Abstract
Diabetes mellitus in human and animal models has been correlated with low sperm count, testicular abnormalities, high levels of germ cell death, and oxidative stress. In this study, we focus on three questions: (1) Is germ cell apoptosis stage-specific in diabetic male rats? (2) Could ascorbic acid (AA) reverse oxidative and histological damage and restore testicular dysfunction? (3) Could AA treatment restore fertility parameters in diabetic rats? Adult Sprague-Dawley rats were divided into four groups: control, diabetic, control plus AA, and diabetic plus AA. Seminiferous tubules underwent severe histological damage, together with a change in frequency of some stages of the seminiferous cycle, and germ cell apoptosis was increased in a stage-dependent manner in diabetic rats. We found a significant decrease in testosterone and higher levels of lipid peroxidation in diabetic rats when compared with controls. A major finding was that AA reversed the histological damage and peroxidation levels to control levels in diabetic rats, but testosterone levels remained unchanged. The pregnancy rate was decreased in females that mated with diabetic rats and those treated with AA, but the litter size was only reduced in the second case. Interestingly, spermatozoa from diabetic and AA-treated rats showed reduced motility and hyperactivation, but only diabetic rats had higher levels of apoptosis when compared with controls. These results suggest that treatment with AA reverses testicular damage in diabetic rats but is insufficient to restore testosterone levels, sperm motility, and fertility in a rat model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a metabolic disease that affects nearly 300 million people worldwide (Agbaje et al. 2007). It is estimated that 366 million people will be diabetic by the year 2030, and so diabetes is considered a public health problem (Pontes et al. 2011). Diabetes is characterized by hyperglycemia attributable to a deficiency in insulin secretion (diabetes mellitus type I) or insulin action (diabetes mellitus type II) and leads to several systemic abnormalities, such as ophthalmopathies, neuropathies, kidney impairments, and cardiovascular and reproductive diseases (Maritim et al. 2003; Pitocco et al. 2010; Zaccardi et al. 2015). With regard to reproductive abnormalities and side effects, diabetes has been associated with sexual dysfunctions in both male and female individuals. In males, for whom fertility problems account for around 50% of all infertility cases at medical consultancy (La Vignera et al. 2012; Poongothai et al. 2009), diabetes causes deleterious effects on endocrinal hormone secretion (hypothalamic-pituitary-gonadal axis), spermatogenesis, and ejaculation (Sexton and Jarow 1997). For instance, low levels of testosterone and androstenedione found in men suggest an effect on Leydig cells (Gluud et al. 1982). Likewise, results from testicular biopsies of individuals with diabetes type I who were also sexually impotent (Cameron et al. 1985) showed decreased tubule diameters, luminal occlusion, and sloughed germ cells. Moreover, diminished sperm count and alterations in motility and morphology have been reported in semen samples of sub-fertile men with diabetes type I (Bartak et al. 1975; Delfino et al. 2007; Padron et al. 1984; Ranganathan et al. 2002). Retrograde ejaculation, an abnormality that occurs when semen is redirected to the urinary bladder instead of being expelled via the urethra, is another pathology found in these patients (Greene and Kelalis 1967). Therefore, diabetes is associated with testicular abnormalities and lower sperm production in male diabetic patients.
With regard to testicular abnormalities in diabetes, animal models have provided similar results to those found in humans suggesting that they are good models for studying this disease. For example, streptozotocin (STZ; 60 mg/kg ip) or alloxan (40 mg/kg iv) has been found to reduce significantly the weight of the testes and epididymis of diabetic rats in comparison with normal rats (Shrilatha and Muralidhara 2007a). In addition, diabetic rats show a decrease of testosterone levels and diameters of the seminiferous tubules (Atalay et al. 2009), atrophy of the tubules and fewer or a total absence of spermatozoa in the luminal space (Arikawe et al. 2006; Atalay et al. 2009; Scarano et al. 2006; Shrilatha and Muralidhara 2007b), and Sertoli cell damage in early stages of STZ treatment (Xu et al. 2014).
Spermatogenesis is a complex process in which testicular stem cells (named spermatogonia) produce spermatozoa under the influence of steroidal hormones, through successive mitosis, meiosis, and the differentiation of haploid spermatids (Hermo et al. 2010). Spermatogenesis can be divided into stages or groups of cells in a specific phase of development (Russell et al. 1990). Even though the purpose of spermatogenesis is constantly to produce spermatozoa throughout the male fertile life, apoptosis or germ cell death have been reported as a physiological event that ensures the quality of gamete production (Blanco-Rodriguez and Martinez-Garcia 1996; Dunkel et al. 1997; Moreno et al. 2011). In this sense, some studies have found elevated levels of apoptosis in the testicular tissue of diabetic rats, an aspect that may be closely related to hypo-spermatogenesis (Kilarkaje et al. 2014). In addition, increased levels of the phosphorylation of JNK (c-Jun NH2-terminal kinase) and the activation of Bax and its effector caspase-3, all of them indicators of apoptosis, have been detected in testicular lysates of rats treated with 60 mg/kg STZ (Koh 2007). Likewise, higher apoptosis, as evaluated by the Annexin V technique, has been determined in spermatogonia, spermatocytes, and spermatids of mice with insulitis, a pathology that produces a phenotype similar to diabetes mellitus (Sainio-Pollanen et al. 1997).
Oxidative stress is an important component of the reproductive system pathogenesis caused by diabetes mellitus. Higher levels of lipid peroxidation, reactive oxygen species (ROS), and lower levels of catalase, glutathione reductase, glutathione peroxidase, and superoxide dismutase antioxidant activity have been found in cytosolic and mitochondrial fractions of testes of diabetic rats treated with STZ when compared with controls (Shrilatha and Muralidhara 2007b). Similar findings have been reported by Bal et al. (2011) for lipid peroxidation and glutathione reductase levels. These observations have been reinforced by data showing that the activity of antioxidant enzymes is re-established when rats are treated with a daily dose of 1.5 mg/kg ascorbic acid (AA; El-Missiry 1999).
