Abstract
Neural tube defect disorders are developmental diseases that originate from an incomplete closure of the neural tube during embryogenesis. Despite high prevalence—1 out of 3000 live births—their etiology is not yet established and both environmental and genetic factors have been proposed, with a heritability rate of about 60%. Studies in mouse models as well as in human have further suggested a multifactorial pattern of inheritance for neural tube defect disorders. Here, we report results obtained from clinical diagnosis and NGS analysis of a cohort composed of 52 patients. Using a candidate gene panel approach, we identified variants in known genes of planar cell polarity (PCP) pathway, although with higher prevalence than previously reported. Our study also reveals variants in novel genes such as FREM2 and DISP1. Altogether, these results confirm the implication of the PCP genes and involve the FRAS/FREM2 complex and Sonic Hedgehog signaling as novel components in the appearance of NTDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural tube defect disorders (NTDs) are common developmental diseases that affect approximately 1 out of 3000 live births (French National Centre for Health statistics 2015). These severe congenital defects originate from a failure of neurulation during embryogenesis that results in an incomplete closure of the neural tube. NTDs show an important phenotypic variability depending both on the site where closure defect occurs and on the severity of this defect. The most frequent NTDs are anencephaly and spina bifida, which result from the failed fusion of the neural tube at the brain or at the spinal region, respectively. Spina bifida exists in two major forms: spina bifida occulta, the closed form, or spina bifida aperta, the open and more severe form. Other open NTDs include myeloschisis, hemimyelomeningocele, hemimyelocele and the most severe and rare form called craniorachischisis, in which both brain and spinal cord remain open (Mohd-Zin et al. 2017).
While severe open lesions that affect the brain are lethal, spina bifida typically results in pain, weakness and incontinence with a profound impact on daily life.
Despite their high prevalence, the etiology of NTDs has not yet been established. Both environmental and genetic factors have been proposed, and the heritability is estimated at 60% (Bassuk and Kibar 2009). Increased recurrence risk for siblings of index cases but decreasing in more distant relatives, concordance observed in twins, and existence of multiplex families with several affected children altogether argue in favour of a polygenic pattern of inheritance (Detrait et al. 2005).
As periconceptional folic acid intake has been described to reduce the occurrence of NTDs (van Gool et al. 2018), genes related to the folate one-carbon metabolism have been studied. However, only few statistically significant associations between NTDs and the folate pathway genes have been identified in case–control studies, notably for variants in MTHFR, MTHFD1 or MTHFD1L genes (Amorim et al. 2007; Zhang et al. 2013).
In addition, the planar cell polarity (PCP) and Wnt pathways have been involved in NTDs by various studies in animal models (Carter et al. 2005; Harris and Juriloff 2007; Zohn 2012). PCP genes are essential for polarized cell movements and for convergent extension movements of the notochord and neural plate (Wallingford and Harland 2002), consistent with their involvement in the formation and closure of the neural tube. Recently, a role of the non-canonical Wnt signaling pathway in guiding the direction of PCP has been described (Humphries and Mlodzik 2018). Model mice homozygous for the PCP genes, Vangl2, Celsr1, Dvls, Scrib1, Fz3/6 or Ptk7, display craniorachischisis (Greene et al. 1998; Hamblet et al. 2002; Lu et al. 2004; Murdoch et al. 2014). Further, several studies of large NTD cohorts have reported that candidate pathogenic variants affecting PCP genes are found in up to 3% of patients, in most cases inherited from a clinically unaffected parent (Kibar et al. 2007; Lei et al. 2013; Robinson et al. 2012). Finally, digenic inheritance pattern involving variants in PCP genes was recently reported in NTD patients (Chen et al. 2018; Wang et al. 2018). Altogether, these results involve PCP and folate metabolism pathways in NTDs and indicate a complex genetic pattern of their inheritance.
The aim of this study was, first, to refine the prevalence of known NTD genes and, second, to identify novel candidate genes. We report results obtained from NGS analysis of 14 families with one or two affected individuals, 25 isolated patients and 9 fetuses, altogether representing 52 patients. NGS analyses focused on a panel of candidate genes, NTDs-related genes or mouse candidate genes, present in the 5504 genes associated with known phenotypes, called medical exome (Okazaki et al. 2016). We could describe prevalence higher than expected for mutations in genes involved in the planar cell polarity pathway such as CELSR1 and PTK7, thus reinforcing the implication of this pathway in the onset of NTDs. We also identified mouse candidate genes like FREM2, a member of the FRAS/FREM complex expressed in neural tube (Mansfield et al. 2011; Timmer et al. 2005), and implicated the Sonic Hedgehog (SHH) pathway in NTDs.
