Fig. 5b, c).
a Frozen section of the bladder wall, mainly occupied by muscle bundles that are roughly in transverse section (in oblique section at the bottom of the micrograph) showing numerous nerve fibres running well within each bundle. At the top right is part of the urothelium, and in this region near the cranial pole, there are no afferent fibres in the mucosa. Bar 20 μm. b A large afferent fibre with prominent varicosities, parallel to a muscle bundle, is shown in a whole-mount preparation. Bar 20 μm
All these fibres, muscular and perivascular, are varicose and the pattern shows a great variability, almost as wide as that of the fibres in the mucosa.
Discussion
The occurrence of distinct structural features in the afferent fibres of the bladder mucosa, as illustrated here, their complexity and variability, and the difficulties of interpretation all highlight how little we understand the basic mechanisms of this sensory system.
The limitations of the present work are those of immuno-fluorescence microscopy, for example that only what is immuno-fluorescent is seen, and that magnification and resolution are quite modest. Nevertheless, the use of whole-mount preparations reduces some of the optical and sampling problems of sections. The whole-mount method is applied on tissues ex vivo, after mild fixation, without sectioning or embedding, without injection of tracers, and it offers a full view of a tissue; there are, however, limitations (large bladders, auto-fluorescence, penetration of the antibodies, more compact connective tissue, for example). Whole mounts of rat bladder mucosa were first used by Yokokawa et al. (1985) and the present results are in full agreement with their work.
The identification of CGRP-immuno-fluorescent fibres with afferent fibres is reasonably safe (Su et al. 1986; Yokokawa et al. 1986) and has been tested in several previous studies on laboratory animals. Even in the human bladder, co-localisation studies have confirmed the value of CGRP for identification of afferent fibres (Smet et al. 1997).
There may be, however, CGRP-negative afferent fibres, in particular in species other than the rat. This does not affect the present conclusions but must allow for additional physiological possibilities.
The distribution of the afferent fibres has functional implications. The gradual transition from a maximal, very high density, near the caudal pole of the bladder, to a virtual absence at the cranial pole, implies that the mucosa is practically insensitive over a large area about the cranial pole; from the equatorial region, there must be a progressive increase in sensitivity with a maximum near the urethral opening.
The openings of the ureters and urethra into the bladder must be providing a highest afferent input to the CNS, which fits with their crucial position and suggests the presence in those regions of factors particularly favourable to the growth of afferent nerves.
The afferent fibres are concentrated in a kind of plexus parallel to the urothelium, very variable in its layout in the distended or the contracted bladder. Fibres branch repeatedly, maintaining their individuality until their ends, running in an irregular fashion and without a preferred orientation, a distribution that fits in the wall of a near-spherical organ, which expands non-directionally.
Where the spatial density is not too high, points of termination of the afferent axons are recognised—and they show no characteristic structure, adding to our uncertainty as where the afferent transduction takes place.
All the fibres within the plexus and elsewhere are invariably varicose, as shown in an earlier documentation of high quality (Tamaki et al. 1992); two new features are presented here.
First, the chains or strings of varicosities are very long, hundreds of varicosities over hundreds of micrometres, and are not affected by branching, with the branching always occurring at a varicosity and not at an intervaricose segment.
Second, the varicose pattern, high characteristic as it is, is also very variable or irregular within a single string and in every fibre. There is no gradient, no changes near the very end of the fibre and no apparent regularity in the repeat, the size and the separation of varicosities. Classifications and quantitative analysis are almost impossible under the criteria we have available. It may be that there are different types of fibre, although the variability is found also within a single fibre.
From the structural variability of varicosities, the suggestion arises that it is a pattern in a dynamic condition with a continuous and rapid structural change, without a rigid well-defined stable structure, which we simply observe statically in one single instant. However, there are no data as yet as to how dynamic these structures are, their turnover, continuous growth, adaptive changes, repair.
Two scientific questions arise directly from the varicose pattern of the fibres.
