Abstract
Prior to fertilization, mammalian sperm undergo several molecular, biochemical and physiological changes in a process termed capacitation. However, the mechanisms explaining the involvement of cytoskeletal remodeling and membrane re-ordering in each process prior to fertilization remain poorly understood. We found that the migration of both flotillin microdomains and Src family kinases towards the apical ridge of guinea pig sperm occurs under capacitating conditions. This re-ordering is associated with spectrin cleavage by calpain. Moreover, Src, Fyn, Lyn and Hck interact with flotillin-1; this interaction increases in a capacitation-dependent manner and the increased autophosphorylation of these kinases is linked to flotillin-1 association. The aforementioned results are prevented by the inhibition of calpain by calpeptin. Thus, spectrin cytoskeleton cleavage during capacitation seems to precede the reorganization of flotillin microdomains and Src family kinases towards the apical ridge of the sperm head in order to initiate the signaling cascade required for proper capacitation and further acrosome reaction. The significance of the Src family kinase reorganization for capacitation is demonstrated by the inhibition of calpain during capacitation also preventing the Src-family-kinase-dependent phosphorylation of FAK at Tyr576/577. Our work further highlights the scaffolding properties of flotillin microdomains and reveals the importance of their large-scale segregation during capacitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshly ejaculated mammalian spermatozoa are unable to fertilize mature oocytes; in order to become competent for fertilization, these cells require a period of time within the female reproductive tract (in vivo) or incubation in a defined medium (in vitro; Austin 1952; Chang 1951). During this time, several biochemical and physiological changes, collectively called capacitation, occur in the spermatozoa (Gangwar and Atreja 2015; Stival et al. 2016). In mammalian sperm, capacitation is defined as a complex process that includes membrane modifications, enzyme activity modulation, cytoskeleton reorganization, membrane hyperpolarization and protein phosphorylation (Aitken and Nixon 2013; Gangwar and Atreja 2015). Furthermore, capacitation is accompanied by an increment in flagellar bend amplitude called sperm hyperactivation (Olson et al. 2011; Suarez 2008). Once capacitation has occurred, sperm are able to undergo a physiologically induced acrosome reaction (AR) in order to become fertilization-competent.
Accumulating evidence has revealed that sperm membranes contain lipid-ordered microdomains termed “lipid rafts” that undergo changes in their spatial distribution under capacitating conditions (Asano et al. 2013; Bou Khalil et al. 2006; Selvaraj et al. 2006) following an apparent increase in the fluidity of lipid microdomains (Hinkovska-Galcheva and Srivastava 1993). These lipid rafts are reorganized towards the apical ridge of the sperm head following capacitation, giving rise to a clustering of raft markers that may favor specific signaling and protein interactions (Nixon and Aitken 2009; Shadan et al. 2004; van Gestel et al. 2005). Nonetheless, the underlying mechanisms and importance of this phenomenon are still poorly understood.
Flotillins and caveolins are commonly used as membrane lipid raft markers and are expressed in mammalian sperm (Cross 2004; Miranda et al. 2009; Travis et al. 2001; van Gestel et al. 2005). The flotillin family is composed of two homologous members (flotillin-1 and flotillin-2), which are evolutionarily conserved across distant phyla (Rivera-Milla et al. 2006). They preferentially associate with each other in the form of oligomeric complexes and are capable of forming their own type of non-caveolar membrane microdomains by the formation of homo- and hetero-tetramers (Solis et al. 2007) with scaffolding properties favoring specific protein interactions and cell signaling (Lang et al. 1998; Stuermer et al. 2001). Flotillins are situated at the cytoplasmic face of the plasma membrane and at membranes of intracellular compartments (Lang et al. 1998) through myristoylation and palmitoylation modifications (Morrow et al. 2002; Neumann-Giesen et al. 2004). Several publications have suggested that the scaffolding properties of the flotillin microdomains regulate cell adhesion and signaling events; however, their precise molecular function remains unknown (Stuermer and Plattner 2005). The selective enrichment of proteins within flotillin microdomains includes glycosylphosphatidylinositol (GPI)-anchored proteins (Stuermer et al. 2001), cytoskeleton-associated proteins (Langhorst et al. 2007) and doubly acylated proteins, such as the Src family kinases (SFKs; Langhorst et al. 2006). Diverse evidence indicates that flotillins interact with signaling proteins such as Src, Fyn and Lyn in various cell types (Liu et al. 2005; Nebl et al. 2002; Neumann-Giesen et al. 2007). Since both SFKs and flotillins are present in the guinea pig sperm cell, an interaction between these proteins seems possible.
To date, the Src family of kinases in mammalian cells includes at least eight members, Src (the original member), together with Blk, Fgr, Fyn, Hck, Lck, Lyn and Yes; they all have a common molecular structure, conserved Src-homology 2 (SH2) and Src-homology 3 (SH3) peptide domains and similar molecular weights in the 52–62 kDa range. Nonetheless, each kinase possesses a unique sequence at their N-terminal region and undergoes different acyl modifications from the others (Brown and Cooper 1996; Okada 2012). In somatic cells, SFKs play key roles in various pathways, such as cell differentiation, proliferation, survival, adhesion, morphology and motility (Thomas and Brugge 1997).
The presence of SFKs in the mammalian sperm cell has been determined; however, depending on the species, their individual localization and abundance are highly uneven, suggesting specific functions for each kinase. In order to elucidate the role of SFKs in mammalian sperm, the entire family has been inhibited by a small-molecule, namely indolinone (SU6656), during capacitation (Goupil et al. 2011; Mitchell et al. 2008), resulting in the discovery that the inhibition decreases tyrosine phosphorylation in human (Mitchell et al. 2008) and murine (Baker et al. 2006) sperm, inhibits the motility of murine sperm (Baker et al. 2006) and blocks the acrosome reaction in human (Tapia et al. 2011), bovine (Etkovitz et al. 2009) and guinea pig (Baltierrez-Hoyos et al. 2012) sperm but also causes a twofold increase in the acrosome reaction in porcine sperm (Bragado et al. 2012). All of the above suggest that perhaps SFKs mediate diverse pathways in mammalian sperm in a species-specific manner.
Various polarized cell types are able to organize and maintain specialized membrane domains in order to coordinate processes within the underlying cortical cytoplasm and cytoskeleton. In order to do so, membrane domains are enriched with selective lipids and proteins stabilized by spectrin, actin, or dystrophin cytoskeletons (Le Rumeur et al. 2010; Machnicka et al. 2014). Spectrin forms a submembranous cytoskeleton comprising α- and β-subunits assembled in antiparallel filamentous heterodimers. The spectrin cytoskeleton is linked to the plasma membrane through ankyrin (Bennett and Healy 2009); however, novel evidence strongly suggests that spectrin associates with membrane domains by means of flotillins in red blood cells and neutrophils (Head et al. 2014; Ludwig et al. 2010; Salzer and Prohaska 2001). The relationship between the spectrin cytoskeleton and flotillins is still poorly understood. On the contrary, the interaction of flotillin-actin has been broadly studied. The immobility and lateral mobility of flotillins depend on the stabilization or disruption of the actin cytoskeleton, respectively (Langhorst et al. 2007).
In the mammalian sperm cell, depending on species, the actin and spectrin cytoskeletons are organized under the plasma membrane in both the head and flagellar regions; however, despite their undoubtedly important role, little is known about the mechanism of cytoskeleton dynamics (Bastian et al. 2010; Camatini et al. 1991; Hernandez-Gonzalez et al. 2000; Xiao and Yang 2007). Actin polymerization is a key process occurring during capacitation, whereas a fast F-actin breakdown is essential to achieve the acrosome reaction (Brener et al. 2003). Moreover, actin dynamics in the head and flagellum are involved in Izumo1 relocation and sperm motility, respectively (Azamar et al. 2007; Finkelstein et al. 2013; Sosnik et al. 2009).