Materials and methods
Patients
This study included a cohort of 52 unrelated patients of European ancestry diagnosed with NTD. Additionally, 36 parents were analysed by medical exome.
14 families were recruited at the genetic medical service of Rennes or Vannes Hospital Centers. Nine families were trios including one affected child and two parents. Two families were composed of two affected children/fetuses and their two parents. Both parental DNAs were not systematically available: one family consisted of two affected children and the mother, and another family consisted of one case and the mother. One family was composed of two affected children without parental DNA.
25 adult isolated cases were recruited through the genetics consultations at the National Reference Center for Spina Bifida located at the Hospital Center of Rennes. We also included 9 fetuses interrupted after ultrasound had revealed a neural tube defect.
The study was approved by the local ethics committee and was declared to the CNIL (French National committee for information and Freedom No. 1873368 v 0). Written informed consent was obtained from all participating individuals or legal representatives.
Sequencing
All samples were prepared using the Agilent Focused Exome preparation kit (which covers 5504 genes associated with known phenotypes and 11,884,205 base pairs) and sequenced on an Illumina HiSeq 1500 sequencer using 2 × 150 bp sequencing kits.
All variants identified by NGS were confirmed by Sanger sequencing (Supplementary Data Fig. 1).
Bioinformatics pipeline
Raw sequencing data were mapped to the GRCh37/hg19 reference genome with the bwa-mem aligner (Houtgast et al. 2018). Variant calling was performed using Freebayes (https://github.com/ekg/freebayes) and the GATK (Unified Genotyper and Haplotype Caller) (De Summa et al. 2017). Whenever possible, i.e., when familial cases were available, the variant calling was conducted in a family structure.
Variants were then filtered based on their sequencing quality: a read depth of ≥ 10 × and a quality score of ≥ 30; frequency in population: only variants with minor allele frequency < 0.1% in any population database or our in-house database were retained.
The resulting variants were annotated with ANNOVAR (February 2016 build) (Wang et al. 2010). A gene-based annotation was performed using RefGene to identify the variant functional classes (missense/intronic/UTR/splicing/frameshift…), the genes and the amino acid changes. For variant frequency annotation, we used allelic population frequency information from six control databases: dbSNP (build 147), 1000 Genomes (August 2015), Kaviar (September 2015), Exome Sequencing Project (ESP, March 2015), Exome Aggregation Consortium (ExAC) and its new version—Genome Aggregation Database (gnomAD). To identify recurring false positives, this annotation step was completed with our in-house exome data (variants frequency during previous runs and annotations conducted in our laboratory).
Potential deleterious effects were assessed using several prediction algorithms (SIFT, PolyPhen2 HDIV, PolyPhen2 HVAR, LRT, MutationTaster, MutationAssessor, FATHMM, MetaSVM, MetaLR, VEST, CADD, GERP++, DANN and PROVEAN) provided by dbNSFP (non-synonymous functional predictions, v3.0) (Liu et al. 2016).
For variants that could potentially alter splicing, we used the library dbscSNV (v1.1) provided by ANNOVAR, which contains predictions of splice sites based on AdaBoost and RandomForest score. Complementary annotations were performed using Alamut Visual v2.8.1 (Interactive Biosoftware, Rouen, France) and custom-made annotations based on OMIM database and/or inheritance mode for each gene. Moreover, for each sample, variants were investigated based on all likely transmission modes (autosomal dominant, autosomal recessive, X-linked recessive in males) and segregation on a family-by-family basis.
Finally, we retained loss of function and frameshift variants, as well as non-synonymous ones with a majority of deleterious predictions given by dbNSFP, dbscSNV and Alamut. The pathogenicity score given by these algorithms was confirmed by UMD Predictor (Salgado et al. 2016) and allowed us to provide a deleterious score (UMD score > 65) to the selected variants, except for intronic and del/ins variants. These scores are reported in Table 1.