AA or Vitamin C is an antioxidant widely prescribed to treat scurvy and to prevent flu. An antioxidant is a natural or synthetic substance that scavenges free radicals and protects against cellular damage. Vitamin C, Vitamin A, Vitamin E, Q10 Coenzyme, and several bioflavonoids appear on the list of antioxidants used by diabetic patients to improve non-reproductive parameters (Maritim et al. 2003).
Thus, in view of these antecedents, we have focused on three questions in the present study: (1) Is germ cell apoptosis stage-specific in diabetic adult male rats? (2) Could AA treatment prevent oxidative and histological damage in diabetic rats and restore testicular dysfunction? (3) How does AA treatment affect fertility parameters in diabetic rats?
Materials and methods
Chemicals and reagents
STZ, Harris hematoxylin solution, sucrose, chlorhydric acid (37%), and acetic acid were purchased from Sigma-Aldrich Chemical (St. Louis, Mo., USA). Sodium citrate and thiobarbituric acid, L-ascorbate, and Entellan were acquired from Merck (Darmstadt, Germany). The terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) kit was obtained from Roche (Roche Diagnostics, Mannheim, Germany). All chemicals used were of analytical grade. All solutions were made up with distilled water.
Induction of diabetes
Male Sprague-Dawley rats (8–9 weeks old; 150 – 200 g) were acquired from the Faculty Animal Facility. Animals were housed in polycarbonate cages under a 12 L:12D cycle and were provided with fresh drinking water and rat chow ad libitum. All animal handling protocols were endorsed by the Chilean National Fund of Science and Technology (Fondecyt).
In order to generate a chemically induced diabetic model, 6– to 8-h-fasted rats were injected intra-peritoneally with STZ, a β-pancreatic cell toxicant, according to the protocol of Wu and Huan (2008). Briefly, STZ was diluted in citrate buffer (pH 4.5), was administrated at a single dose of 65 mg/kg body weight, and was followed by the administration of 10% sucrose water during the following 24 h. For the control group, an equal volume of citrate buffer was injected, and plain water was provided. Blood glucose was measured by using reagent strips (Accu-Check Glucometer, Roche). Animals were considered diabetic if blood glucose values were higher than 200 mg/dl (Hernandez-Salinas et al. 2013; Scarano et al. 2006; Shrilatha and Muralidhara 2007a). Glucose and weight were monitored weekly for 60 days under conditions described previously. All individuals that did not reach the glucose limit were not considered diabetic (200 mg/dl) and were excluded from the study.
AA therapy
Animals were randomly divided into four groups of four animals per cage, and the antioxidant therapy was daily administered by gavage. This therapy started 31 days after diabetes was confirmed and was continued for another 30 days. The groups were as follows:
-
(1)
Control group: non-diabetic rats receiving distilled water.
-
(2)
Control + AA group: non-diabetic rats receiving an aqueous solution of AA by gavage and at a concentration of 70 mg/kg rat weight.
-
(3)
STZ-diabetic group: STZ-induced diabetic rats receiving distilled water.
-
(4)
STZ-diabetic + AA group: STZ-induced diabetic rats receiving an aqueous solution of AA by gavage, at a concentration of 70 mg/kg of rat weight.
The dose of AA was chosen according to previous studies performed by our group (Hernandez-Salinas et al. 2015, 2013).
Assessment of stages and morphological analysis
Routine histology was performed to assess cycle stages and morphology. Briefly, testes were dehydrated in ascending concentrations of ethanol and embedded in paraffin. Thin sections (5–7 μm) were cut and stained with periodic acid-Schiff and hematoxylin. Sections were mounted with Entellan and examined under a light microscope (Olympux CX41, Tokyo, Japan) at a magnification of ×600.
Seminiferous tubule stages were classified into groups: I-III, IV-VI, VII-VIII, IX-XI, and XII – XIV (Leblond and Clermont 1952; Russell et al. 1990). To evaluate the frequency of stages, 200 cross sections of seminiferous tubules were randomly analyzed per individual. In addition, an important group of seminiferous tubules that could not be classified into any stage because of their high grade of damage were designated as unidentified. Seminiferous tubule diameters were measured in rounded-shaped seminiferous tubules and expressed as an average diameter (μm). An average of TUNEL(+) cells were estimated by counting all TUNEL(+) cells present in 300 cross sections of seminiferous tubules in each animal. All of these studies were carried out by light microscopy.
Status of lipid peroxidation
Lipid peroxidation was estimated through levels of thiobarbituric-acid-reactive substances (TBARS). It was assessed in testicular tissue homogenates by using the method described by Ramanathan et al. (1994) with slight modifications and malondialdehyde as a standard (MDA; Sigma-Aldrich, St. Louis, Mo., USA). Briefly, testicular homogenates were mixed with SDS (8% w/v), TBA (0.8% w/v), and acetic acid (20% v/v) and heated for 60 min at 90 °C. Precipitated materials were removed by centrifugation, and the absorbance of the supernatant was determined at 532 nm. Levels of TBARS were calculated by using a calibration curve with MDA.
TUNEL staining
Fragmentation of DNA was evaluated by immunohistochemical detection with the In Situ Cell Death Detection Kit, POD (Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s protocol with some modifications. In short, testicular tissue sections that had been previously dewaxed were placed in a Couplin jar containing 0.01 citrate buffer pH 6.0. They were blocked with 9% hydrogen peroxide in TRIS-HCl (0.1 M, pH 7.2) containing 3% bovine serum albumin (BSA). Slides were rinsed with phosphate-buffered saline (PBS), and 50 μl TUNEL reaction mixture was added followed by incubation at 37 °C. Subsequently, 50 μl POD-converter was added to each section, and slides were incubated for 30 min at 37 °C. After three PBS rinses, 50 μl diaminobenzidine substrate was added, and the slides were rinsed again. Finally, Entellan (Merck, Darmstadt, Germany) was used to mount the sections, which were then analyzed by light microscopy (Olympus Cx31, Japan).