Calpains are proteases responsible for the degradation of several cytoskeletal elements (Briz and Baudry 2016) and have been associated with motility and the acrosome reaction (Aoyama et al. 2001; Ozaki et al. 2001; Ben-Aharon et al. 2005), because during capacitation, calpain inhibition blocks motility and the acrosome reaction. We have shown that, by inhibiting spectrin cytoskeleton cleavage via calpain, the normal course of the acrosome reaction is blocked in guinea pig sperm (Bastian et al. 2010). Interestingly, flotillin microdomains have the property of spatially organizing the plasma membrane and allowing specifically located signaling (i.e., active SFKs) by interacting with the cell cortical cytoskeleton, specifically with actin, spectrin and myosin 2a (Langhorst et al. 2007; Ludwig et al. 2010). Significant aggregation of flotillin-1 in the apical acrosomal region has been reported as being a capacitation marker in boar sperm (van Gestel et al. 2005); however, the mechanisms regulating such an aggregation and its involvement in capacitation and the acrosome reaction have not been fully elucidated. Consequently, the purpose of this work has been to study the role of the interaction of flotillin microdomains with SFKs and the relocation of the microdomains with respect to the remodeling of the actin and spectrin cytoskeletons during capacitation of guinea pig sperm.
Materials and methods
Antibodies and reagents
Rabbit polyclonal antibodies against flotillin-1 (H104), Src (N16), Fyn (FYN3), Lyn (44), Hck (N30) FAK (A-17), p-FAK (Tyr576/577; sc-21831) and Rac-1 (C-11) and mouse anti-flotillin-2 monoclonal antibody (B6) were purchased from Santa Cruz Biotechnology (Dallas, Tex., USA). Rabbit polyclonal antibody against Src family kinases phosphorylated at Tyr416 (PK1109), polyvinylidene fluoride (PVDF) blotting membranes, calpeptin (03340051) and Immobilon horseradish peroxidase (HRP) substrate (WBKLS0500) were purchased from Merck Millipore (Billerica, MA, USA). The anti-utrophin 71 antibody (Up71) was kindly donated by Dr. Dominique Mornet from INSERM U592 (Paris, France). HRP-conjugated anti-rabbit, HRP-conjugated anti-mouse, HRP-conjugated anti-goat, tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-rabbit and fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa., USA). The Clean-Blot Detection IP Reagent AP (21230) was bought from Thermo Fisher Scientific (Rockford, Ill., USA). Latrunculin A (L5163), anti-chicken spectrin antibody (S1390), Triton X-100, sodium bicarbonate, calcium chloride dihydrate, sodium chloride, sodium pyruvate, sodium azide, polyvinyl alcohol (PVA), glycerol, formaldehyde, glutaraldehyde, bovine serum albumin (BSA), DL-lactic acid 85 %, iodoacetamide, benzamidine, aprotinin, leupeptin, pepstatin, phenylmethylsulfonyl fluoride (PMSF), TRIS base (Trizma), soybean trypsin inhibitor, sodium orthovanadate, rhodamine phalloidin, sodium fluoride and chlortetracycline (c-4881) were purchased from Sigma-Aldrich (St. Louis, Mo., USA). Complete mini protease inhibitor cocktail was purchased from Hoffmann La-Roche (Basel, Switzerland). Acrylamide, N,N'-methylenebisacrylamide and sodium dodecyl sulfate (SDS) were purchased from Bio-Rad Laboratories (Hercules, Calif., USA). Amersham ECL (RPN2106), Hyperfilm ECL (28906836) and Hypercassette autoradiography cassette (RPN11649) were purchased from GE Healthcare Bio Sciences (Pittsburgh, Pa., USA). Fixer and replenisher (1901875), developer and replenisher (1900943) and medical X-ray blue/MXB film (1668144) were bought from Carestream Health (Rochester, N.Y., USA).
Animals
Male Dunkin-Hartley guinea pigs (Cavia porcellus) were bred and maintained until they reached 800-900 g. They were housed in a vivarium at Cinvestav-IPN on a 12 h light / 12 h darkness schedule with access to food and water ad libitum. All animal handling procedures and experimental designs were approved by the Internal Committee for the Care and Use of Laboratory Animals, Cinvestav-IPN (CICUAL No. 321-02), following American Veterinary Medical Association guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
Sperm capacitation
Capacitation was performed as described by Rogers and Yanagimachi (1975). Sperm were obtained from the ductus deferens of the guinea pigs and then washed in 154 mM NaCl solution. The sperm cells (35 × 106 cell/ml) were capacitated by incubation at 37 °C in minimal culture medium (MCM-PL) containing: 105.9 mM sodium chloride, 1.71 mM calcium chloride, 25.07 mM sodium bicarbonate, 0.25 mM sodium pyruvate, 20 mM DL-lactic acid 85 %, pH 7.8. Capacitation was conducted for the desired time.
Calpeptin and latrunculin A treatments
In order to avoid spectrin cytoskeleton degradation, sperm cells were treated with calpeptin, a specific calpain inhibitor. Sperm were obtained and washed in a 154 mM NaCl solution supplemented with calpeptin 10 μM. The sperm cells (35 × 106 cell/ml) were capacitated by incubation at 37 °C in MCM-PL supplemented with 10 μM calpeptin. When cells attained 60 min of capacitation, the MCM-PL was supplemented with another dose of calpeptin to reach a final concentration of 20 μM and to preserve the inhibition of calpain until 90 min of capacitation. Additionally, a set of protease inhibitors was supplemented with 10 μM calpeptin and stored at −70 °C. Since the actin cytoskeleton is involved in flotillin microdomain re-ordering and since actin is polymerized during capacitation, sperm were capacitated in MCM-PL supplemented with 50 μM latrunculin A in order to prevent actin polymerization.
Immunofluorescence assays
Ductus deferens sperm were fixed with 3 % (v/v) formaldehyde and 0.2 % (v/v) glutaraldehyde in phosphate-buffered saline (PBS). After 1 h, the sperm were collected by centrifugation. The pelleted sperm (600 g for 3 min) were incubated in 50 mM NH4Cl for 10 min and rinsed twice with PBS and finally with bi-distilled water. Microscope slides were prepared by using this suspension, air-dried at room temperature overnight and stored at 4 °C. Sperm cells were permeabilized in acetone for 7 min at −20 °C and washed with PBS. Flotillin-1 (1:50), flotillin-2 (1:50), Src (1:25), Fyn (1:50), Lyn (1:50) and Hck (1:25) antibodies were diluted in PBS with 1 % BSA (blocking solution) and incubated on the slides overnight at room temperature. The slides were incubated for 2 h at 37 °C with the appropriate TRITC- or FITC-conjugated secondary antibody diluted in blocking solution. The samples were mounted with glass-coverslips by using Gelvatol, sealed adequately and stored at −20 °C until observed. The stained cells were imaged under a confocal laser scanning microscope (Leica TCS SP8) and analyzed by using the imaging software LAS AF Lite (Ver. 2.6.3).
Western blotting
Ductus deferens sperm were obtained, washed in 154 mM NaCl solution and capacitated when needed. They were centrifuged (600 g) for 3 min and suspended in RIPA buffer (25 mM TRIS HCl pH 7.6, 150 mM NaCl, 1 % NP-40, 1 % sodium deoxycholate, 0.1 % SDS) supplemented with protease inhibitors (5 mg/ml soybean trypsin inhibitor, 100 mg/ml benzamidine, 30 mg/ml pepstatin, 30 mg/ml leupeptin, 30 mg/ml aprotinin, 1 mM PMSF diluted in dimethylsulfoxide, 20 mg/ml iodoacetamide, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10 % glycerol, 2.5 % complete mini protease inhibitor cocktail [1 tablet diluted in 1 ml H2O]). The samples were then incubated for 20 min on ice and centrifuged (14,000 g) for 20 min at 4 °C. The supernatants were collected and their protein concentration was determined (Bradford 1976). For the reduction of protein disulfide bonds, supernatant aliquots were boiled for 5 min in 3× Laemmli buffer (720 mM TRIS-base, 6 % SDS (w/v), 30 % glycerol, 0.03 % bromophenol blue (w/v), pH 10). They were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10-12 % polyacrylamide non-gradient gels and subsequently transferred to PVDF membranes and blocked with 5 % skimmed milk in 1× PBS/1 % Triton X-100 (pH 7.5). When probed for spectrin, proteins were initially run on 6 % polyacrylamide gels and transferred to nitrocellulose membranes as performed by Bastian et al. (2010). For other proteins, 10 % polyacrylamide gels were used. The membranes were incubated overnight at 4 °C with the primary antibody. The dilutions for the primary antibodies were as follows: flotillin-1 (1:500), flotillin-2 (1:500), Src (1:500), Fyn (1:500), Lyn (1:500), Hck (1:250), FAK (1:500), p-FAK (1:500) and Rac-1 (1:250). Membranes were next incubated with their respective HRP secondary antibody: HRP-conjugated anti-rabbit (1:5000), HRP-conjugated anti-mouse (1:1250), or HRP-conjugated anti-goat (1:2000). The PVDF membranes were bathed with Amersham ECL and exposed to a MXB film inside an autoradiography cassette. Gel loading was normalized to Up71 (1:500). When the antibody for Src family kinases phosphorylated at Tyr416 (anti-p-Tyr416, 1:1000) was used, membranes were blocked for 2 h with 5 % BSA in 1× PBS/1 % Triton X-100 (pH 7.5) and incubated overnight at 4 °C with 3 % BSA in 1× PBS/1 % Triton X-100 (pH 7.5), followed by the Clean-Blot Detection IP Reagent HRP-conjugate (1:2000; this reagent is optimized for post-immunoprecipitation) in order to minimize heavy and light chain IgG bands. Finally, the PVDF membranes were bathed with Immobilon HRP substrate and exposed to a Hyperfilm ECL inside an autoradiography cassette to optimize resolution and reduce exposure time.