To identify relevant variants for NTD on a physiopathological basis, we have prioritized variants present in a list of 230 candidate genes (Supplementary data Table 1). This list contains genes selected from literature reviews (Harris and Juriloff 2007; Ishida et al. 2018), from human genes associated with OMIM genetic disorders (https://www.omim.org), from MGI database (Mouse genome informatics—http://www.informatics.jax.org) integrating genetic, genomic and biological and from the human diseases database Malacards website (https://www.malacards.org).
We used the website provided by the French Exome Project FREX (http://lysine.univ-brest.fr/FrExAC) to estimate the frequency of these variants in the French population (574 unrelated French individuals) and to evaluate their specific enrichment in the NTD cohort.
Results
For each affected individual, clinical and molecular findings were collected and are summarized in Table 1. The numbers quoted in the text correspond to the patient identification numbers indicated in Table 1.
Variants in genes previously associated with human NTDs
Nine patients carried deleterious variants in PCP genes. Among those, we report variants in CELSR1, a gene essential for neural tube closure, in the four isolated patients described below.
The first patient (#1) had a spina bifida aperta and a family history with a cousin presenting with anencephaly. The detected mutation is a missense variant (NM_014246.1: c.1898A > G; p.(His633Arg)) located in the region encoding the cadherin domain of CELSR1, and is observed with a frequency of 0.0004% in gnomAD.
The second CELSR1 variant (NM_014246.1:c.4366C > T; p.(Arg1456Trp)) has been identified in a 7-year-old child (#2), presenting with a myelomeningocele. Her mother presented with a spina bifida occulta that was also observed in one of her cousins. This variant is predicted to be highly deleterious and is observed with a frequency of 0.02% in gnomAD.
One fetus (#3) also presented with a myelomeningocele, but associated with Arnold Chiari malformation, did not show family history and carried an intronic variant in CELSR1 (NM_014246.1 (CELSR1):c.8205 + 9C > T) that is described in gnomAD with a frequency of 0.0083%.
In the fourth patient (#4), who presented with spina bifida aperta, we identified a likely highly deleterious CELSR1 variant in exon 1 (NM_014246.1:c2609A > G; p.(Pro870Leu)) present at a very low frequency in gnomAD (0.0047%). Noteworthily, this variant was associated with another variant that affected FREM2 (c.4485C > G; p.(Ile1495Met)) and described in gnomAD with a frequency of 0.004% (see next result section).
Additional variants in distinct PCP genes were found in our cohort. Interestingly, family trio analysis identified a de novo mutation (NM_001270398.1:c.2780A > T; p.(Glu927Val)) in exon 18 of the protein tyrosine kinase 7 (PTK7) gene (Jung et al. 2002) in a fetus with encephalocele (patient #5). This variant has never been observed in the general population (GnomAD) and is predicted to be highly deleterious.
A variant in FRIZZLED 6 (FZD6) was identified in an isolated case (#6) presenting with myelomeningocele and craniostenosis without family history. This variant (NM_001164615.1:c.1526G > A; p.(Arg509Gln)) was described in gnomAD with a frequency of 0.0016% and is predicted to impact deleteriously protein function. FZD6 belongs to the Frizzled gene family, the members of which are known to activate the PCP and Wnt pathways (De Marco et al. 2012).
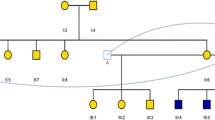
In familial cases #7, two affected live-born children presented with myelomeningocele and cleft lip, while cleft lip and palate have been also reported in the mother and the grandmother. The two affected children shared a maternally inherited variant in PRICKLE1 (NM_001144881.1 (PRICKLE1):c.1222T > C; p.(Trp408Arg)), a crucial gene of the PCP pathway (Tao et al. 2009).
Altogether, we identified eight variants present in genes involved in the PCP pathway. All of these variants are very rare or even absent in the general population and predicted to be deleterious at the protein level. These results thus confirm and reinforce the crucial role of the PCP pathway in normal and pathological development of the neural tube.
We also report variants in genes that do not relate to the PCP pathway and that have been previously linked to human NTDs.
