Abstract
The inordinately long midgut of hemipterans is devoid of peritrophic membranes described for many other insects. These membranes separate apical microvilli of midgut cells from contents of the lumen. In hemipterans, by contrast, contents of the lumen are separated from apical surfaces of midgut epithelia by secretion of additional plasma membranes (perimicrovillar membranes) containing digestive enzymes. In the lace bug Corythucha ciliata, precursors for these perimicrovillar membranes arise in smooth endoplasmic reticula (SER) as stacked, coiled membranes and are continually expelled into the lumen along the entire length of the midgut as stacked, tubular membranes; these membranes undergo changes in form as they pass from the SER to the midgut lumen. Rather than adopting the double membrane configuration in the gut lumen that was first described for hemipteran perimicrovillar membranes, these modified perimicrovillar membranes of the Corythucha gut line apical surfaces of midgut apical lamellae and intermix with the contents of the lumen; foregut and hindgut epithelial cells are devoid of vesicles containing coiled membranes observed abundantly in midgut epithelia. Rather than achieving renewal of adult midgut epithelial cells through the divisions of regenerative cells as observed in many adult insects, prolific generation of perimicrovillar membranes apparently maintains the integrity of this lengthy hemipteran midgut epithelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemipteran guts are characterized by disproportionately long midguts and disproportionately short hindguts. The short foreguts and hindguts are straight; however, to accommodate the long midgut in the body cavity, several convolutions of the midgut canal form with species-specific constant and predictable topology. The exact juxtapositioning of specific midgut regions relative to one another implies the occurrence of important osmotic exchanges of water between these regions.
Arthropods have adopted several strategies for maintaining the integrity of adult midgut monolayers that are continually exposed to ingested food. Extracellular peritrophic membranes line midgut lumens of certain arthropod species and separate ingested material from the apical microvilli of the midgut cells. Peritrophic membranes provide a protective barrier between ingested matter and the delicate plasma membranes of microvilli (Terra 2001). In some of the same and additional species, regenerative cells interspersed throughout the midgut epithelium continually divide and differentiate to replace the differentiated midgut cells that continually die (Nardi et al. 2011; Ohlstein and Spradling 2005). But for other species, neither peritrophic membranes nor regenerative cells can account for the resilience and vitality of adult midguts through which food is continually passing and being processed. Rather than replacing entire cells in the midgut monolayer, organelles and membranes of these cells are degraded and recycled by the complex autophagic machinery of the cell; without autophagy, cells accumulate damaged molecules and succumb to self-destruction (Mizushima 2007).
As more hemipteran guts have been examined, ultrastructural features of hemipteran midguts first described as perimicrovillar membranes or as double plasma membranes lining the midgut lumen (Silva et al. 1995; Lane and Harrison 1979) have been revised to include the distinctive midgut membranes of aphids (Cristofoletti et al. 2003) referred to as modified perimicrovillar membranes that are discharged into the midgut lumen from a midgut luminal surface where microvilli have been replaced by apical lamellae. To the surfaces of these perimicrovillar membranes are bound such digestive enzymes as aminopeptidase, α-glucosidase, and cysteine proteinase. By being anchored to these secreted membranes, enzymes remain in the lumen without being flushed out with honeydew and feces.
The complex configurations of hemipteran midguts and the diversity of hemipteran diets argue that surveying the ultrastructural features underlying these differences would provide information on whatever diverse strategies have been adopted not only to maintain the integrity of the midgut epithelium but also to process the variety of ingested nutrients from plant and animal sources.
In another plant-feeding hemipteran Corythucha ciliata, generation of the modified luminal membranes first reported for aphids occurs along the entire length of a midgut that is also lined with apical lamellae rather than microvilli. The production of these membranes at the smooth endoplasmic reticulum (SER) and trafficking of vesicles containing these stacked, coiled membranes to the luminal surfaces of epithelial cells continually renew the apical surfaces of these midgut cells.
Materials and methods
Collection of specimens
Adult individuals of Corythucha ciliata were collected in Urbana, Illinois, on the under surfaces of sycamore leaves. Ten were processed for sectioning; 20 were prepared as whole mounts for light microscopy/immunolabeling.