Plasma testosterone determination
After sedation of the rats with CO2, blood was collected in tubes containing clot activator and centrifuged (4000 rpm, 10 min, 4 °C) in order to obtain plasma for the assessment of testosterone. Values for testosterone were determined by radioimmunoassay (RIA) by using the kits from the WHO Matched Reagent Programme (World Health Organization – Program for the Provision of Matched Assay Reagents for the Radioimmunoassay of Hormones in Reproductive Physiology, Genève, Switzerland, 1980), following the manufacturer’s protocol. The sensitivity for this assay was 380 fM.
Sperm isolation and capacitation assay
Mature rat cauda epididymal sections were separated from the epididymis and placed in 1 ml non-capacitation medium at 37 °C under mineral oil. Three incisions were made with fine scissors, and the non-capacitation medium drops containing the tissue were incubated for 20 min to allow the spermatozoa to swim out. Media were prepared according to Kaplan and Kracier (1978) with double-distilled and deionized water. Stock solution A contained 87.9 mM NaCl, 5.1 mM KCI, 1.5 mM KHPO4, 1,5 mM MgSO4, 14 mM CaCl2, 31.25 mM HEPES (N-2-hydroxietil piperazine-N′ -2-etanosulfonate), and 4 mg/ml phenol red. Stock A was titrated to pH 7.2. Stock solution B contained 167.1 mM NaHCO3 and 3 mg/ml phenol red. The final solution for the capacitation medium was prepared by mixing 80 ml solution A and 15 ml solution B and by adding 0.54 mM glucose, 10,000 units penicillin, 5 mg streptomycin, and 400 mg BSA to give a final volume of 100 ml (Kaplan and Kraicer 1978). Non-capacitation medium was based on the solution described above, but NaHCO3 and BSA were substituted by NaCl and HEPES. Aliquots of the original suspension were diluted to 5 × 106 cells/ml fresh capacitation or non-capacitation medium and incubated for up to 2.5 h at 37 °C under an atmosphere of 5% CO2.
Annexin V-PI assay
Phosphatidylserine exposure was evaluated by flow cytometry (fluorescence-activated cell sorting [FACS]) with the Annexin V-FITC and propidium iodide (PI) kit following the manufacturer’s recommendations (Invitrogen, Carlsbad, Calif., USA) with some modifications. Mature rat sperms were recovered from the cauda epididymis in tempered PBS, concentrated to 1 × 106 cells/ml, and washed twice in cold PBS. The suspension was split into four tubes of 100 μl each. One tube was used to evaluate autofluorescence, the second was incubated with 0.5 μM PI, the third with annexin V-FITC, and the fourth with Annexin V-FITC plus 0.5 μM PI. The samples were vortexed and incubated for 15 min at room temperature in darkness. Fluorescence was evaluated in a FACS Canto Cytometer (BD Biosciences, San Jose, Calif., USA), and 10,000 gated events were acquired for each condition. All data were analyzed by using FCS express V2.0 software (De Novo Software, Los Angeles, Calif., USA).
Dicholorofluorescein-diacetate-PI assay
Oxidative stress was evaluated by flow cytometry by using the dichlorofluorescein diacetate (DCF-DA) and PI kit following the manufacturer’s recommendations (Molecular Probes D399, Invitrogen, Carlsbad, Calif., USA). Rat sperm were recovered from epididymis in tempered PBS and concentrated to 1 × 106 cells/ml. The suspension was split into four tubes of 100 μl each. One tube was used to evaluate autofluorescence, the second was incubated with 0.5 μM PI, the third with 25 μM DCF-DA, and the fourth with 25 μM DCF-DA plus 0.5 μM PI. Fluorescence was evaluated in a FACS Canto Cytometer (BD Biosciences), and 10,000 gated events were acquired for each condition. All data were analyzed by using FCS express V2.0 software (De Novo Software).
Computer-assisted sperm analysis
Sperm motility was analyzed at Centro de Espermiogramas Digitales Asistidos por Internet (CEDAI; http://www.cedai.cl) by analytical computational tools developed in SCIAN-Lab (http://www.scian.cl) based on Interactive Data Language (IDL) 8.2 (ITT, Bolder, Colo., USA). The CEDAI system has been standardized and validated by three experts in human semen analysis, and CEDAI has shown a high correlation with the CASA SCA system (http://www.micropticsl.com/products/sperm-class-analyzer-casa-system/). The algorithm for the quantification of human semen parameters has been previously adapted and published for the analysis of mouse spermatozoa (Busso et al. 2014). Head size and head shape parameters were adjusted for rat sperm detection by visual analysis by an expert. The evaluation of tail movement was included in the motility classification criteria. Three sperm motility groups (Progressive, Slow, and Hyperactivated) were selected from test tracks to check/define rat motility parameters and thresholds. Image acquisition was carried out at xy resolution (750/580 pixels, 650/484 mm, 8-bit intensity scale), and one-second videos were captured at 30 Hz with a charge-coupled device (CCD) scA780–54 gc camera (Basler, Ahrensburg, Germany) with a light-emitting diode (LED)-illuminated Zeiss microscope (Axio Lab.A1; L5 objective; Dark Field). Home-made software (CEDAI-GRABBER) controlled image acquisition. Sperm samples (10 μl) were added into a Neubauer chamber (10 μm deep) to obtain microscope acquisitions. At least three samples from each animal and time point were analyzed following the same procedure. Interactive computational tools for sperm head and tail segmentation and tracking were adapted from a system optimized/optimized for human sperm motility analysis at CEDAI. Tracks of 15–30 points were accepted. Four types of sperm trajectories, namely hyperactivated (HY), progressive (PR), slow, and static, were classified according to a combination of head and tail motility patterns (VSL: straight line velocity; VCL: curvilinear velocity; ALH: lateral head displacement; LIN: linearity; WOB: balancing). Head motility patterns were as follows: Static (VSL ≤ 10 μm/s), Slow (VCL ≤ 50 μm/s), PR (VCL > 50 μm/s), HY (VCL > 100 μm/s; LIN ≤ 51%; ALH ≥ 2.0 μm). Tail motility patterns: Static (VSL ≤ 10 μm/s), Slow (VCL ≤ 50 μm/s), PR (VCL > 50 μm/s), HY (LIN ≤ 38.0%, ALH ≥ 0.88 μm, WOB ≥ 16 μm).