Co-immunoprecipitation assay
Co-immunoprecipitation experiments were performed by using the Crosslink Immunoprecipitation Kit (26147) purchased from Thermo Fisher Scientific. This method involves the capture of 20 μg IP antibody on Protein A/G beaded agarose resin and covalently immobilizing it on the support by crosslinking with 2.5 mM disuccinimidyl suberate (DSS). The antibody resin is then incubated at 4 °C for 12 h with 500 μg pre-cleared guinea pig sperm protein extracts, allowing the antibody-antigen complex to form. Proteins bound to the respective antibodies were eluted, recovered by low speed centrifugation (3000 g) and stored at −20 °C. The entire procedure was performed in a microcentrifuge spin cup. Only the antigen is eluted in the procedure, enabling it to be identified and further analyzed with minimal interference from antibody fragments. For protein disulfide reduction, supernatant aliquots were boiled for 5 min in 3× Laemmli buffer (pH 10) containing 2-mercaptoethanol and the proteins were then separated by SDS-PAGE. Next, proteins were transferred to PVDF membranes for immunodetection. A secondary antibody (Clean-Blot Detection Reagent AP) specific for IP assays was used to eliminate detection-interference from both heavy and light chain IgG fragments.
Phalloidin staining assays
Epididymal sperm cells were fixed on slides as described above. The cells on the slides were permeabilized in acetone for 7 min at −20 °C and washed with PBS. In order to detect F-actin staining patterns, cells were incubated for 1 h at 37 °C with TRITC-phalloidin (1:20) and washed 5 times in PBS. The samples were mounted on glass-coverslips by using Gelvatol, sealed adequately, and stored at −20 °C. The stained cells were imaged under an optic epifluorescence microscope (Olympus BX50) and analyzed by using the imaging software NIS-Elements AR (Ver. 3.1). All washes were carried out 5 times during a period of 5 min with PBS.
Chlortetracycline fluorescence assay
This procedure, which was previously described by Ward and Storey (1984), was modified for guinea pig sperm as follows. At the time of the assay, 45 μl non-capacitated or capacitated sperm was mixed with 45 μl chlortetracycline (CTC) solution (750 mM CTC in 130 mM NaCl, 5 mM cysteine, 20 mM TRIS, pH 7.8) and incubated for 20 s in a water bath at 37 °C. Immediately after incubation, the CTC-sperm suspension was fixed with 0.75 μl glutaraldehyde (12.5 %) in 0.5 mM TRIS (pH 7.4) to give a final concentration of 0.1 % glutaraldehyde, at room temperature. A 10-μl sample of the CTC-sperm suspension was smeared onto a glass slide and covered with a coverslip, adequately sealed and stored for 48 h at -20 °C to clear strong background fluorescence. The CTC solution was kept in a light-shielded container at 4 °C at all times. All fluorescence images were obtained by using a fluorescence microscope (Olympus BX5). In each sample, 100 spermatozoa were classified as expressing one of three CTC staining patterns: “F”, with faint fluorescence in the acrosome region, as is characteristic of non-capacitated acrosome-intact cells; “B”, with bright fluorescence in the acrosomal region and with a band along the equatorial segment, as is characteristic of capacitated acrosome-intact cells; “AR”, with fluorescence in the post-acrosomal region, as is characteristic of physiologically capacitated acrosome-reacted cells. If the sperm samples did not match the statistic criteria, the samples were not used for further experiments. The presence or absence of the acrosomal cap on each cell was verified under phase-contrast illumination.
Statistical analysis
Statistical analyses were performed by using the t-test with SigmaPlot version 12.0. Statistical significance is indicated in the figure legends.
Results
Flotillins are re-ordered during capacitation
For the first time, flotillin-1 and flotillin-2 were detected by Western blot analyses in sperm extracts from guinea pigs at their respective Mr of ∼50 kDa (Fig. 1a). Since flotillin-1 and -2 have almost 44 % of amino acid sequence identity (Babuke and Tikkanen 2007) and to avoid a false positive band on flotillins detection, we used antibodies raised against the non-conserved region of flotillins of human origin, from the amino acid sequences 324-427 and 151-240, for the detection of flotillin-1 and flotillin-2, respectively. Immunofluorescence analyses revealed that flotillin-1 (Fig. 1c) and flotillin-2 (Fig. 1c’) were located in the acrosomal region and mid-piece of non-capacitated sperm (Fig. 1c–c’’); however, under capacitating conditions, both proteins relocated to the apical ridge of the sperm head, whereas their presence in the mid-piece remained without change (Fig. 1d–d’’). The entire sperm population with intact acrosomes exhibited two types of flotillin-1 immunolabelling patterns in the sperm head: a dispersed pattern in the acrosome, which is a characteristic of non-capacitated cells (P1) and an apical ridge re-ordered pattern, which is a characteristic of capacitated cells (P2). The re-ordering of flotillin-1 (P2) showed more than a four-fold increment when the sperm samples underwent 90 min of capacitation (Fig. 1e). As a capacitation control, we determined chlortetracycline (CTC) fluorescence patterns to evaluate the physiological states of the guinea pig sperm samples. After 90 min of capacitation, sperm displayed approximately 70 % of pattern B (Fig. S1), which correlated with the number of sperm that exhibited the flotillin re-ordering pattern after capacitation (Fig. 1e). All of the above results suggest that flotillin re-ordering is associated with capacitation in guinea pig sperm and that the association of flotillin-1 with flotillin-2 remains intact under capacitation conditions.
Flotillin-1 (Flot-1) and flotillin-2 (Flot-2) remain associated after capacitation and re-order in a capacitation-dependent manner. a Whole sperm extracts from non-capacitated (−) and 90-min (+) capacitated (Cap) guinea pig sperm were subjected to immunoblotting in order to determine the presence of flotillins and possible changes that these proteins could undergo during capacitation. The protein utrophin of 71 kDa (Up71) was used to ensure equal protein loading (bottom). Western blot images are representative of three independent experiments. b Co-immunoprecipitation (IP) assays were carried out with total sperm extracts from non-capacitated and 90-min capacitated sperm by using anti-flotillin-1 antibody. The precipitated proteins were subjected to SDS–PAGE and Western blot analysis and were probed for flotillin-1 as a positive control and with anti-flotillin-2 antibody to determine its association. The reciprocal strategy was performed with anti-flotillin-2 antibody. Western blot images are representative of three independent experiments. c–d’’’ Flotillin-1 and flotillin-2 were immunolabelled in non-capacitated (c–c’’’) and capacitated (d–d’’’) sperm (90 min) with intact acrosome. Co-localization was analyzed by confocal microscopy. P1 represents a dispersed pattern in the acrosome, and P2 represents an apical clustered pattern (BF bright field images). Images are representative of at least three independent experiments. Bars 10 μm. e Quantification of patterns P1 and P2 showed a significant difference in both patterns under capacitating conditions, pattern P1 decreased (to 15 ± 3.60) and pattern P2 increased (to 71 ± 4.58). Graph represents means ± SEM, n = 3 independent experiments, 300 cells per experiment were counted
Next, we carried out co-immunoprecipitation assays with flotillin-1 obtained from whole cell extracts of non-capacitated and capacitated sperm cells, resulting in the co-immunoprecipitation of flotillin-2 (Fig. 1b). The association of flotillin-1 with flotillin-2 remained constant regardless of capacitation. Since the immunofluorescence patterns of both flotillins were strongly co-localized in the majority of the sperm population and since immunoprecipitation assays revealed that both flotillins remained associated upon capacitation, we chose to use flotillin-1 for pattern quantification and further experiments.