Dissection of tissues
For dissection, well-chilled lace bugs that had been placed on ice for 15 min were transferred to petri dishes in which black Sylgard (Dow Corning) had been added as a substrate. The insects were submerged in cold Grace’s insect culture medium (pH 6.5, Invitrogen). To the silicone surface of Sylgard, tissues were pinned with stainless steel minutien pins (0.1 mm diameter). After dissection with finely sharpened forceps and tungsten needles, tissues were either processed for (1) sectioning or for (2) preparation of whole mounts.
Preparation of sections and whole mounts for microscopy
After the addition of fixative, the pinned tissue retained its configuration. The two different fixatives used are described below.
Specimens chosen for preparation of whole mounts were fixed for 30 min in a solution of phosphate-buffered saline (PBS) containing 4% paraformaldehyde. Fixative was removed by washing tissues three times over a 30–60-min period in PBS.
Six whole mounts were labeled with the rabbit anti-mitosis antibody anti-phosphohistone H3 (Millipore). This primary antibody was diluted 1:100 in blocking buffer (PBS + 10% normal goat serum + 0.1% Triton X-100) and incubated with tissue overnight at 4 °C. After three rinses in blocking buffer, tissue was then incubated for 2 h at room temperature with 7.5 mg/ml fluorescein isothiocyanate (FITC)-coupled goat anti-rabbit antibody (Vector). A 1:1000 dilution of nuclear-specific 4′,6-diamidino-2-phenylindole (DAPI, 1 mg/ml distilled water) was added to label all nuclei.
Following three more washes with blocking buffer, the labeled tissues were mounted on glass slides in a mixture of Tris (pH 9.0):glycerin (3:7) and imaged with a Nikon E600 fluorescence microscope (Nardi et al. 2010).
Specimens for sectioning and subsequent examination with electron microscopy and light microscopy were fixed at 4 °C in a primary fixative of 2.5% glutaraldehyde and 0.5% paraformaldehyde dissolved in a rinse buffer of 0.1 M cacodylate (pH 7.4) containing 0.18 mM CaCl2 and 0.58 mM sucrose. After 3 h in this fixative, tissues were washed three times with rinse buffer before being transferred to the secondary fixative (2% osmium tetroxide in rinse buffer). Tissues remained in this solution for 4 h in the cold and were then washed three more times with rinse buffer. To enhance membrane contrast, rinsed tissues were placed in filtered, saturated uranyl acetate for 30 min immediately before being gradually dehydrated in a graded ethanol series (10–100%).
From absolute ethanol, tissues for sectioning were transferred to propylene oxide and infiltrated with mixtures of propylene oxide and resin before being embedded in pure LX112 resin. Resin was polymerized at 60 °C for 3 days followed by an additional overnight treatment in an 80 °C oven.
Embedded tissues were sectioned with a diamond knife either at 0.5 μm for light microscopy or at ~ 0.09 μm for electron microscopy. Sections for light microscopy were mounted on glass slides and stained with a solution of 0.5% toluidine blue in 1% borax. Thin sections of those regions of alimentary canal chosen for ultrastructural examination were mounted on copper grids and stained briefly with saturated aqueous uranyl acetate and Luft’s lead citrate to enhance contrast. Images were taken with a Hitachi 600 transmission electron microscope operating at 75 kV.
Results
Global organization of the Corythucha alimentary canal
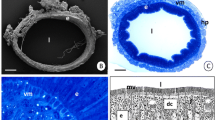
The configuration of the Corythucha midgut in situ arises from multiple folding of the midgut epithelial canal (Fig. 1). The central folded stretch of the midgut that lies between the anterior end and the posterior end of the midgut is folded five times. Figure 2 shows the partial unfolding of the gut and labels the locations of the five folds. Light microscopic images of sections at different anterior-posterior levels along the lengthy gut provide an overview for the ultrastructural images that follow.