Statistical analysis
Data are expressed as means ± SEM. Comparisons between groups were made by using an analysis of variance (ANOVA) or a non-parametric test, namely the Mann-Whitney U-test, with GraphPad Prism 5.0 (La Jolla, Calif., USA). Differences were considered significant at P < 0.05.
Results
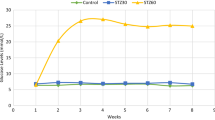
In order to induce diabetes, 6– to 8-h-fasted animals were injected with 65 mg/kg STZ according to the protocol described above (see Materials and methods). In animals treated with STZ, blood glucose levels rose approximately three-fold during the first week when compared with the control group (Fig. 1a). By the end of treatment (63 days), blood glucose values had steadily increased up to 5 times when compared with controls. On the other hand, blood glucose levels of control rats did not change during the studied period. Body weight of controls steadily increased, whereas the weight of diabetic rats only slightly changed during the 63 days after STZ injection (Fig. 1b, P < 0.001). Treatment with AA did not change blood glucose levels or body weight in control rats. To find out if AA treatment was an effective antioxidant therapy, we estimated the levels of MDA as a measure of lipid peroxidation in testicular homogenates (Fig. 2). We found a significant two-fold increase of MDA levels in diabetic rat testes (1.10 ± 0.22 nmol/mg protein) when compared with the control group (0.40 ± 0.09 nmol/mg protein, P = 0.02). The treatment with AA reduced the MDA levels in diabetic rats to values similar to those of controls (Fig. 2).
Ascorbic acid (AA) does not modify weight or glycemia in diabetic adult rats. Diabetes was induced in adult rats on week 0 by a streptozotocin (STZ) injection at a dose of 65 mg/kg followed by oral administration of 10% sucrose water for 24 h. Ascorbic acid was administered by gavage. Blood glucose (a) and body weights (b) were monitored weekly. Each point represents mean ± SEM. Mann Whitney U-test (*P < 0.05, ***P < 0.001 vs values in control rats)
Thus, our results showed that STZ promoted elevated blood sugar level and decreased body weight together with its effect on oxidative stress in our rat model. In addition, treatment with AA decreased oxidative damage in testes but did not have any effect on blood glucose levels or body weight.
Diabetes induce testicular damage
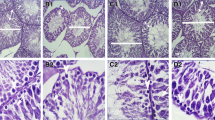
The next step was to determine the extent of testicular damage in diabetic rats. The relative weight of testes in animals from the four groups was similar (Table 1). The diameter of seminiferous tubules and epithelium thickness decreased in diabetic rats when compared with those of control rats (Table 1, Fig. 3a, b). Diabetic rats treated with AA showed a partial reversion of these parameters but did not reach control levels (Table 1, Fig. 3f). AA treatment did not modify the diameter of seminiferous tubules or epithelium thickness in control rats (Fig. 3f, Table 1). In addition, diabetic rat seminiferous tubules showed empty spaces in the seminiferous epithelium (also named vacuolization, Fig. 3c, arrow), and some of them showed individual or groups of sloughed cells in the lumen (Fig. 3d, arrowhead). Quantification of these alterations showed that they were significantly higher in diabetic rats when compared with controls (Table 1). Interestingly, the vacuolization of seminiferous tubules and cell sloughing were reduced in animals treated with AA (Fig. 3e, Table 1).
AA prevents testicular histological damage in diabetic rats. a Seminiferous tubules from control rats showing complete spermatogenesis. b-d Seminiferous tubules from diabetic rats showing a decreased diameter and disorganized morphology (arrow vacuolization, arrowhead desquamation). e Control testes treated with AA exhibiting normal histological appearance. f Testes of diabetic rats treated with AA. Note the amelioration of seminiferous tubule damage when AA was administrated. Magnification ×400. Bar 100 μm
Next, we studied whether the seminiferous cycle was affected in diabetic rats. Results showed that diabetic rats presented a significant proportion of seminiferous tubules whose identity could not be ascribed to a particular stage (Fig. 4). In addition, we found a significant decrease in the frequency of stages VII-VIII and a concomitant increase in stages XII-XIV in diabetic rats, when compared with control rats (Fig. 4). This dramatic effect observed in diabetic rats might have been attributable to a significant decrease in circulating plasma levels of testosterone in comparison with control rats (0.19 ± 0.02 v/s 2.06 ± 0.66 ng/ml, Fig. 5). When diabetic rats were treated with AA, the frequency of stages VII-VIII and XII-XIV was restored, and the number of unidentified tubules reduced (Fig. 4). However, the plasma testosterone level was not reinstated to that observed in control rats with or without AA (Fig. 5). Thus, these results suggest that the changes in stage frequency observed in diabetic rats are probably related to oxidative damage and not to testosterone levels.