First: what are the physiological properties imparted to a fibre by the varicose pattern?
Second: what are the morphogenetic processes, during development, growth, renewal, and repair, that bring about the varicose pattern?
It seems that nothing can be said for now about the morphogenesis. Varicose fibres, afferent and efferent, are very common at the peripheral sites of the autonomic nervous system and the problem should be examined in a broader perspective. In the bladder, one may wonder what it is that keeps the mucosa of its cranial regions nerve-free: an unfavourable terrain for axonal growth? inhibiting factors? losing in the competition with other areas?
As to the first question, considering the autonomic nerves in general, the ultrastructural evidence in the literature strongly suggests that the varicosities of autonomic efferent (motor) fibres are points of transmitter release. They are packed with axonal vesicles, they include areas uncovered by a glial wrapping, and they lie within 40–60 nm from the surface of smooth muscle cells. This is particularly evident in the bladder detrusor and it leads to the conclusion that these are proper neuro-muscular junctions (Gabella 1995; reviewed by Hirst et al. 1996; Bennett 1996).
One is tempted to consider whether there is a similar arrangement also in afferent (sensory) varicose autonomic fibres, with active points (that is sites of sensory transduction) distributed in a chain of sites along a terminal axonal branch. Scores of afferent junctions would then be spread over the terminal length of a single axonal branch.
This interpretation is substantially at variance from the standard paradigm of sensory input, but it would fit well with the present structural evidence. It would also agree with the presence of varicose CGRP-positive fibres in the pelvic ganglion (terminals or in transit), which are very close to ganglion neurons and sometimes form synaptic junctions on them (Papka and McNeill 1992, 1993; Senba and Tohyama 1988). Similar data exist on the intramural ganglion neurons that are present in the bladder of some species, such as man and guinea pig (Gillespie et al. 2006).
A novel observation (which had a passing mention in our paper of 1998) is that some of these terminal afferent axons divide but then the two branches merge again re-forming a single axon.
The possibility of a misinterpretation is high, given the limited resolution of fluorescence microscopy (axons may cross each other giving a false appearance of fusion, or they can run so close to each other to appear as a single axon, for example).
Therefore, the interpretation must be cautious. However, the occurrence of looping axons is so common and was observed in preparations of more than ten animals, that it is likely to be an authentic structure, and one of rather intriguing significance. While the process of axonal branching is well known, its opposite, a kind of de-branching, has not been shown in previous studies.
Afferent fibres are also abundant in the musculature, without obvious regional differences. Afferent fibres are present in each muscle bundle and are aligned with the muscle cells. The ‘target’ here is the muscle cells, but the points of nerve-muscle closeness are spread over a chain of many varicosities, that is tens of micrometres.
The afferent innervation of the bladder cannot yet be put on a quantitative basis, but in terms of amount and distribution of fibres and varicosities, is very extensive, unmatched by that found in the gut or airways.
The fibres originate from some 4000 ganglion neurons in dorsal root ganglia, on each side of the body, roughly matching the number of motor neurons to the bladder from the pelvic ganglia of female rats (Gabella 1999).
Repeated branching of the fibres takes place once they are inside the bladder wall (Gabella 1999) and branching occurs also in the terminal, varicose portions of each fibre.
Such a widespread distribution of fibres, their interconnectedness and abundance of their varicosities indicate that afferent impulses originate from every part of the bladder, while responding to a wide variety of stimuli. Therefore, from the peripheral afferent network, the CNS receives sufficient input to construct a holistic representation of the bladder, in all its sensory aspects (mechanical, physical, chemical, thermal, and so on, including pain).
Structural characteristics—such as the interconnectedness of the nerves of various tissues, the distribution of varicosities over hundreds of micrometres along an axonal branch, the absence of clear target points for the fibres and the apparent irregularity in the sequence of varicosities—are all features that do not match a physiological model based on rigidly wired circuits and distinct sensory pathways.