The exceptional length of the midgut is emphasized in this outstretched gut of Corythucha labeled with the DNA-specific stain DAPI. Between the lines marking divisions between the foregut and midgut (FG-MG) and the midgut and hindgut (MG-HG), four prominent regions of the midgut are indicated based on their diameters: M1, expanded anterior-most region; M2, narrow region connecting M1 and M3; M3, expanded region that lies between the two constricted regions of the midgut, M2 and M4; and M4 is the posterior-most stretch of the midgut that borders on the hindgut. In situ, the midgut folds five times. The location of each fold is indicated with numbers 1–5. Sections of the gut have been cut at nine locations along its anterior-posterior axis (a–i) and provide global views of the epithelial regions whose ultrastructure is depicted in subsequent figures of electron micrographs
If the convoluted alimentary canal of Corythucha is straightened, two expanded, distinct regions of the midgut can be discerned along the anterior-posterior axis—M1 and M3 that are separated by a narrow M2 stretch of the midgut. Another stretch of narrow midgut (M4) lies between M3 and the hindgut (Fig. 2). Region M1 assumes a linear configuration between the foregut and the more posterior multiply folded portion of the midgut that includes region M2 at its anterior end; the basal surfaces of region M1 are surrounded by the hemocoel. M1’s basal surfaces do not physically interact with other gut regions. However, region M3 interacts along its basal surfaces not only with hemolymph but also with the basal surfaces of more posterior stretches of the midgut. These folded lengths of the midgut physically adhere along their basal surfaces. Presumably, exchanges of water and nutrients occur to some extent across these surfaces of M2, M3, and M4.
The thickness of the epithelial monolayer of the more anterior region M1 exceeds the thickness of the epithelium of region M3 (Fig. 2). Since the two regions have approximately the same outside diameters, the luminal volume of region M3 is correspondingly greater than that of region M1. All luminal surfaces of the midgut are lined with extensions of the epithelial apical surface that resemble microvilli; these apical extensions referred to as lamellae, however, are often fused laterally (Fig. 3a, b). Figures 4 and 5d are arranged in order along the anterior-posterior axis of the midgut, from the anterior end of M1 to the posterior end of M4 that borders the hindgut.
a The lumen (L) of the midgut epithelium from region M4 (shown in Fig. 2) is lined by extensions of the apical surface referred to as lamellae (arrows) rather than microvilli. These lamellae are fused laterally. Copious electron-dense matter (asterisk) is discharged into the lumen. Opaque, white granules (g), each covered by an electron-dense film are also present in the lumen. b At the anterior end of region M1, electron-dense vesicles (arrows) pass between the apical lamellae en route to the L
a At level b shown in Fig. 2, many apical lamellae lie within deep invaginations (arrows). The lumen (L) is filled with electron-dense matter and opaque, white granules (g). b Level e shown in Fig. 2. Apical extensions are broad (arrows) with gaps separating these extensions. These gaps are filled with electron-dense material that is being expelled into the L. cm, circular muscles of epithelial basal surface. Between the apical extensions and the circular muscles lie extensive smooth endoplasmic reticulum (ser) and a number of vesicles with stacked, coiled membranes (arrowheads)
The epithelial cells of the posterior midgut region M4 expel an abundance of electron-dense matter into the midgut lumen (L) from their apical lamellae (ap). a Level f in Fig. 4a. As in Fig. 4a, ap lie within deep invaginations of the midgut epithelium. Electron-dense matter is abundantly present among these lamellae. b Between level f and level g in Fig. 4a. In this narrow stretch of the midgut, the L is filled with electron-dense matter and white granules (g) having rather uniform dimensions (~ 1–5 μm). An electron-dense film surrounds each granule. Also see granules in Figs. 3, 5, and 10. c At level g in Fig. 4a, the ap line a highly convoluted L. ly, lysosomes. d Level h of Fig. 4a is located at the posterior-most stretch of the midgut. ap line a L mostly devoid of electron-dense matter. cm, circular muscles; lm, longitudinal muscles
Along the entire length of the midgut, electron-dense vesicles are expelled at the bases of these apical lamellae. An electron-dense core separates the plasma membrane lipid bilayers of adjacent lamellae; these features can be resolved at magnifications > × 20,000 (Fig. 6a, a'). As electron-dense vesicles pass between lamellae en route to the midgut lumen, the alignment of structures within these vesicles is evident (Fig. 6b).