The above results indicated that flotillin microdomains underwent a re-ordering triggered by capacitation in the guinea pig sperm. Subsequently, we performed co-immunoprecipitation assays to determine whether spectrin and flotillin-1 were associated in the guinea pig sperm, as proposed in other cell types (Head et al. 2014; Ludwig et al. 2010; Salzer and Prohaska 2001). The Western blot analysis of co-immunoprecipitated proteins revealed that spectrin co-immunoprecipitated together with flotillin-1 in non-capacitated sperm, whereas in capacitated sperm, a significantly lower amount of spectrin co-immunoprecipitated with flotillin-1 (Fig. 2a, b). To define spectrin cytoskeleton participation, spectrin degradation was inhibited by using calpeptin, a specific inhibitor of calpain. We found that the amount of spectrin that co-immunoprecipitated with flotillin-1 on capacitated sperm in the presence of calpeptin was similar to that found in non-capacitated guinea pig sperm (Fig. 2a, b). Additionally, reciprocal assays were carried out and similar results were obtained; flotillin-1 co-immunoprecipitated together with spectrin at a lower amount in capacitated sperm than in non-capacitated sperm. We also found that the amount of flotillin-1 that co-immunoprecipitated with spectrin on capacitated sperm in the presence of calpeptin was similar to that found in non-capacitated guinea pig sperm (Fig. 2a, b).
Spectrin-flotillin association is reduced during capacitation. a Left Co-immunoprecipitation assays with flotillin-1 (Flot-1) were carried out on total sperm extracts (500 μg) from non-capacitated 90-min capacitated, and 90-min capacitated cells in the presence of calpeptin (10 μM). The precipitated proteins were subjected to SDS–PAGE and Western blot (WB) analysis with flotillin-1 as a positive control and probed with anti-spectrin antibody to determine its association. Right The reciprocal strategy was performed with anti-spectrin antibody. Western blot images are representative of three independent experiments. b Densitometric analysis of co-immunoprecipitated proteins. The results are expressed as the ratio N/N0, where N is the total amount of co-immunoprecipitated spectrin or flotillin-1 and N0 is the total amount of immunoprecipitated flotillin-1 or spectrin. Means ± SEM, n = 3 independent experiments
Flotillin-1 associates and re-orders with SFKs
Previous studies strongly suggest the presence of SFKs in guinea pig sperm (Baltierrez-Hoyos et al. 2012). We therefore first sought to identify, for the first time, the presence and localization of four selected Src family kinases. Through Western blot analyses, we found that four SFKs (Src, Fyn, Lyn, Hck) were present in their respective ∼58- to 60-kDa band region and their quantity remained constant in both non-capacitated sperm and sperm capacitated for 90 min (Fig. 3a; for a negative control, see Fig. S2). Then, by immunofluorescence confocal microscopy analysis, we showed that flotillin-1 and the four Src family members (Src, Fyn, Lyn, Hck) had identical immunolabeling patterns on the acrosome and mid-piece of non-capacitated guinea pig sperm (Fig. 4a’’, c’’, e’’, g’’). Under capacitating conditions, these four kinases migrated towards the sperm head apical ridge and strongly co-localized with flotillin-1; co-localization in the mid-piece remained intact during capacitation of guinea pig sperm (Fig. 4b’’, d’’, f’’, h’’). To identify whether the association of flotillin-SFKs became stronger or weaker, we performed co-immunoprecipitation assays with non-capacitated and capacitated sperm extracts. Immunoprecipitation of flotillin-1 from whole cell extracts of non-capacitated sperm resulted in the co-immunoprecipitation of Src, Fyn, Lyn and Hck (Fig. 3b, c). When a similar assay was performed on whole cell extracts of capacitated guinea pig sperm, the amount of co-immunoprecipitated protein significantly increased for Src, Fyn, Lyn and Hck (Fig. 3b), as evidenced by densitometric analysis (Fig. 3c).
Src family kinases (SFKs) increase their association with flotillin-1 during capacitation in guinea pig sperm. a Whole guinea pig sperm extracts (300 μg) from non-capacitated (−) and 90-min (+) capacitated (Cap) cells were probed for Src (N16), Fyn (FYN3), Lyn (44) and Hck (N30) antibodies to determine their presence in sperm and the changes that these proteins can undergo during capacitation. Following SFK detection, the membranes were stripped and re-probed with anti-Up71 to ensure equal protein loading (bottom). Western blot images are representative of three independent experiments. b Co-immunoprecipitation assays with flotillin-1 were performed with total sperm extracts (500 μg) from non-capacitated and capacitated (90 min) sperm. The precipitated proteins were subjected to SDS–PAGE and Western blot analysis probed for flotillin-1 (Flot-1) as a positive control and probed with each SFK antibody to determine its association. Western blot images are representative of three independent experiments. c Densitometric analysis of co-immunoprecipitated proteins. Results are expressed as the ratio N/N0, where N is the total amount of protein co-immunoprecipitated together with flotilin-1 (SFKs and flotillin-1) and N0 is the total amount of immunoprecipitated flotillin-1. Means ± SEM, n = 3 independent experiments
SFKs co-localize and re-order with flotillin-1 during capacitation. Non-capacitated (a–a’’’, c–c’’’, e–e’’’, g–g’’’) and 90-min capacitated sperm (b–b’’’, d–d’’’, f–f’’’, h–h’’’) were doubly stained for flotillin-1 (Flot-1) and for the following SFKs: Src (a–b’’’), Fyn (c–d’’’), Lyn (e–f’’’) and Hck (g–h’’’). Their co-localization was analyzed by confocal microscopy. The first column shows the marker with respect to the flotillin-1 antibody visualized with an FITC-conjugated secondary antibody (green), whereas the second column shows the marker with respect to the corresponding SFK antibody coupled to a TRITC-conjugated secondary antibody (red). The third column shows the merge between flotillin-1 and the respective SFK, whereas the fourth column shows the respective bright field (BF) micrographs. Arrows indicate the re-ordering towards the apical ridge of SFKs in the capacitated sperm. Images are representative of five independent experiments. Bars 10 μm
Cytoskeleton remodeling precedes flotillin re-ordering
To shed light into the functional significance of the dynamics of the cortical cytoskeleton on flotillins mobility, our next step was to determine whether the re-ordering of flotillin-SFKs depended on cytoskeleton remodeling. First of all, spectrin cleavage was inhibited during capacitation: sperm were capacitated in the presence of calpeptin and after 90 min of incubation, flotillin-1 and SFKs were immunolocalized. By inhibiting spectrin cytoskeleton degradation during capacitation, flotillin-1 and SFKs were not re-ordered, since the staining patterns were similar to those of non-capacitated sperm (Fig. 5a–e). The actin cytoskeleton is known also to regulate the mobility and stabilization of flotillin domains (Langhorst et al. 2007). Therefore, we carried out assays to determine whether the actin cytoskeleton participated in flotillin re-ordering. When the capacitation-associated actin polymerization was inhibited by inducing capacitation in the presence of latrunculin A in the capacitation medium, the immunolocalization of flotillin-1 and SFKs showed that, although no actin polymerization occurred (Fig. 5f–f’’), the re-ordering of these proteins remained unaltered (Fig. 5g–k). These results demonstrate, for the first time, that flotillin microdomain mobility is spectrin-cleavage dependent in guinea pig sperm.