What sort of map the CNS makes out of the impulses (often referred to as ‘signals’) arriving from the bladder is impossible to tell.
However, it may be that to some extent it is a map that develops post-natally with ‘experience’ (rather than being entirely a rigidly wired circuit) and one that includes more integration at the periphery and both a wide distribution of source points and a broad range of physical detections.
References
Alm P, Alumets J, Brodin E, Hakanson R, Nilsson G, Sjöberg N-O, Sundler F (1978) Peptidergic (substance P) nerves in the genito-urinary tract. Neuroscience 3:419–425
Andersson KE, McCloskey KD (2014) Lamina propria: the functional center of the bladder. Neurourol Urodynam 33:9–16
Applebaum AE, Vance WH Coggeshall RE (1980) Segmental localization of sensory cells that innervate the bladder. J Comp Neurol 192:203–209
Apodaca G (2004) The uroepithelium not just a passive barrier. Traffic 5:117–128
Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M (2008) Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol 15:681–687
Bennett MR (1996) Autonomic neuromuscular transmission at a varicosity. Prog Neurobiol 50:505–532
Birder LA (2014) Nervous network for lower urinary tract function. Int J Urol 20:4–12
Birder L, Andersson KE (2013) Urothelial signaling. Physiol Rev 93:653–680
Burcher E, Zeng X-P, Stigas J, Shang F, Millard RJ, Moore KH (2000) Autoradiographic localization of tachykinin and calcitonin gene-related peptide receptors in adult urinary bladder. J Urol 163:331–337
de Groat WC, Yoshimura N. (2009) Afferent nerve regulation of bladder function in health and disease. Handbook Exp Pharmacol. 2009;194: 91–138. [In: Sensory Nerves, edited by Canning B & Spina D]
Dixon JS, Gilpin CJ (1987) Presumptive sensory axons of the human urinary bladder: a fine structural study. J Anat 151:199–207
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol 505: 503–11
Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hökfelt T, Fischer JA (1987) Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptide 8:399–410
Gabella G (1995) The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol 24:159–187
Gabella G, Davis C (1998) Distribution of afferent axons in the baldder of rats. J Neurocytol 27:141–155
Gabella G (1999) Structure of the intramural nerves of the rat bladder. J Neurocytol 28:615–637
Gillespie JI, Markeling-van Ittersum M, de Vente J (2006) Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325:33–45
Gosling JA, Dixon JS (1974) Sensory nerves in the mammalian urinary tract. An evaluation using light and electron microscopy. J Anat 117:133–144
Gulbenkian S, Merighi A, Wharton J, Varndell IM, Polak JM (1986) Ultrastructural evidence for the coexistence of calcitonin gene-related peptide and substance P in secretory vesicles of peripheral nerves in the guinea pig. J Neurocytol 15:535–542
Hirst GDS, Choate JK, Cousins HM, Edwards FR, Klemm MF (1996) Transmission by postganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience 73:7–23
Hökfelt T, Schultzberg M, Elde R, Nilsson G, Terenius L, Said S, Goldstein M (1978) Peptide neurons in peripheral tissues including the urinary tract: immunohistochemical studies. Acta Pharmacol Toxicol 43(II):78–89
Kanai AJ, Andersson KE (2010) Bladder afferent signaling: recent findings. J Urol 2010(183):1288–1295
Lundberg JM, Franco-Cereceda A, Alving K, Delay-Goyet P, Lou Y-P (1992) Release of calcitonin gene-related peptide from sensory neurons. Ann N Y Acad Sci 657:187–193
Maggi CA, Santicioli P, Abelli L, Parlani M, Capasso M, Conte B, Giuliani S, Meli A (1987) Regional differences in the effects of capsaicin and tachykinins on motor activity and vascular permeability of the rat lower urinary tract. Naunyn Schmiedeberg’s Arch Pharmacol 335:636–645