a, a' Level e in Fig. 2. Multivesicular bodies (arrows in a) are abundant in epithelial cells. In the magnified view of the apical lamellae (a'), the structure of their membrane bilayers (white arrows in a') and the electron-dense material separating these bilayers is evident. b Level g in Fig. 2. Electron-dense particles (arrows) of relatively uniform prolate shapes and sizes containing polarized contents are being expelled between the apical lamellae
These vesicles arise from the abundant SER evident in basal regions of epithelial cells (Figs. 3, 4b, and 7a–e). The routes followed by these electron-dense structures from the apical surfaces of the midgut epithelium to the lumen pass between these lamellae (Figs. 3b, 4, 5, and 6).
a–e Vesicles with stacked, coiled membranes (arrowheads) and lysosomes (ly) are ubiquitous throughout the cytoplasm of the midgut epithelium. lm, longitudinal muscles. a Level b in Fig. 2. This section of the midgut epithelium includes both the epithelial basal surface with longitudinal muscles (fm in Fig. 7a and lm in Fig. 7b) and the apical surface with lamellae bordering the lumen (L). Between the apical and basal surfaces lie extensive smooth endoplasmic reticula (ser). b Level g in Fig. 2. Opaque, white granules (g), each covered by an electron-dense film, are present in the L. The granules are surrounded by electron-dense particles observed throughout the lumen. Between the lumen and basal surface lies an extensive ser. c–e Level d in Fig. 2. Vesicles (arrowheads) contain stacked, coiled membranes. ly, lysosomes
Luminal membranes
Within the electron-dense packets that are expelled at the bases of these modified microvilli lie stacked, coiled membranes (Fig. 8a–f). As these stacked, coiled membranes pass between apical lamellae en route to the lumen, they undergo a configurational change to stacked, tubular membranes (Figs. 9 and 10). Figure 9 shows the double membrane that surrounds each vesicle as it emerges from the smooth endoplasmic reticulum containing coiled membranes separated by regular 5-nm spacing. Microtubules whose diameters measure 23 nm are indicated for comparison. The stacked membranes exhibit changes in morphology as they traverse the epithelial cell from the smooth endoplasmic reticulum to the lumen. In Fig. 9b, the double-membrane vesicle fuses at the base of the apical lamellae to release its membrane contents shown at higher magnification in Fig. 10. The periodicity of the stacked membranes that pass between apical lamellae shows a spacing of 12.5 nm. When sectioned in a specific plane, these stacked membranes are revealed to be tubular (Fig. 10). These should not be mistaken for the abundant, highly oriented 23-nm microtubules that occupy the adjacent cytoplasm of the apical lamellae (Fig. 10b). The stacked membranes partially retain their stacked configuration after passage into the midgut lumen (Fig. 10c).
a–f Vesicles containing stacked, tightly coiled plasma membranes occupy the cytoplasm of midgut epithelial cells at all levels along the epithelial anterior-posterior axis. Insets in each figure show the regular 5-nm periodicity of the membranes. The locations of these images in Fig. 2 are as follows: a, level b; b, level f; c, level b; d, level g; e, level g; f, level h
a In this magnified view of Fig. 7d (upper right corner), the double membranes of vesicles containing stacked, coiled membranes with their 5-nm periodicity are marked with arrowheads. Microtubules (~ 23 nm) are indicated with arrows for comparison. b Vesicles containing stacked, tubular membranes (arrow) fuse at the base of apical lamellae (arrowhead). Lumen is at the top of the figure
a A close-up of the region marked with arrow and arrowhead in Fig. 9b shows the organization of the membranes that are being expelled between the apical lamellae. Arrowheads point to the stacked, tubular membranes with the periodicity of 12.5 nm. b Within a given apical lamella, microtubules (arrowheads) are aligned along its long axis. These lamellae and microtubules have been sectioned transversely. At this high magnification, tubulin subunits of microtubules are evident. c Within the midgut lumen at level d in Fig. 2, more stacked membranes of 12.5-nm periodicity (arrowheads) are visible among the contents of the lumen
In the midgut lumen, the diet of Corythucha blends with these secreted membranes. These membranes are discharged into the lumen along the entire length of the midgut. Whatever enzymes reside in these secreted membranes digest plant tissue/sap that is ingested. The digested material is eventually absorbed at the surfaces of midgut cells.