Calpain inhibition suppresses flotillin-1 and SFK re-ordering in guinea pig sperm. Immunostaining of 90-min capacitated (Cap) sperm in presence of calpeptin (+Calpeptin; a–e’) or latrunculin A (+Lat-A; g–k’) with intact acrosomes by antibodies against flotillin-1 (Flot-1; a, g), Src (b, h), Fyn (c, i), Lyn (d, j) and Hck (e, k). Analysis by confocal microscopy. The first and third columns show the marker with respect to the primary antibody visualized with a TRITC-conjugated secondary antibody (red), whereas the second and fourth columns show the respective bright field (BF) micrographs. Arrows indicate the re-ordering of the selected proteins towards the apical ridge. Images are representative of at least three independent experiments. f–f’’ As a positive control for latrunculin A treatment on actin cytoskeleton remodeling, the sperm were stained with TRICT-phalloidin. Bars 10 μm
SFK autophosphorylation is related to spectrin remodeling
Once we had defined that SFKs associate with and relocate with flotillin-1 during capacitation and that spectrin degradation precedes this microdomain aggregation, our next step was to determine whether the re-ordering of flotillin microdomains impacted on SFK Tyr416 autophosphorylation. Therefore, we immunoprecipitated Src, Fyn, Lyn and Hck (Fig. 6a–d) from whole sperm protein extracts by using different capacitation times in the presence or absence of calpeptin and probed them with a specific antibody that recognizes the Tyr416 phosphorylation of any SFK member (anti-SFK-pTyr416). Western blot analysis showed that p-Tyr416 on Src, Fyn, Lyn and Hck was almost absent in non-capacitated sperm (time: 0 min) and also during the first 15 min of capacitation. However, after 30 min of capacitation, Tyr416 phosphorylation increased in a coordinated and significant manner, reaching the highest levels of activation at 90 min of capacitation (Fig. 6a–d). The increment in p-Tyr416 previously described for Src, Fyn, Lyn and Hck was statistically lower when the sperm were capacitated in the presence of calpeptin (Fig. 6a’–d’). These results suggest that both spectrin degradation and SFK relocalization are prerequisites for SFK autophosphorylation in sperm and allow physiological capacitation and further acrosome reaction.
Calpain inhibition suppresses the SFK Tyr416 autophosphorylation that occurs during the course of capacitation. Left Co-immunoprecipitation assays were carried out with total sperm extracts (500 μg) from non-capacitated (0 min) and capacitated (Cap: 15, 30, 60, and 90 min) sperm in the presence or absence of calpeptin. Co-immunoprecipitation assays were performed for the following SFKs: Src (a), Fyn (b), Lyn (c) and Hck (d). Immunoprecipitated proteins were probed with a specific antibody that recognizes any SFK member phosphorylated at Tyr416. Western blot images are representative of three independent experiments. Right Graphs showing densitometric analyses for the following SFKs: Src (a’), Fyn (b’), Lyn (c’) and Hck (d’). The respective SFK phosphorylation ratio is expressed as the ratio of SFK phosphorylated at Tyr416/total SFK. Means ± SEM, n = 3 independent experiments
Spectrin remodeling and SFK re-ordering precede FAK Tyr576/Y577 phosphorylation
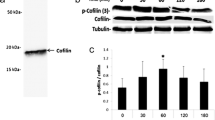
Finally, to elucidate the importance of SKF relocalization and autophosphorylation in the guinea pig sperm, we analyzed whether FAK Tyr576/577 phosphorylation was affected by the inhibition of SFK re-ordering and activation. Tyr576/577 of FAK is a site specifically phosphorylated by SFK members (Calalb et al. 1995). As we previously showed, the inhibition of spectrin cleavage by calpeptin prevented SKF relocalization and autophosphorylation. Therefore, before and during capacitation, sperm were incubated in the presence of calpeptin. Western blot analysis showed that FAK Tyr576/577 phosphorylation increased in capacitated sperm, an increment that was not reached when the sperm were treated with calpeptin (Fig. 7a, b).
Calpain inhibition prevents capacitation-dependent FAK Tyr576/Y577 phosphorylation. a Whole guinea pig sperm extracts (300 μg) from non-capacitated, 60-min capacitated and 60-min capacitated sperm in presence of calpeptin (10 μM) were probed with anti-FAK Tyr576/Y577 antibody. Membranes were stripped and re-probed with anti-FAK antibody to ensure equal protein loading. Western blot images are representative of three independent experiments. b Densitometric analysis of FAK Tyr576/577 phosphorylation during capacitation (Cap) in the presence and absence of calpeptin. Means ± SEM, n = 3 independent experiments
Discussion
The spectrin-actin cytoskeletal meshwork associated with the plasma membrane forms a structural platform that stabilizes membrane microdomains for further focalized signal triggering (Machnicka et al. 2012). This cytoskeletal network, through scaffold proteins such as ankyrin and flotillins, anchors several signaling proteins at the membrane domains, e.g., receptors, ion channels, transporters, kinases and adhesion molecules (Bennett and Healy 2009; Bennett and Lorenzo 2016). The spectrin cytoskeleton is known to be associated with membrane microdomains defined by flotillins (Domingues et al. 2010; Salzer and Prohaska 2001); however, the precise molecular function of spectrin and flotillin in mammalian sperm is still not known. In the present study, we provide initial evidence concerning the role of sperm flotillin microdomains that function as a scaffolding structure for Src family kinases involved in capacitation and the acrosome reaction and whose re-ordering and activation during capacitation are probably driven by spectrin cleavage.
A hallmark event that occurs during capacitation is the redistribution of membrane microdomain markers (Gadella and Boerke 2016). Flotillins and caveolins are the principal protein markers in the membrane microdomains and are found dispersed throughout the acrosome region. During capacitation, these proteins undergo a reorganization that results in their increased concentration at the apical ridge of the acrosome (van Gestel et al. 2005). Our results are in agreement with the finding that, in an entire sperm population with intact acrosomes, two types of flotillin-1 and -2 patterns are found in the sperm head: a dispersed pattern in the acrosome, characteristic of non-capacitation (Fig. 1c–c’’) and an apical ridge re-ordered pattern, characteristic of capacitated sperm (Fig. 1d–d’’). Therefore, we suggest that the re-ordering of the flotillins is an important capacitation event that can be considered as a marker of this spermatic process; our proposal is supported by our results in which approximately 70 % of the capacitated sperm exhibit pattern P2 (Fig. 1e), a similar percentage being reported for capacitated porcine (∼75 %) and mouse (∼90 %) sperm (Boerke et al. 2013; Angeles-Floriano et al. 2016). We also questioned whether the localization of each flotillin varies, finding that, in sperm, the flotillins strongly co-localize and co-immunoprecipitate, suggesting that both flotillins coexist in the same microdomain, in accordance with flotillin properties (Solis et al. 2007). These results show that flotillin-1 and flotillin-2 share a similar behavior, which is probably the result of both proteins forming hetero-oligomers that are not dissociated during capacitation (Fig. 1b, c). Even though the capacitation of guinea pig sperm in vitro is performed in the absence of BSA or another cholesterol-depletion molecule, guinea pig sperm are fertile (Rogers and Yanagimachi 1975) and the reorganization of flotillin-1 and -2 occurs normally (Fig. 1d–d’’, e). Our results are in agreement with the finding that cytoskeleton dynamics are responsible for raft mobilization (Langhorst et al. 2007) and that albumin-mediated sterol depletion preferentially forms non-lipid raft pools on the surface of the sperm (van Gestel et al. 2005). Our results also support the proposal that lipid membrane microdomains are not disrupted during capacitation (Shadan et al. 2004) and that the accumulation of microdomain markers such as caveolin-1 and flotillins in the apical ridge surface area characterizes the capacitation-dependent reorganization of these microdomains (van Gestel et al. 2005). Depending on the species, the sperm membrane might be reinforced with different sterol compositions, which may be the reason that sperm from other species require BSA or another cholesterol depletion molecule to induce protein relocalization and to achieve a capacitated state in vitro.