One patient (#8) was described with a myelomeningocele associated with hydrocephaly and Arnold Chiari malformation. Cleft lip and palate cases are reported in her family. A predicted deleterious variant (NM_198174(GRHL3):c.1409G > A; p.(Arg470His)) in the GRHL3 gene (gnomAD 0.0008%) was identified. GRHL3, grainyhead-like transcription factor 3, is a transcription factor that has been implicated both in epidermal integrity and wound healing (Gustavsson et al. 2008) as well as in the regulation of E-cadherin during epithelial-to-mesenchymal transition (Alotaibi et al. 2015).
Patient #9 presented with a myelomeningocele associated with Arnold Chiari malformation. No family history was reported for this fetus. A variant in PAX3 was selected (NM_181459.3(PAX3):c. 1036T > C; p.(Ser346Pro)) due to its deleterious UMD score and its very low frequency in gnomAD (0.0014%). PAX3 is a paired box transcription factor that is a key developmental regulator of the neural crest.
Patient #10 belongs to a family of ten children, five of whom presented with a spina bifida aperta. In this patient, a heterozygous frameshift variant NM_003181.3(T):c.1221_1222ins14 (Thr408Alafs*22) in the last exon 9 of the gene T (Brachyury) was identified. This protein crucially acts in the formation and differentiation of posterior mesoderm and in the axial development of all vertebrates (Chang et al. 2019).
A sporadic spina bifida aperta case (#11) carried a deleterious variant in the GLDC gene (NM_000170.2(GLDC):c.2618C > G; p.(Ser873Cys)) that has never been observed in the general population (gnomAD). This variant is located in the gene encoding the glycine system P protein, one of the enzymes of the glycine cleavage system involved in the mitochondrial folate one-carbon metabolism (FOCM) (Leung et al. 2017), the first biological pathway that was linked to NTDs.
Variants in genes selected based on their implication in the appearance of neural tube defects in mouse
Two cases presented with variants in FREM2. An isolated patient (#12) harboured a rare variant (gnomAD 0.0057%) in FREM2 (NM_207361.5(FREM2):c. 5494C > A; p.(Gln1832Lys)) predicted to be deleterious. She had a myelolipoma associated with a tethered spinal cord and a family history of lumbar sacralization. A second case (#13) carried a variant in FREM2. For this fetus with spina bifida, the mother’s DNA sample was available. The variant (NM_207361.5(FREM2):c.4558C > T; p.(Arg1520Trp)) in the FREM2 gene, non-inherited from the mother, was selected. This variant was present in gnomAD at a frequency of 0.0069%. FREM2- or FRAS1-related extracellular matrix protein is an extracellular matrix protein that localizes in the lamina densa of epithelial basement membranes and has been described to be underexpressed in anencephalic fetal adrenal glands (Mansfield 2011). Depending on the genetic background, mouse models with mutation in Frem2 develop a phenotype including exencephaly and eye defects (Timmer et al. 2005).
In family #14, DNA samples from two sibling fetuses affected with spina bifida and Arnold Chiari malformation were studied, but their parents’ DNA were not available. The two patients shared a frameshift mutation in APAF1 (NM_18186.1(APAF1):c1610del; p.(Asp537Valfs*14)). APAF1, apoptotic protease-activating factor 1, has been shown to be important for neural tube closure in mouse (Cecconi 1999) where, remarkably, hypomorphic alleles of Apaf1 induce spina bifida (Honarpour et al. 2001). The frameshift variant, absent in GnomAD, is observed in exon 12 of the APAF1 gene containing 27 exons and is therefore expected to result in an important shortening of the protein. Altogether these observations support the pathogenic impact of the frameshift variant identified in these siblings and link for the first time APAF1 to NTD.
Variant in DISP1, a developmental gene never formerly linked to NTDs
In family #15, two affected fetuses shared a variant in the DISP1 gene that acts in the SHH signaling pathway by mediating basolateral secretion of SHH (Tian et al. 2005). The identified variant (NM_032890.4(DISP1):c.3296C > T; p.(Pro1099Leu)), predicted to be deleterious, was absent in the mother (paternal DNA was not available) and is present in gnomAD at a frequency of 0.0020%.
Cases with combination of deleterious variants
Three cases with digenic inheritance pattern were observed in our cohort.
The first case of variant combination concerns patient #4, (already described in the first paragraph of the result section) and is associated with variants in CELSR1 and FREM2 genes.