Maintenance of midgut integrity
Midgut regenerative cells are not present in epithelial sections. Neither do whole mounts of Corythucha gut epithelia label with the mitosis marker anti-phosphohistone H3. The integrity of the midgut epithelium must be maintained without the continual replacement of cells. The liquid, nonabrasive meals that are consumed by these herbivorous Hemiptera are not associated with extensive replacement of midgut epithelial cells by regenerative cells. Rather than having nests of stem cells to replace damaged or dying cells, continuous synthesis of luminal membranes apparently maintains the health and integrity of Corythucha midgut epithelial cells.
Discussion
Examining the structure of the alimentary canal of Corythucha ciliata at closely spaced intervals along its entire length provides a detailed survey of how positional information is specified for cells of these cylindrical epithelia. Cells at different locations along the organ’s anterior-posterior axis exhibit a combination of features not described for other insects whose digestive tracts have been examined.
Perimicrovillar membranes
Presumably, the relatively sturdy peritrophic membranes consisting of chitin and proteins that line the midgut lumens of non-hemipteran insects protect the delicate membranes of the midgut microvilli from abrasion by food particles. This extracellular membrane also partitions digestive enzymes into compartments: the endoperitrophic space within the confines of the peritrophic membrane and the ectoperitrophic space lying between the microvilli and outer surface of the peritrophic membrane (Terra 2001).
For Hemiptera, however, in the location occupied by the peritrophic membrane lie additional plasma membranes that enshroud the plasma membranes coating the luminal surfaces of midgut epithelial cells. These membranes are located between the lumen of the midgut and the microvilli or apical lamellae of the midgut epithelium. The characteristic double plasma membranes lining the midgut were first described in the bug Rhodnius prolixus as perimicrovillar membranes (Lane and Harrison 1979). In this blood-feeding hemipteran, the second membrane surrounds the luminal cell membrane of each microvillus. The microvilli are separated from this second membrane by a regular space of approximately 10 nm. These perimicrovillar membranes are known to contribute a great variety of enzymes to digestion (Terra and Ferreira 1994).
Membranes typically arise from Golgi regions of cells. More recently, Silva et al. (1995) offered evidence that the perimicrovillar membranes of a plant-feeding bug Dysdercus peruvianus are derived from the inner membrane of double-membrane cytoplasmic vesicles that bud from double-membrane Golgi cisternae. The double-membrane vesicles fuse at the luminal surface of midgut epithelial cells. The outer membrane of the vesicle fuses first with the microvillar membrane; the inner membrane of the vesicles subsequently joins with existing perimicrovillar membrane.
In examining a diversity of hemipteran midguts (Silva et al. 2004), however, this precise description of the modification of the luminal membrane of the hemipteran midgut and its origin does not seem to apply universally to other members of the insect order. For the aphid midgut, Cristofoletti et al. (2003) described “modified perimicrovillar membranes” that extend into the lumen. In place of regularly arrayed microvilli, a network of luminal or apical lamellae covers the midgut epithelial surface. Following their birth in the Golgi bodies of the midgut cytoplasm, these presumptive modified perimicrovillar membranes pass along the lateral spaces between apical lamellae until they extend from the luminal surface of the lamellae as mature modified perimicrovillar membranes (MPMs). These MPMs were described as being amorphous membrane masses (Cristofoletti et al. 2003). Immunocytochemical localization of aminopeptidase, alpha-glucosidase, and cysteine proteinase demonstrated that these digestive enzymes occur not only within the cytoplasm of midgut epithelial cells but also extracellularly within these modified perimicrovillar membranes.
Although the modified perimicrovillar membranes of the aphid midgut were described as amorphous (Cristofoletti et al. 2003), similar apparently amorphous membranes have also been observed throughout the midgut of Corythucha that, upon closer examination, are highly structured prior to being expelled in the midgut lumen. Higher-resolution images of these electron-dense structures within the epithelial cytoplasm reveal that they represent tightly coiled membranes. These coiled membranes are found along the entire length of the midgut epithelium (Figs. 3, 4b, 7a, 8, and 9a). As these highly organized membranes enter the spaces between apical lamellae and finally the lumen, they transition from the stacked, coiled membranes to stacked, tubular membranes. Membranes are capable of complex transformations (Lipowsky 1995); membrane polymorphism apparently accompanies the formation of these modified perimicrovillar membranes.