Despite flotillin reorganization being a capacitation-associated event, the mechanism that regulates the redistribution and its importance in capacitation are still unknown (Boerke et al. 2014; van Gestel et al. 2005). One explanation for the observations mentioned above is that the removal of selected glycosylphosphatidylinositol (GPI)-anchored proteins from the capacitating sperm is associated with the reorganization of lipid membrane microdomains and with the acrosome reaction (Watanabe and Kondoh 2011). However, the experiments performed are not conclusive in explaining the mechanism of the way that flotillins are re-ordered (Boerke et al. 2014). The results of the present study suggest another possible mechanism related to the spectrin cytoskeleton. Two important pieces of evidence from the literature suggest the participation of spectrin in this mechanism: (1) spectrin and flotillins are found in lipid membrane microdomains of various cell types (Nebl et al. 2002) and (2) the spectrin cytoskeleton binds to the inner face of the plasma membrane and creates a physical restrictive barrier that maintains a specific protein localization by avoiding the free diffusion of cytoplasmic and plasma membrane proteins (Bennett and Lorenzo 2016; Susuki et al. 2016). Thus, our results strongly imply that spectrin and flotillin-1 are associated with each other in non-capacitated sperm, a physiological state in which the meshwork of spectrin keeps its integrity, possibly by inhibiting the diffusion of spectrin-associated proteins such as flotillin-1 and -2. This spectrin meshwork changes during the course of capacitation in such a way that the spectrin barrier is disassembled when it is cleft by calpain (Bastian et al. 2010); as a result, flotillin-1 dissociates from spectrin and reorganizes at the acrosomal ridge (Fig. 1d–d’’, Fig. 2a, b). Therefore, our proposed mechanism is supported by the evidence that, when spectrin cleavage is avoided by calpain inhibition, it prevents the dissociation of flotillin-1 from spectrin (Fig. 2a, b) and also inhibits flotillin-1 redistribution (Fig. 5a–e).
In various cell types, the cortical actin cytoskeleton modulates the organization and lateral mobility of flotillin microdomains (Langhorst et al 2007). Nonetheless, flotillin-1 redistribution in sperm cells is not perturbed by latrunculin A, a specific drug that inhibits actin polymerization (Spector et al. 1989). In spite of the low levels of F-actin displayed by sperm treated with latrunculin A, flotillin-1 still migrates towards the acrosomal ridge. This suggests that actin cytoskeleton polymerization might not be necessary for flotillin mobilization. The role of actin cytoskeleton polymerization might be to stabilize the re-ordered flotillin microdomains, since the accumulation of F-actin in this region is observed after capacitation (Fig. 5f’). Even though the mechanism proposed in this study can partially explain the way that flotillins are re-ordered in a capacitation-dependent manner, we do not exclude the idea that GPI-anchored protein removal has a function in this process; hence, further experiments are necessary to clarify the manner in which the two mechanisms regulate flotillin redistribution.
The four SFKs studied here were localized in the mid-piece and acrosomal region, which is a similar localization previously reported for Src and Fyn in human, mouse and rat sperm (Goupil et al. 2011; Kierszenbaum et al. 2009; Mitchell et al. 2008). In mouse sperm, Lyn was detected in the acrosomal region and principal end pieces but was absent from the mid-piece, whereas Hck was restricted to the principal piece of the flagellum (Goupil et al. 2011). This suggests that all these different localizations depend on each Src family member in a species-specific manner. In spite of the discrepancies in SFK localization found among sperm from various species, SFKs are key regulators of capacitation and the acrosome reaction, because the inhibition of their activity prevents the normal course of both processes (Krapf et al. 2010; Stival et al. 2015; Varano et al. 2008).
Membrane microdomains participate in focalized signaling events by assembling specific signaling molecules and by providing a structure in which proteins can meet and initiate a downstream signaling process (Foster et al. 2003). Membrane microdomain markers, such as caveolin-1 and flotillins, accumulate at the apical ridge and may function as protein scaffolds that concentrate specific molecules in regions in which they are required. Interestingly, several SFKs have been localized in specialized membrane microdomains (Simons and Toomre 2000; Yasuda et al. 2002). Accordingly, Src and Fyn have been found associated and co-clustered at various regions in the N-terminal sequence of flotillins in adipocyte cells (Liu et al. 2005). Similarly, we show that SFKs co-immunoprecipitate with flotillin-1 and their interaction increases under capacitating conditions (Fig. 3b, c); this might be related to the increment in SFK autophosphorylation (Fig. 6a–d’). Furthermore, during the course of capacitation, Src, Fyn, Lyn and Hck migrate towards the apical ridge of the sperm head. Together, the above evidence suggests that, during capacitation, the SFKs interact strongly with flotillin microdomains. Nevertheless, we failed to demonstrate whether the flotillin-1-SFK interaction occurs in a direct manner or through other proteins present in the flotillin microdomain.
Among all SFK members, the autophosphorylation of Tyr416 is highly conserved and is required to promote the kinase activity of all family members (Roskoski 2015). In other cell types, Fyn associates constantly with flotillin-1 through the SPFH domain (Liu et al. 2005), whereas the interaction of Lyn with flotillin-1 increases after Lyn activation (Kato et al. 2006). On the same note, our data also suggest that the association of SFKs with flotillins in sperm is important for SFK autophosphorylation. Indeed, SFK autophosphorylation might depend on two steps: first, at spectrin cleavage and secondly, on association with flotillin-1. This occurs in such a way that, when the spectrin cleavage is inhibited, SFK autophosphorylation is greatly avoided (Fig. 6a–d’). Based on these results, we suggest that the cytoskeleton meshwork organized by spectrin stabilizes membrane microdomains from the acrosomal region and does not allow a free redistribution of flotillins, with a consequent lack of activation of signaling proteins being required for proper capacitation and the acrosome reaction, as for the tyrosine kinase Src (for a review, see Bailey 2010).
Flotillins not only are important scaffold proteins but also influence the activation of various signaling proteins, because their mis- and downregulations affect the high activation levels of Rho-GTPases such as Rac, Rho and Cdc42, which modify actin cytoskeleton dynamics (Stuermer 2011). Furthermore, in mammalian sperm, SFKs are not the only proteins whose activity is related to membrane microdomain lipid changes; phospholipase B is activated by sterol removal (Asano et al. 2013). Adding our data concerning SFKs and flotillins to the work from others, we reinforced the proposal that flotillins are scaffold proteins that concentrate signaling molecules in specific sperm regions.
Flotillin-1 and SFKs co-localize in the mid-piece (Fig. 4a’’, c’’, e’’, g’’) but do not undergo changes in their localization during capacitation (Fig. 4b’’, d’’, f’’, h’’). Interestingly, this sperm region lacks spectrin (Bastian et al. 2010) but is rich in actin-F (Fig. 5f–f’’); probably, the complex flotillin-1-SFK present in the mid-piece is stabilized by the actin cytoskeleton. These results support the importance of spectrin in structuring sperm domains and suggest that flotillin-1-SFKs influence mitochondrial functions, as proposed by Hebert-Chatelain (2013).
Our data show that the inhibition of spectrin cleavage had two important effects on Src, Fyn, Lyn and Hck autophosphorylation: (1) SFK re-ordering occurs late during capacitation (Fig. 4b’’, d’’, f’’, h’’) and (2) the increase in the phosphorylation Tyr416 that occurs during the normal course of capacitation is significantly restricted (Fig. 6a–d’). The localization of the various Src family tyrosine kinases in membrane microdomains is known to be crucial for their activation in several cell lines, because they have substantially lower kinase activity outside of the membrane microdomains (Hitosugi et al. 2007; Kato et al. 2006; Young et al. 2003). Additionally, the inhibition of spectrin cleavage prevents the phosphorylation increment of FAK Tyr576/Y577 (Fig. 7a, b), which are SFK-mediated phosphorylation sites when FAK is previously Tyr397 phosphorylated (Schlaepfer et al. 1999). In the guinea pig sperm, FAK contributes to acrosome integrity by activating several signaling pathways and by regulating actin polymerization (Roa-Espitia et al. 2016). FAK Tyr397 autophosphorylation increases in the apical ridge and mid-piece in guinea pig sperm during capacitation (Roa-Espitia et al. 2016), regions in which SFKs reside and relocate. Therefore, we propose that FAK phosphorylation and the autophosphorylation of Src, Fyn, Lyn and Hck in guinea pig sperm depends on spectrin cleavage in order to release flotillin-1, thereby allowing a relocation and a stronger interaction with SFKs and a further focalized signaling process by p-FAK Tyr576/577 and other SFK targets. Our results imply that a fine regulation of SFK autophosphorylation occurs at the right time and space within the cell. With reference to time, the autophosphorylation of SFKs increases after 30 min of capacitation; before this time, SFK autophosphorylation is extremely low, since capacitation cannot be initiated until the required levels of Ca2+ are present to cleave spectrin through an activated calpain. Concerning space, the highest SFK autophosphorylation ratio is reached when these kinases are carried by flotillin-1 to the apical region of the acrosome (Fig. 4a–h’’’, 6a–d’), where possibly target proteins are found, such as p-FAK Tyr397 (Roa-Espitia et al. 2016), since most of the sperm, after 60 min of capacitation, show the pattern 2 staining that is correlated with the highest levels of p-Tyr416 for SFKs (Fig. 6a–d’).