The second case (#16) presented with a spina bifida aperta with no familial history. Two putative pathogenic variants were selected: a deleterious variant in PTK7 (NM_001270398.1 (PTK7):c.1108C > T; p.(Arg370Trp)) present in gnomAD at a very low frequency (0.0004%). This variant is located in exon 7, which encodes the fourth Ig-like loop domain within the extracellular domain of the protein. We selected another variant, in SCRIB (NM_182706.4(SCRIB):c.2410G>A; p.(Gly804Ser)), predicted to be deleterious and found in gnomAD with a frequency of 0.0044%. SCRIB is a PCP gene encoding a protein that is a member of the LAP protein family (leucine-rich repeats, LRR, and PSD-95/Discs large/ZO-1, PZD). In mouse models, mutations in Scrib1 cause a severe form of NTD as a result of a defective planar cell polarity (PCP) signaling (Kharfallah et al. 2017). The LRR region and PZD domains have an important role for SCRIB localization and stabilization at the plasma membrane (Albertson and Doe 2003; Zeitler et al. 2004). The c.2410G>A variant is located in the PZD domains, which are described to interact physically and functionally with VANGL2, a member of the PCP protein family implicated in NTD (Kallay et al. 2006).
The third case with digenic inheritance was observed in a fetus (#17), who was interrupted after anencephaly/exencephaly was diagnosed. The first predicted deleterious variant (NM_001172412.1(VANGL1):c.248C > T; p.(Ser83Leu)) concerned VANGL1, one of the best-known PCP genes associated with NTD (Kibar et al. 2007). This variant is present in gnomAD with a frequency of 0.0065%. The second variant affects PTCH1 (NM_000264.4(PTCH1):c.3590C > A; p.(Ser1197Tyr)) and has never been reported in gnomAD. PTCH1 is the receptor of SHH, which plays an important role in the formation of the neural plate.
In total, predicted pathogenic variants in known or potential NTD genes were detected in up to 36% of the family cases (5/14). The same percentage was found in the unrelated cases (9/25 patients) (Table 1).
Discussion
NTDs are characterized by a high level of phenotypic heterogeneity and their genetic origin still remains largely elusive. Due to high-throughput sequencing, the list of genes involved in human diseases is exponentially increasing. Though whole exome sequencing (WES) is the gold standard for the diagnosis of neurodevelopmental disorders, it remains expensive for some genetic centers. Our recent experience indicated that commercially available gene panels that comprise all OMIM-referenced genes represent an affordable alternative strategy to WES (Cherot et al. 2017). We thus applied this new-generation sequencing-targeted exome on 5504 genes to a cohort of 52 patients with a large spectrum of neural tube defects, aiming at improving our knowledge about OMIM genes associated with NTD. In total, we were able to identify variants in 20 patients (14 isolated cases and 3 siblings) in 15 genes.
A first important result is that candidate pathogenic variants in known or potential NTD genes were detected in up to 36% for both family and unrelated cases (Table 1), which highlights a high rate of candidate gene variants in the studied population. In addition to this overall assessment, the second main result of this study is the major role of PCP pathway in the etiology of NTD. Indeed, among the 15 genes presenting rare and predicted deleterious variants in patients, six of them (CELSR1, PRICKLE 1, FZD6, SCRIB, PTK7 and VANGL1) belong to the PCP pathway and altogether account for half of the identified variants (10 out of 20). All these genes have been previously implicated in neural tube defect disorders (Kibar et al. 2007; Bosoi et al. 2011). Of note is that CELSR1 variants were identified in 4 out of 52 NTD cases (8%), a rate slightly higher than the 3% previously reported in other cohort studies (Allache et al. 2012; Lei et al. 2014; Robinson et al. 2012). The second gene, PRICKLE1, codes a core PCP protein thought to be recruited by VANGL to the cell membrane, which is crucial for establishing cell polarity (Tao et al. 2009; Wang et al. 2011). Misexpression of the planar cell polarity member PRICKLE1 is known to cause neural tube defects in humans and its RNAi-mediated inactivation causes NTD phenotype in chicken embryos (Dady et al. 2014). Furthermore, PRICKLE1 variants were found in cleft lip and palate patients at an elevated frequency (Yang et al. 2014), which is coherent both with myelomeningocele and cleft lip observed in our two patients (case #7) carrying a maternally inherited PRICKLE1 variant and with cleft lip presented by the mother. Consistent with the involvement in NTD of another major PCP gene, FZD6 variants were associated with an increased risk of developing neural tube defects in a cohort of Northern Han Chinese (Shi et al. 2014) as well as in a cohort of Italian and Canadian NTD patients (De Marco et al. 2012). Similarly, mutations in SCRIB have been reported and associated with severe forms of human NTD such as craniorachischisis (Robinson et al. 2012), with spina bifida (Lei et al. 2013) and in fetuses with anencephaly (Wang et al. 2018), altogether underlining the important role of this gene in NTDs. Finally, PTK7 variants were previously reported with a frequency of 1.1% in a cohort of 473 patients, emphasizing the potential risk factor of this gene in NTDs (Wang et al. 2015).