AA reverts changes in the frequency of stages in testes from STZ-induced diabetic rats. Frequencies were obtained during histological examination of 100 cross sections by light microscopy. Values represent means ± SEM observed frequency of stages. Control n = 5; diabetic n = 5; Control AA n = 3; Diabetic + AA n = 5. Significant differences from controls are indicated (*P < 0.05, **P < 0.01)
AA did not restore plasma testosterone levels in diabetic rats. Plasma levels of testosterone were measured 2 months after treatment with a single dose of STZ; radioimmunoassay (RIA) technique. Depicted columns are means ± SEM (*P < 0.05). Control (n = 4), control + AA (n = 5), diabetic (n = 6), diabetic + AA (n = 3)
Diabetes promotes a stage-specific increase of germ cell apoptosis
In order to determine the degree of apoptosis, we quantified the number of TUNEL(+) cells in randomized chosen seminiferous tubules (total of 300 tubules per animal; n = 3). As a whole, results showed a tendency to increased apoptosis (TUNEL(+) cells) in diabetic rats when compared with controls (Fig. 6, P = 0.059). The percentage of tubules with three apoptotic cells in diabetic rats was significantly higher than that in controls, and in some tubules, four (P = 0.22), five (P = 0.1), and six (P = 0.1) apoptotic cells (Fig. 7) could be seen. The apoptosis rate in seminiferous tubules of diabetic rats treated with AA was similar to that in controls (Fig. 6a, 7). Interestingly, AA treatment reduced physiological apoptosis in control rats (control = 0.79 ± 0.09 versus diabetic = 0.20 ± 0.12, P < 0.05), suggesting that oxidative stress is important in this process during normal spermatogenesis (Fig. 6a). We observed that spermatocytes were more likely to be marked positively for TUNEL staining regardless of the origin of the tubules (from control or treated rats; Fig. 6b-e). In order to detect subtle changes in the apoptosis rate in seminiferous tubules, we decided to quantify TUNEL(+) cells in the various stages of the seminiferous epithelium. The rate of apoptosis was corrected by the frequency of the stages found in normal (control) and diabetic rats. Results showed a significant increase in levels of apoptosis in diabetic rat testes at stages I- IV (0.67 ± 0.08), IV-VI (control 0.5 ± 0.09 vs. diabetic 1.98 ± 0.42; P = 0.05), VII-VIII (control = 0.51 ± 0.21 vs. diabetic = 1.07 ± 0.16; P = 0.03), and unidentified seminiferous tubules (control = 0.41 ± 0.25 versus diabetic = 1.91 ± 0.44; P = 0.02). Administration of AA to diabetic rats significantly reduced apoptosis to values similar to that of the controls at stages I-VIII and XII-XIV, but not in the unidentified group (Fig. 6g). In addition, we found san increase in the percentage of seminiferous tubules with 3 apoptotic cells, and after AA treatment, the percentage of tubules with 4, 5, and 6 cells significantly decreased (Fig. 7). Thus, these results suggest that AA prevents stage-specific germ cell apoptosis in diabetes rats.
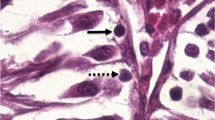
AA restores germ cell apoptosis of diabetic rats to control levels. a Diabetic rat exhibited a tendency to higher levels of apoptosis than control (control = 0.79 ± 0.09 versus diabetic = 1.41 ± 0.28). Treatment with AA restored germ cell apoptosis in diabetic rats to a value similar to that of controls (control = 0.20 ± 0.12 vs diabetic = 0.68 ± 0.16). No significant differences were found. b–f Micrographs of seminiferous tubules in stages VII-VIII from (b) control, (c) diabetic, (d) control + AA, (e) diabetic + AA (arrowheads apoptotic cells). Note that no signal was detectable in negative control (f) or Sertoli cells. Bar 100 μm. g Seminiferous tubules in stages (IV-VI) and (VII-VIII) and unidentified seminiferous tubules showed a higher level of apoptosis than controls. When ascorbic acid was administered to diabetic rats, these levels returned towards control values. Control n = 4; diabetic n = 5; control AA n = 3; diabetic + AA n = 4
Finally, we wanted to explore whether spermatozoa were also affected in diabetic rats. Results showed that the percentage of hyperactive spermatozoa in control mice was higher in capacitating medium (CM) when compared with non-capacitating medium (NCM; Fig. 8a). Hyperactivation was similar in CM and NCM in spermatozoa from the other three experimental groups and was significantly reduced when compared with controls. The percentage of progressive motility and slow-moving spermatozoa was similar in the four experimental groups regardless of incubation in CM or NCM (Fig. 8b, c). On the other hand, the percentage of static spermatozoa (showing no type of movement) was significantly higher in diabetic, diabetic + AA, and control + AA samples when compared with controls in CM and NCM (Fig. 8d). Since we found an increased proportion of static spermatozoa, we wondered about their viability, and so we quantified results following the exposure of spermatozoa to phosphatidylserine (Annexin-V positive cells) and PI incorporation by flow cytometry. Spermatozoa from diabetic rats showed an increased proportion of Annexin-V- and PI-positive cells when compared with controls and those treated with AA (Fig. 9a, b). In addition to PI incorporation, we assessed oxidative stress through the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCF), which is a sensor of reactive oxygen species (ROS). Interestingly, spermatozoa from diabetic and AA-treated animals showed an increased percentage of positive cells for DCF (Fig. 9a), but only spermatozoa from diabetic rats were positive for both DCF and PI (Fig. 9d). This suggests that, despite the higher levels of oxidative stress in spermatozoa from AA-treated rats, only those from diabetic animals were dead. Thus, higher levels of oxidative stress (ROS) correlate with an increase in death and decreased motility in rat spermatozoa.