The similarity in the structural features of the midgut epithelial cells of aphids and lace bugs (i.e., presence of apical lamellae and modified perimicrovillar membranes) implies a similarity in their function. The apical lamellae on luminal surfaces of aphid midgut cells have been postulated to offer resistance to hydrostatic pressure generated by the hyperosmotic diets of aphids (Cristofoletti et al. 2003); however, (1) the aphid diet of phloem sap and (2) the presence of endosymbiotic bacteria that modulate the osmotic pressure within their midguts (Pompon et al. 2011) differ markedly from the diet of lace bugs that lack microbial symbionts as discussed in the next two sections.
Comparing the diet of Corythucha with the diet of sap-feeding aphids
Lace bugs with their piercing mouthparts ingest liquid diets; however, in addition to sap from phloem and xylem cells, lace bug stylets can also apparently extract cytoplasm from cells of leaf mesophyll as implied from the pattern of feeding damage that is confined to clusters of leaf cells circumscribed by leaf veins. This feeding pattern can account for the stippled and bleached foliage characteristic of lace bug-feeding damage. Goodchild (1966) described members of the related hemipteran families Tingidae and Miridae as “feeding on mesophyll cells with the aid of a toxic saliva.” A recent survey of the salivary gland genes responsible for extra-oral digestion in the closely related mirid bug Lygus revealed the presence of 45 polygalacturonase enzymes that break down plant cell walls (Zhu et al. 2016). This abundance of enzymes that degrade cell walls indicates a diet more diverse and more nutritious than that consumed by those phloem sap and xylem sap feeders, i.e., aphids and leafhoppers, respectively.
The midgut lumen of Corythucha contains numerous white, opaque granules (Figs. 3, 4, 5b, and 7b). Each of these is surrounded by an electron-dense film or membrane. These white granules are never observed within epithelial cells but only within the lumen. The density of these structures increase in more posterior portions of the midgut, and the structures are also numerous in the hindgut lumen and feces (not shown).
Undigested starch granules that resemble these opaque, white granules observed in the hindgut lumens of Corythucha have been frequently observed in hindguts of beetles such as Tenebrio and Sitophilus. One study that addressed the activity of amylase on starch granules demonstrated that amylase digestion is limited on intact starch granules and granules with lipids bound to their surfaces (Baker and Woo 1992). Removal of these lipids with ethanol or detergent from granules increased their susceptibility to amylase. Mechanical damage to intact starch granules was also effective in increasing their digestion by insect α-amylase. Lipids are known to bind surfaces of starch granules, and after reaction of lipids with osmium used during fixation, these bound lipids would appear as electron-dense films. The opaque, white granules in Corythucha guts are presumed to represent undigested starch granules from the plant cell cytoplasm, each surrounded by such an electron-dense film.
Corythucha lacks the microbial symbionts found in other plant-feeding hemipterans
In addition to providing certain nutrients that are missing from the diets of their hosts (Engel and Moran 2013; Douglas 2003), microbial endosymbionts can contribute to reducing the sugar concentration within the midgut. Aphids whose intracellular symbionts have been eliminated with antibiotics (aposymbiotic) have a higher hemolymph osmotic potential than aphids with symbionts and compensate for this difference by consuming more low-osmolarity xylem sap than their symbiotic counterparts (Pompon et al. 2011).
If the convoluted alimentary canal of other plant-feeding hemipterans such as pentatomids, cydnids, and alydids is straightened, four distinct regions of the midgut can be discerned along the anterior-posterior axis, designated M1–M4 (Kikuchi et al. 2011). For most of these hemipterans, the most posterior region M4 contains midgut caeca that are densely inhabited by microbes.