In conclusion, our results support the hypothesis that the cytoskeleton structure of spectrin stabilizes various membrane domains where scaffolding proteins reside and interact with diverse signaling molecules, thereby affecting their activity. Therefore, we consider that the spectrin cytoskeleton is one of the molecular structures that inhibits capacitation in guinea pig sperm and that, when it is disassembled, it triggers the apical aggregation of flotillin microdomains and various signaling cascades important for capacitation and the acrosome reaction. The observations generated by this study demonstrate that spectrin not only serves as a structural protein but is also involved in membrane microdomain stabilization prior to capacitation. Our findings show, for the first time, that, once spectrin cytoskeleton cleavage occurs during capacitation, it allows flotillin microdomains to migrate towards the head apical ridge, together with SFKs, to trigger FAK to its maximal catalytic state, like other targets, to initiate a localized intense signaling cascade required for the proper capacitation and further acrosome reaction that are essential for guinea pig sperm to become fertilization-competent.
References
Aitken RJ, Nixon B (2013) Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 19:785–793
Angeles-Floriano T, Roa-Espitia AL, Baltiérrez-Hoyos R, Cordero-Martínez J, Elizondo G, Hernández-González EO (2016)Absence of aryl hydrocarbon receptor alters CDC42 expression and prevents actin polymerization during capacitation. Mol Reprod Dev 83:1015–1026
Aoyama T, Ozaki Y, Aoki K, Kunimatsu M, Tada T, Sasaki M, Suzumori K (2001)Involvement of mu-calpain in human sperm capacitation for fertilization. Am J Reprod Immunol 45:12–20
Asano A, Nelson-Harrington JL, Travis AJ (2013) Phospholipase B is activated in response to sterol removal and stimulates acrosome exocytosis in murine sperm. J Biol Chem 288:28104–28115
Austin CR (1952) The capacitation of the mammalian sperm. Nature 170:326
Azamar Y, Uribe S, Mujica A (2007) F-actin involvement in guinea pig sperm motility. Mol Reprod Dev 74:312–320
Babuke T, Tikkanen R (2007)Dissecting the molecular function of reggie/flotillin proteins.Eur J Cell Biol 86:525–532
Bailey JL (2010) Factors regulating sperm capacitation. Syst Biol Reprod Med 56:334–348
Baker MA, Hetherington L, Aitken RJ (2006) Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 119:3182–3192
Baltierrez-Hoyos R, Roa-Espitia AL, Hernandez-Gonzalez EO (2012) The association between CDC42 and caveolin-1 is involved in the regulation of capacitation and acrosome reaction of guinea pig and mouse sperm. Reproduction 144:123–134
Bastian Y, Roa-Espitia AL, Mujica A, Hernandez-Gonzalez EO (2010) Calpain modulates capacitation and acrosome reaction through cleavage of the spectrin cytoskeleton. Reproduction 140:673–684
Ben-Aharon I, Brown PR, Etkovitz N, Eddy EM, Shalgi R (2005) The expression of calpain 1 and calpain 2 in spermatogenic cells and spermatozoa of the mouse. Reproduction 129:435–442
Bennett V, Healy J (2009) Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol 1:a003012
Bennett V, Lorenzo DN (2016) An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr Top Membr 77:143–184
Boerke A, Brouwers JF, Olkkonen VM, van de Lest CH, Sostaric E, Schoevers EJ, Helms JB, Gadella BM (2013)Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization.Biol Reprod 88:21
Boerke A, Lit J van der, Lolicato F, Stout TA, Helms JB, Gadella BM (2014) Removal of GPI-anchored membrane proteins causes clustering of lipid microdomains in the apical head area of porcine sperm. Theriogenology 81:613–624
Bou Khalil M, Chakrabandhu K, Xu H, Weerachatyanukul W, Buhr M, Berger T, Carmona E, Vuong N, Kumarathasan P, Wong PT, Carrier D, Tanphaichitr N (2006) Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev Biol 290:220–235
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bragado MJ, Gil MC, Martin-Hidalgo D, Hurtado de Llera A, Bravo N, Moreno AD, Garcia-Marin LJ (2012) Src family tyrosine kinase regulates acrosome reaction but not motility in porcine spermatozoa. Reproduction 144:67–75
Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, Breitbart H (2003) Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod 68:837–845
Briz V, Baudry M (2016) Calpains: master regulators of synaptic plasticity. Neuroscientist 2016:1073858416649178, doi:10.1177/1073858416649178
Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287:121–149
Calalb MB, Polte TR, Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15:954–963
Camatini M, Colombo A, Bonfanti P (1991) Identification of spectrin and calmodulin in rabbit spermiogenesis and spermatozoa. Mol Reprod Dev 28:62–69
Chang MC (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168:697–698
Cross NL (2004) Reorganization of lipid rafts during capacitation of human sperm. Biol Reprod 71:1367–1373
Domingues CC, Ciana A, Buttafava A, Casadei BR, Balduini C, Paula E de, Minetti G (2010) Effect of cholesterol depletion and temperature on the isolation of detergent-resistant membranes from human erythrocytes. J Membr Biol 234:195–205
Etkovitz N, Tirosh Y, Chazan R, Jaldety Y, Daniel L, Rubinstein S, Breitbart H (2009) Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev Biol 334:447–457
Finkelstein M, Megnagi B, Ickowicz D, Breitbart H (2013) Regulation of sperm motility by PIP2(4,5) and actin polymerization. Dev Biol 381:62–72
Foster LJ, De Hoog CL, Mann M (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A 100:5813–5818
Gadella BM, Boerke A (2016) An update on post-ejaculatory remodeling of the sperm surface before mammalian fertilization. Theriogenology 85:113–124
Gangwar DK, Atreja SK (2015) Signalling events and associated pathways related to the mammalian sperm capacitation. Reprod Domest Anim 50:705–711
Gestel RA van, Brewis IA, Ashton PR, Helms JB, Brouwers JF, Gadella BM (2005) Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol Hum Reprod 11:583–590
Goupil S, La Salle S, Trasler JM, Bordeleau LJ, Leclerc P (2011) Developmental expression of SRC-related tyrosine kinases in the mouse testis. J Androl 32:95–110
Head BP, Patel HH, Insel PA (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838:532–545
Hebert-Chatelain E (2013) Src kinases are important regulators of mitochondrial functions.Int J Biochem Cell Biol 45:90–98
Hernandez-Gonzalez EO, Lecona-Valera AN, Escobar-Herrera J, Mujica A (2000) Involvement of an F-actin skeleton on the acrosome reaction in guinea pig spermatozoa. Cell Motil Cytoskeleton 46:43–58
Hinkovska-Galcheva V, Srivastava PN (1993) Phospholipids of rabbit and bull sperm membranes: structural order parameter and steady-state fluorescence anisotropy of membranes and membrane leaflets. Mol Reprod Dev 35:209–217
Hitosugi T, Sato M, Sasaki K, Umezawa Y (2007) Lipid raft specific knockdown of SRC family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells. Cancer Res 67:8139–8148
Kato N, Nakanishi M, Hirashima N (2006) Flotillin-1 regulates IgE receptor-mediated signaling in rat basophilic leukemia (RBL-2H3) cells. J Immunol 177:147–154
Kierszenbaum AL, Rivkin E, Talmor-Cohen A, Shalgi R, Tres LL (2009) Expression of full-length and truncated Fyn tyrosine kinase transcripts and encoded proteins during spermatogenesis and localization during acrosome biogenesis and fertilization. Mol Reprod Dev 76:832–843
Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE (2010) Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem 285:7977–7985
Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, Plattner H, Stuermer CA (1998) Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol 37:502–523
Langhorst MF, Reuter A, Luxenhofer G, Boneberg EM, Legler DF, Plattner H, Stuermer CA (2006) Preformed reggie/flotillin caps: stable priming platforms for macrodomain assembly in T cells. FASEB J 20:711–713
Langhorst MF, Solis GP, Hannbeck S, Plattner H, Stuermer CA (2007) Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett 581:4697–4703
Le Rumeur E, Winder SJ, Hubert JF (2010) Dystrophin: more than just the sum of its parts. Biochim Biophys Acta 1804:1713–1722
Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR (2005) The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem 280:16125–16134
Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ (2010) Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol 191:771–781
Machnicka B, Grochowalska R, Boguslawska DM, Sikorski AF, Lecomte MC (2012) Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci 69:191–201
Machnicka B, Czogalla A, Hryniewicz-Jankowska A, Boguslawska DM, Grochowalska R, Heger E, Sikorski AF (2014) Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim Biophys Acta 1838:620–634
Miranda PV, Allaire A, Sosnik J, Visconti PE (2009) Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol Reprod 80:897–904
Mitchell LA, Nixon B, Baker MA, Aitken RJ (2008) Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod 14:235–243
Morrow IC, Rea S, Martin S, Prior IA, Prohaska R, Hancock JF, James DE, Parton RG (2002) Flotillin-1/reggie-2 traffics to surface raft domains via a novel Golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J Biol Chem 277:48834–48841
Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ (2002) Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem 277:43399–43409
Neumann-Giesen C, Falkenbach B, Beicht P, Claasen S, Luers G, Stuermer CA, Herzog V, Tikkanen R (2004) Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J 378:509–518
Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R (2007) Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci 120:395–406
Nixon B, Aitken RJ (2009) The biological significance of detergent-resistant membranes in spermatozoa. J Reprod Immunol 83:8–13
Okada M (2012) Regulation of the SRC family kinases by Csk. Int J Biol Sci 8:1385–1397
Olson SD, Fauci LJ, Suarez SS (2011) Mathematical modeling of calcium signaling during sperm hyperactivation. Mol Hum Reprod 17:500–510
Ozaki Y, Blomgren K, Ogasawara MS, Aoki K, Furuno T, Nakanishi M, Sasaki M, Suzumori K (2001) Role of calpain in human sperm activated by progesterone for fertilization. Biol Chem 382:831–838
Rivera-Milla E, Stuermer CA, Malaga-Trillo E (2006) Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci 63:343–357
Roa-Espitia AL, Hernandez-Rendon ER, Baltierrez-Hoyos R, Munoz-Gotera RJ, Cote-Velez A, Jimenez I, Gonzalez-Marquez H, Hernandez-Gonzalez EO (2016) Focal adhesion kinase is required for actin polymerization and remodeling of the cytoskeleton during sperm capacitation. Biol Open 5:1189–1199
Rogers BJ, Yanagimachi R (1975) Retardation of guinea pig sperm acrosome reaction by glucose: the possible importance of pyruvate and lactate metabolism in capacitation and the acrosome reaction. Biol Reprod 13:568–575
Roskoski R Jr (2015) Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res 94:9–25
Salzer U, Prohaska R (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97:1141–1143
Schlaepfer DD, Hauck CR, Sieg DJ (1999) Signaling through focal adhesion kinase. Prog Biophys Mol Biol 71:435–478
Selvaraj V, Asano A, Buttke DE, McElwee JL, Nelson JL, Wolff CA, Merdiushev T, Fornes MW, Cohen AW, Lisanti MP, Rothblat GH, Kopf GS, Travis AJ (2006) Segregation of micron-scale membrane sub-domains in live murine sperm. J Cell Physiol 206:636–646
Shadan S, James PS, Howes EA, Jones R (2004) Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol Reprod 71:253–265
Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39
Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, Stuermer CA (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J 403:313–322
Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, Johnson GR, Visconti PE (2009) Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci 122:2741–2749
Spector I, Shochet NR, Blasberger D, Kashman Y (1989) Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil Cytoskeleton 13:127-144
Stival C, La Spina FA, Baro Graf C, Arcelay E, Arranz SE, Ferreira JJ, Le Grand S, Dzikunu VA, Santi CM, Visconti PE, Buffone MG, Krapf D (2015) Src kinase is the connecting player between protein kinase A (PKA) activation and hyperpolarization through SLO3 potassium channel regulation in mouse sperm. J Biol Chem 290:18855–18864
Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D (2016) Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 220:93–106
Stuermer CA (2011) Reggie/flotillin and the targeted delivery of cargo. J Neurochem 116:708–713
Stuermer CA, Plattner H (2005) The “lipid raft” microdomain proteins reggie-1 and reggie-2 (flotillins) are scaffolds for protein interaction and signalling. Biochem Soc Symp 2005:109-118
Stuermer CA, Lang DM, Kirsch F, Wiechers M, Deininger SO, Plattner H (2001) Glycosylphosphatidyl inositol-anchored proteins and Fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol Biol Cell 12:3031–3045
Suarez SS (2008) Control of hyperactivation in sperm. Hum Reprod Update 14:647–657
Susuki K, Otani Y, Rasband MN (2016)Submembranous cytoskeletons stabilize nodes of Ranvier.Exp Neurol 283:446–451
Tapia S, Rojas M, Morales P, Ramirez MA, Diaz ES (2011) The laminin-induced acrosome reaction in human sperm is mediated by Src kinases and the proteasome. Biol Reprod 85:357–366
Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609
Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS (2001) Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and guinea pig spermatozoa. Dev Biol 240:599–610
Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M (2008) Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod 23:2652–2662
Ward CR, Storey BT (1984) Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol 104:287–296
Watanabe H, Kondoh G (2011) Mouse sperm undergo GPI-anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J Cell Sci 124:2573–2581
Xiao X, Yang WX (2007) Actin-based dynamics during spermatogenesis and its significance. J Zhejiang Univ Sci B 8:498–506
Yasuda K, Nagafuku M, Shima T, Okada M, Yagi T, Yamada T, Minaki Y, Kato A, Tani-Ichi S, Hamaoka T, Kosugi A (2002) Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J Immunol 169:2813–2817
Young RM, Holowka D, Baird B (2003) A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem 278:20746–20752
Acknowledgements
We thank the staff of Unidad de Microscopía Confocal (Dpto. Biología Celular, CINVESTAV-IPN) for providing confocal facilities. We also thank Dr Liora Shoshani Z. and Dr. Dominique Mornet for their generous antibody donations. This work was supported by a Consejo Nacional de Ciencia y Tecnología (CONACYT) grant (79921) to Enrique O. Hernández-González, and with doctoral scholarships to Deneb Maldonado-García (322154), Tania Reyes-Miguel (262875) and Monica L. Salgado-Lucio (263011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists that could be perceived as prejudicing the impartiality of this scientific work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Chlortetracycline (CTC) staining patterns after capacitation of guinea pig sperm. Sperm were incubated under capacitation conditions and, after 90 min, were stained with CTC. Fluorescence images corresponding to three different CTC staining patterns of the guinea pig sperm. The patterns scored were: F corresponds to non-capacitated sperm, B corresponds to capacitated sperm, AR corresponds to acrosome-reacted sperm. Means ± SEM, n = 3 independent experiments. (JPEG 33.4 kb)
Supplementary Figure 2
Immunoprecipitation of Rac-1 as a negative control. Co-immunoprecipitation assays with Rac-1 were performed with total sperm extracts (500 μg) from non-capacitated and 90-min capacitated sperm. The precipitated proteins were subjected to SDS–PAGE and Western blot analysis probed for Rac-1 as a positive control and probed with anti-flotillin-1 (Flot-1), anti-flotillin-2 (Flot-2), anti-spectrin and anti-SFKs antibodies to determine its associations. Western blot images are representative of three independent experiments. (JPEG 34.1 kb)
Rights and permissions
About this article
Cite this article
Maldonado-García, D., Salgado-Lucio, M.L., Roa-Espitia, A.L. et al. Calpain inhibition prevents flotillin re-ordering and Src family activation during capacitation. Cell Tissue Res 369, 395–412 (2017). https://doi.org/10.1007/s00441-017-2591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2591-2