Spermatozoa motility analysis in control and diabetic rats. Mature cauda epididymal spermatozoa were incubated in capacitating or non-capacitating medium and then incubated for 2.5 h. Hyperactivated spermatozoa, speramatozoa exhibiting progressive and slow motility, and static spermatozoa were analyzed by CASA. Graphs represent the percentage of spermatozoa in each category from controls, control plus ascorbic acid (AA), diabetic, and diabetic plus AA. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
Oxidative stress and apoptosis in spermatozoa. Mature cauda epididymal spermatozoa isolated from control and diabetic rats, with or without AA treatment, were incubated with fluorescent probes in order to determine the levels of oxidative stress (DCF 2′,7′-dichlorodihydrofluorescein diacetate) and apoptosis (Annexin V and Propidium Iodide [PI]). Control n = 3; diabetic n = 4; control + AA n = 3; diabetic + AA n = 3. Two-way analysis of variance and post hoc Bonferroni test. *P < 0.05, **P < 0.01, ***P < 0.0001
Discussion
In this work, we show that diabetes induced by STZ in male rats promotes several histological alterations, changes in the frequency of seminiferous epithelium stages, increase of oxidative stress, and germ cell apoptosis together with a decrease in plasma testosterone, sperm motility, and fertility. Treatment with AA reduced the levels of peroxidation and histological alterations in seminiferous tubules but did not restore the plasma levels of testosterone, fertility, or sperm motility.
Previous studies have shown that diabetic rats present severe damage in seminiferous tubules together with increased apoptosis (El-Missiry 1999; Kilarkaje et al. 2014; Koh 2007; Xu et al. 2014). Our data supported the previous findings and revealed that more than 50% of the tubules have some type of damage such as vacuolization and/or cell detachment from the basal membrane. We also found that diabetic rats present a decrease in the diameter of their seminiferous tubules. One of the questions that we wished to answer in this work was whether alterations in seminiferous tubules were dependent on the seminiferous epithelium cycle. Interestingly, we determined that the frequency of stages VII-VIII and XII-XIV in diabetic rats was significantly different from that of control animals. Moreover, we discovered that the stages of a significant portion of seminiferous tubules could not be assessed in diabetic rats. During stages VII-VIII, fully mature spermatids are known to be released into the seminiferous lumen, whereas during stages XII-XIV, zygotene spermatocytes develop into pachytene, and meiosis II takes place giving rise to haploid round spermatids. Furthermore, spermatids type 11 advance to spermatids type 14 (Hermo et al. 2010; Russell et al. 1990). Therefore, our findings suggest a detention and/or slowdown of the cell cycle at stages XII-XIV possibly leading to a decrease in the production of fully mature spermatids. On the other hand, the reduction of tubules in stages VII-VIII suggests a decrease in the release of fully mature spermatids into the seminiferous tubule lumen. Our data might explain the reduction in sperm production of diabetic rats observed in this study and previous work (Fernandes et al. 2011a; La Vignera et al. 2012; Scarano et al. 2006).
Our data reveal that a two-fold increase occurs in the MDA levels in diabetic rat testes. Previous studies have shown that Wistar rats treated with 50 mg/kg STZ and maintained during five weeks exhibit an increase of ∼ 2.6 times in lipid peroxidation levels compared with control rat levels (192.8 ± 21.5 vs 74. 9 ± 3.1 nmol/ml MDA, P < 0.001; Bal et al. 2011). In another study of rats treated with 40 mg/kg STZ, MDA levels in diabetic rats were found to be double those in control levels (Kong et al. 2016). Other work determined a ratio of ∼1.7 times between the two groups (diabetic = 2.60 ± 0.24 vs control = 1.67 ± 0.09 nmol/mg protein; Orman et al. 2015). Finally, another study in Wistar rats treated with STZ (60 mg/kg) showed that MDA levels were ∼1.5 times higher than the control levels, and that, after AA administration for 21 consecutive days (30 mg i.p / day), those levels were reduced to one third (Naziroglu 2003). Thus, the level of MDA found in the present study is within the ranges previously reported. Curiously, our findings demonstrate that the treatment with 70 mg/kg AA does not restore the testosterone levels in diabetic rats but recovers the frequency of seminiferous tubules stages to values similar to those of controls. Only two studies in diabetic rats have shown that treatment with 150 mg Vitamin C per day or 429 mg/kg (considering 350 g as being rat weight) restores the levels of plasma testosterone to values similar to those of controls (Fernandes et al. 2011a, 2011b). However, the dose in those previous studies was 7 times higher than that used in this study and 15 times higher than the highest dose in humans (28 mg/kg, human weight 70 kg) based on a daily intake of 2000 mg (Mayo-Clinic 2015: http://www.mayoclinic.org/vitamin-c/expert-answers/FAQ-20058030?p=1). Thus, the results of those studies do not fully apply to humans, and their results may be skewed because of the non-physiological dosage of Vitamin C. Interestingly, treatment of STZ-induced diabetic rats with insulin did not restore apoptotic levels or histological seminiferous tubule damage to control levels, as we report here with AA (Xu et al. 2014). This might be because of the broader spectrum of action that AA has in reducing oxidative damage and thus in reversing direct and indirect effects of insulin signaling. On the other hand, STZ has been shown to induce cytotoxicity by increasing ROS/RNS production, oxidative stress, and mitochondrial dysfunction (Raza and John 2012). Thus another plausible interpretation of the results of this study is that STZ directly damages the testes, and that AA reverts this cytotoxicity.