Hemipterans that feed on mesophyll cells rather than solely on phloem and/or xylem sap do not encounter the challenges of a diet that is lacking essential nutrients. Likewise, the midguts of these mesophyll-feeding bugs do not encounter the high osmotic potential posed by the high sugar concentration of ingested phloem sap. The long, convoluted midgut of Corythucha; its four distinct regions; and the hindgut are completely devoid of caeca and microbes. Endosymbionts and bacteriocytes such as those found in aphids are also absent from the body cavity of Corythucha.
Conclusions
Regenerative cells have not been observed to renew the midgut epithelium of the lace bug Corythucha ciliata. Along the entire length of the lace bug midgut, however, vesicles containing stacked, coiled membranes pass from the smooth endoplasmic reticulum in basal regions of epithelial cells to the apical surfaces of these cells where they are expelled as membranes of altered morphology between apical lamellae. These polymorphic membranes continually renew the perimicrovillar membranes that line the luminal face of the midgut. Among other plant-feeding hemipterans, Corythucha is distinctive in its (a) lack of association with microbial symbionts and its (b) diet of both plant sap and plant cytoplasm.
References
Baker JE, Woo SM (1992) Digestion of starch granules by α-amylases from the rice weevil, Sitophilus oryzae: effect of starch type, fat extraction, granule size, mechanical damage, and detergent treatment. Insect Biochem Mol Biol 22:529–537
Cristofoletti FT, Ribeiro AF, Deraison C, Rahbé Y, Terra WR (2003) Midgut adaptation and digestive enzyme distribution in a phloem-feeding insect, the pea aphid Acyrthosiphon pisum. J Insect Physiol 49:11–24
Douglas AE (2003) The nutritional physiology of aphids. Adv Insect Physiol 31:73–140
Engel P, Moran NA (2013) The gut microbiota of insects—diversity in structure and function. FEMS Microbiol Rev 37:699–735
Goodchild AJP (1966) Evolution of the alimentary canal in the Hemiptera. Biol Rev 41:97–140
Kikuchi Y, Hosokawa T, Fukatsu T (2011) An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460
Lane NJ, Harrison JB (1979) An unusual cell surface modification: a double plasma membrane. J Cell Sci 39:355–372
Lipowsky R (1995) The morphology of lipid membranes. Curr Opin Struct Biol 5:531–540
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Nardi JB, Bee CM, Miller LA (2010) Stem cells of the beetle midgut epithelium. J Insect Physiol 56:296–303
Nardi JB, Bee CM, Miller LA, Mathur D, Ohlstein B (2011) Cell renewal in adjoining intestinal and tracheal epithelia of Manduca. J Insect Physiol 57:487–493
Ohlstein B, Spradling A (2005) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474
Pompon J, Quiring D, Goyer C, Giordanengo P, Pelletier Y (2011) A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J Insect Physiol 57:1317–1322
Silva CP, Ribeiro AF, Gulbenkian S, Terra WR (1995) Organization, origin and function of the outer microvillar (perimicrovillar) membranes of Dysdercus peruvianus (Hemiptera) midgut cells. J Insect Physiol 41:1093-1103
Silva CP, Silva JR, Vasconcelos FF, Petretski MDA, DaMatta RA, Ribeiro AF, Terra WR (2004) Occurrence of midgut perimicrovillar membranes in paraneopteran insect orders with comments on their function and evolutionary significance. Arthropod Struct Dev 33:139–148
Terra WR (2001) The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol 47:47–61
Terra WR, Ferreira C (1994) Insect digestive enzymes: properties, compartmentalization and function. Comp Biochem Physiol 109B:1–62
Zhu Y-C, Yao J, Luttrell R (2016) Identification of genes potentially responsible for extra-oral digestion and overcoming plant defense from salivary glands of the tarnished plant bug (Hemiptera: Miridae) using cDNA sequencing. J Insect Sci 16(1):60
Acknowledgments
We thank Dorothy Loudermilk of the School of Chemical Sciences Graphic Artist Services (University of Illinois) for the care and skill with which she prepared images in this manuscript. We are grateful to an anonymous reviewer whose constructive comments helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nardi, J.B., Miller, L.A. & Bee, C.M. Luminal membranes in the midgut of the lace bug Corythucha ciliata. Cell Tissue Res 375, 685–696 (2019). https://doi.org/10.1007/s00441-018-2943-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2943-6