Masunaga K, Yoshida M, Inadome A, Iwashita H, Miyamae K, Ueda S (2006) Prostaglandin E2 release from isolated bladder strips in rats with spinal cord injury. Int J Urol 13:271–276
Mattiasson A, Ekblad E, Sundler F, Uvelius B (1985) Origin and distribution of neuropeptide Y-, vasoactive intestinal polypeptide- and substance P-containing nerve fibers in the urinary bladder of the rat. Cell Tissue Res 239:141–146
Nakagomi H, Yoshiyama M, Mochizuki T, Miyamoto T, Komatsu R, Imura Y, Moizawa Y, Hiasa M, Miyaji T, Kira S, Araki I, Fujishita K, Shibata K, Shigetomi E, Shinozaki Y, Ichikawa R, Uneyama H, Iwatsuki K, Nomura M, de Groat WC, Moriyama Y, Takeda M, Koizumi S (2016) Urothelial ATP exocytosis: regulation of bladder compliance in the urine storage phase. Scientific Report 6: 1–14
Papka RE, McNeill DL (1992) Is there a synaptic innervation of pelvic neurons by CGRP-immunoreactive sensory nerves. Ann N Y Acad Sci 657:477–480
Papka RE, McNeill DL (1993) Light- and electron-microscopic study of synaptic connections in the paracervical ganglion of the female rat: special reference to calcitonin gene-related peptide-, galanin- and tachykinin (substance P and neurokinin A)-immunoreactive nerve fibers and terminals. Cell Tissue Res 271:417–428
Schueth A, Spronck B, van Zandvoort MAMJ, van Koeveringe GA (2017) Computer-assisted three-dimensional tracking of sensory innervation in the murine bladder mucosa with two-photo microscopy. J Chemical Neuroanat 85:43–49
Senba E, Tohyama M (1988) Calcitonin gene-related peptide containing autonomic efferent pathways to the pelvic ganglia of the rat. Brain Res 449:386–390
Sengupta JN, Gebhart GF (1994) Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72:2420–2430
Shea VK, Cai R, Crepps B, Mason JL, Perl ER (2000) Sensory fibers of the pelvic nerve innervating the rat’s urinary bladder. J Neurophysiol 84:1924–1933
Smet PJ, Moore KH, Jonavicius J (1997) Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Investig 77:37–49
Su HC, Wharton J, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JFB, Ballesta J, Bloom SR (1986) Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunocytochemistry. Neuroscience 18:727–747
Tamaki M, Iwanaga T, Takeda M, Adachi I, Satu S, Fujita T (1992) Calcitonin gene-related peptide (CGRP)-immunoreactive nerve terminals in the whole mount preparations of the dog urethra. Arch Histol Cytol 55:1–11
Vera PL Nadelhaft I (1990) Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Res 520:83–89
Wakabayashi Y, Tomoyoshi T, Fujimiya M, Arai R, Maeda T (1993) Substance P-containing axon terminals in the mucosa of the human urinary bladder: pre-embedding immunohistochemistry using cryostat sections for electron microscopy. Histochemistry 100:401–407
Yokokawa K, Sakanaka M, Shiosaka S, Toyama M, Shiotani Y, Sonoda T (1985) Three-dimensional distribution of substance P-like immunoreactivity in the urinary bladder of the rat. J Neural Transmission 63:209–222
Yokokawa K, Tahyama M, Shiosaka S, Shiotani Y, Sonoda T, Emson PC, Hillyard CV, Girgis S, MacIntyre I (1986) Distribution of calcitonin gene-related peptide-containing fibers in the urinary bladder of the rat and their origin. Cell Tissue Res 244:271–278
Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S (2006) Non-neuronal cholinergic system in human bladder urothelium. Urology 67:425–430
Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ Gregory SJ (2007) Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol 585:147–163
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gabella, G. Afferent nerve fibres in the wall of the rat urinary bladder. Cell Tissue Res 376, 25–35 (2019). https://doi.org/10.1007/s00441-018-2965-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2965-0