Previous findings and those presented in this study show a reduction in the levels of testosterone in diabetic rats, suggesting that this effect is linked to alterations in spermatogenesis stage frequency (Fernandes et al. 2011a, 2011b; La Vignera et al. 2012; Scarano et al. 2006; Shrilatha and Muralidhara 2007a; Wu and Huan 2008). The reduction in testosterone levels, in diabetic rats, has been linked to a decrease in 3-β-hydroxysteroid-dehydrogenase (HSD) and 17α-HSD enzyme activities in Leydig cell because of oxidative stress, since treatment with an antioxidant such as Vitamic C reverts the hormone levels to those similar to control levels (Chang et al. 2007; Sen Gupta et al. 2004). We have shown here that treatment with 70 mg/kg AA does not improve the levels of testosterone but reduces histological damage and apoptosis in testes from diabetic rats. These results suggest that the oxidative damage rather than the decrease in testosterone levels causate seminiferous tubules damage and increased apoptosis in diabetic rats. However, alternative pathways, not related to oxidative stress, might trigger testicular damage and decrease sperm motility and viability. This scenario may be the eason that AA restores testicular histology, but not fertility.
Studies from other authors have shown correlation between the levels of AA and the percentage of morphologically abnormal human spermatozoa (Colagar and Marzony 2009; Das et al. 2009). In addition, the levels of AA have been found to be lower in infertile men (Colagar and Marzony 2009). However, Silver et al. (2005) determined no correlation between the index of DNA fragmentation in sperm and the daily dose of Vitamin C in a healthy non-smoking population. Moreover, Rolf et al. (1999) detected no effect on semen parameters in patients with asthenozoospermia or moderate oligoasthenozoospermia treated with high-dose vitamin C and vitamin E. Thus, the scarce data in humans is still controversial. We have found here that most spermatozoa from diabetic rats do not move because they are dead, as evaluated by PI and Annexin-V. However, this finding does not explain the great proportion of motionless spermatozoa found in AA-treated rats. In this regard, all static (motionless) spermatozoa show high levels of ROS as evaluated by the probe DCF, which suggests that, despite having high levels of oxidative stress, AA treatment protects them from death, but not from a decrease in motility. These results may partly explain the reduced fertility observed in diabetic and AA-treated animals when compared with controls. Our results agree with previous studies in human spermatozoa showing a correlation between higher ROS production and lower cell viability and motility (Dona et al. 2011). Interestingly, we found that AA treatment in non-diabtetic rats decreased sperm motility, a finding that might be attributable to an effect of AA on ROS in mature spermatozoa. When generated at lower and controlled levels, ROS act as second messengers (de Lamirande and Gagnon 1992; de Lamirande and O’Flaherty 2008), and its balance is important for mammalian spermatozoa to maintain motility and achieve capacitation. In this context, exogenous AA might imbalance ROS production and alter the signaling pathways involved in sperm motility.
Diabetes is a complex pathology involving multiple steps and affecting various organs, among them testes and sperm production. We have shown that, in a rat model, diabetes impairs testicular organization and affects sperm motility, mainly by increasing oxidative stress, which contributes to the decreased fertility of these males. Our results suggest that Vitamin C might be a good choice for reverting testicular damage, but not sperm motility or fertility.
References
Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE (2007) Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod 22:1871–1877
Arikawe AP, Daramola AO, Odofin AO, Obika LF (2006) Alloxan-induced and insulin-resistant diabetes mellitus affect semen parameters and impair spermatogenesis in male rats. Afr J Reprod Health 10:106–113
Atalay B, Ilhan F, Gulyuf F, Karaca M, Oner A (2009) Assessment of the histopathological changes ocurring in the testis of the mice suffering from experimental diabetes induced using alloxan. J Anim Vet Adv 10:1929–1935
Bal R, Turk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, Gur S, Nedzvetsky VS, Tykhomyrov AA, Andrievsky GV, Baydas G, Naziroglu M (2011) Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology 282:69–81
Bartak V, Josifko M, Horackova M (1975) Juvenile diabetes and human sperm quality. Int J Fertil 20:30–32
Blanco-Rodriguez J, Martinez-Garcia C (1996) Spontaneous germ cell death in the testis of the adult rat takes the form of apoptosis: re-evaluation of cell types that exhibit the ability to die during spermatogenesis. Cell Prolif 29:13–31
Busso D, Oñate-Alvarado MJ, Balboa E, Castro J, Lizama C, Morales G, Vargas S, Härtel S, Moreno RD, Zanlungo S (2014) Spermatozoa from mice deficient in Niemann-Pick disease type C2 (NPC2) protein have defective cholesterol content and reduced in vitro fertilising ability.Reprod Fertil Dev 26:609-621
Cameron DF, Murray FT, Drylie DD (1985) Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat Rec 213:53–62
Colagar AH, Marzony ET (2009) Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr 45:144–149
Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY (2007) Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol 218:196–203
Das P, Choudhari AR, Dhawan A, Singh R (2009) Role of ascorbic acid in human seminal plasma against the oxidative damage to the sperms. Indian J Clin Biochem 24:312–315
de Lamirande E, Gagnon C (1992) Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 13:368–378
de Lamirande E, O’Flaherty C (2008) Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta 1784:106–115
Delfino M, Imbrogno N, Elia J, Capogreco F, Mazzilli F (2007) Prevalence of diabetes mellitus in male partners of infertile couples. Minerva Urol Nefrol 59:131–135
Dona G, Fiore C, Andrisani A, Ambrosini G, Brunati A, Ragazzi E, Armanini D, Bordin L, Clari G (2011) Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum Reprod 26:3264–3273
Dunkel L, Hirvonen V, Erkkila K (1997) Clinical aspects of male germ cell apoptosis during testis development and spermatogenesis. Cell Death Differ 4:171–179
El-Missiry MA (1999) Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp Biochem Physiol Part C 124:233–237
Fernandes GS, Fernandez CD, Campos KE, Damasceno DC, Anselmo-Franci JA, Kempinas WD (2011a) Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol 9:100
Fernandes GS, Gerardin DC, Assumpcao TA, Campos KE, Damasceno DC, Pereira OC, Kempinas WD (2011b) Can vitamins C and E restore the androgen level and hypersensitivity of the vas deferens in hyperglycemic rats? Pharmacol Rep 63:983–991
Gluud C, Madsbad S, Krarup T, Bennett P (1982) Plasma testosterone and androstenedione in insulin dependent patients at time of diagnosis and during the first year of insulin treatment. Acta Endocrinol 100:406–409
Greene LF, Kelalis PP (1967) Retrograde ejaculation of semen dueto diabetic neuropathy. J Urol 98:696
Hermo L, Pelletier RM, Cyr DG, Smith CE (2010) Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 73:241–278
Hernandez-Salinas R, Decap V, Leguina A, Caceres P, Perez D, Urquiaga I, Iturriaga R, Velarde V (2015) Antioxidant and anti hyperglycemic role of wine grape powder in rats fed with a high fructose diet. Biol Res 48:53
Hernandez-Salinas R, Vielma AZ, Arismendi MN, Boric MP, Saez JC, Velarde V (2013) Boldine prevents renal alterations in diabetic rats. J Diabetes Res 2013:593672
Kaplan R, Kaicer P (1978) Effect of elevated calcium concentration on fertilization of rat oocytes in vitro. Gamete Res 1:282–285
Kilarkaje N, Al-Hussaini H, Al-Bader MM (2014) Diabetes-induced DNA damage and apoptosis are associated with poly (ADP ribose) polymerase 1 inhibition in the rat testis. Eur J Pharmacol 737:29–40
Koh PO (2007) Streptozotocin-induced diabetes increases apoptosis through JNK phosphorylation and Bax activation in rat testes. J Vet Med Sci 69:969–971
Kong WY, Tong LQ, Zhang HJ, Cao YG, Wang GC, Zhu JZ, Zhang F, Sun XY, Zhang TH, Zhang LL (2016) The calcium-sensing receptor participates in testicular damage in streptozotocin-induced diabetic rats. Asian J Androl 18:803-808
La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE (2012) Diabetes mellitus and sperm parameters. J Androl 33:145–153
Leblond CP, Clermont Y (1952) Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat 90:167–215
Maritim AC, Sanders RA, Watkins JB 3rd (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Moreno RD, Urriola-Munoz P, Lagos-Cabre R (2011) The emerging role of matrix metalloproteases of the ADAM family in male germ cell apoptosis. Spermatogenesis 1:195–208
Naziroglu M (2003) Enhanced testicular antioxidant capacity in streptozotocin-induced diabetic rats: protective role of vitamins C and E and selenium. Biol Trace Elem Res 94:61–72
Orman D, Vardi N, Ates B, Taslidere E, Elbe H (2015) Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats. Tissue Cell 47:284–290
Padron RS, Dambay A, Suarez R, Mas J (1984) Semen analyses in adolescent diabetic patients. Acta Diabetol Lat 21:115–121
Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G (2010) Oxidative stress, nitric oxide, and diabetes. Rev Diabetic Studies 7:15–25
Pontes DA, Fernandes GS, Piffer RC, Gerardin DC, Pereira OC, Kempinas WG (2011) Ejaculatory dysfunction in streptozotocin-induced diabetic rats: the role of testosterone. Pharmacol Rep 63:130–138
Poongothai J, Gopenath TS, Manonayaki S (2009) Genetics of human male infertility. Singapore Med J 50:336–347
Ramanathan R, Das NP, Tan CH (1994) Effects of gamma-linolenic acid, flavonoids, and vitamins on cytotoxicity and lipid peroxidation.Free Radic Biol Med 16:43-48
Ranganathan P, Mahran AM, Hallak J, Agarwal A (2002) Sperm cryopreservation for men with nonmalignant, systemic diseases: a descriptive study. J Androl 23:71–75
Raza H, John A (2012) Streptozotocin-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human hepatoma HepG2 cells. Int J Mol Sci 13:5751–5767
Rolf C, Cooper TG, Yeung CH, Nieschlag E (1999) Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod 14:1028–1033
Russell L, Ettlin R, Hikim A, Clegg E (1990) Histological and histopathological evaluation of the testis. Cache River, Clearwater
Sainio-Pollanen S, Henriksen K, Parvinen M, Simell O, Pollanen P (1997) Stage-specific degeneration of germ cells in the seminiferous tubules of non-obese diabetic mice. Int J Androl 20:243–253
Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG (2006) Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl 29:482–488
Sen Gupta R, Kim J, Gomes C, Oh S, Park J, Im WB, Seong JY, Ahn RS, Kwon HB, Soh J (2004) Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol Cell Endocrinol 221:57–66
Sexton WJ, Jarow JP (1997) Effect of diabetes mellitus upon male reproductive function. Urology 49:508–513
Shrilatha B, Muralidhara (2007a) Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol 23:578–587
Shrilatha B, Muralidhara (2007b) Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the streptozotocin-diabetic rat. Int J Androl 30:508–518
Silver EW, Eskenazi B, Evenson DP, Block G, Young S, Wyrobek AJ (2005) Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J Androl 26:550–556
Wu KK, Huan Y (2008) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 40:5.47.1–5.47.14. 10.1002/0471141755.ph0547s40
Xu Y, Lei H, Guan R, Gao Z, Li H, Wang L, Song W, Gao B, Xin Z (2014) Studies on the mechanism of testicular dysfunction in the early stage of a streptozotocin induced diabetic rat model. Biochem Biophys Res Commun 450:87–92
Zaccardi F, Rocca B, Pitocco D, Tanese L, Rizzi A, Ghirlanda G (2015) Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev 31:402–410
Acknowledgements
This work was financed by CONICYT (grants 1110778 and 1150352) to R.D.M. We thank Susana Vargas for helping with semen analysis in CEDAI (grant 14SSAF26061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguirre-Arias, M.V., Velarde, V. & Moreno, R.D. Effects of ascorbic acid on spermatogenesis and sperm parameters in diabetic rats. Cell Tissue Res 370, 305–317 (2017). https://doi.org/10.1007/s00441-017-2660-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2660-6