Some of the genes identified in this study do not belong to the PCP pathway, such as GRHL3 that codes for the grainyhead-like transcription factor 3 protein, a transcription factor involved in epidermal integrity and wound healing (Gustavsson et al. 2008). GRHL3 has been previously implicated in NTD both in mouse models (Gustavsson et al. 2008) and in humans (Lemay et al. 2017). The GRLH3 variant we identified is located close to the mutation previously reported as deleterious (Lemay et al. 2017), i.e., in exon 11 that encodes the dimerization domain of the protein, Furthermore, GRLH3 mutations have already been reported in Van der Woode syndrome, the most common syndromic form of cleft lip and palate (Peyrard-Janvid et al. 2014) and a feature that is also described in patient #8’s relatives.
PAX3 was retained as candidate gene, since homozygous Pax3 mutant embryos exhibit NTD such as spina bifida and exencephaly (Burren et al. 2008). Two domains are important in this transcription factor, PAX and HOX, but the variant we identified (NM_181459.3(PAX3):c. 1036T > C; p.(Ser346Pro)) in patient #9 is located downstream from the HOX domain. However, Lemay et al. reported mutations in PAX3 in a patient presenting the same clinical features as patient #9, i.e., myelomeningocele and Arnold Chiari malformation (Lemay et al. 2015).
A variant in the development gene T (Brachyury) NM_003181.3(T):c.1221_1222ins14 (Thr408Alafs*22) was observed in patient #10, belonging to a family with recurrent cases of severe spina bifida. A homozygous substitution in the T gene was previously reported in four individuals from three unrelated consanguineous families with sacral agenesis, abnormal ossification of the vertebral bodies and a persistent notochordal canal (Postma et al. 2014). More recently, a missense homozygous variant in T was also described in a large consanguineous NTD family (Shaheen et al. 2015). Though no segregation analysis could be performed in our large family (parental DNA not available), these previous reports support the pathogenic property of the variant we identified in this patient.
One variant was found in GLDC (patient #11). This gene was considered as an NTD gene because GLDC variants were previously shown to predispose to neural tube defects in humans and also in mice, due to the inability of these animals to use glycine as a one-carbon donor to the folate cycle (Narisawa et al. 2012; Leung et al. 2017; Pai et al. 2015; Shah et al. 2016). It represents the only gene linked to the folate metabolism we identified in this cohort; although genes related to folate metabolism have been intensively studied, their relation to NTD is mainly due to associations of SNPs in MTHFR gene and notably the c.677C > T SNP. Similar positive associations have been reported for other genes such as DHFR, MTHFD1 or MTRR (Boyles et al. 2005; Shaw et al. 2009), which suggests that folate metabolism contribution to NTD etiology is significant but, as only few deleterious mutations in folate metabolism have been described to date, it might act via environmental cues rather than through genetic forms.
We also identified variants in genes that were selected based on mouse models exhibiting neural tube defects. Importantly, we identified the novel candidate genes APAF1 and FREM2, solely due to their implication in NTD in animal models. Indeed, their haploinsufficiency is associated with mouse NTDs in the spontaneous mutants “fog” and “myelencephalic blebs” (Harris et al. 1997; Harris and Juriloff 2007). FREM2 has been proposed to act as a general mediator of epithelial adhesion during development, where it is thought to facilitate the cellular rearrangements during the neural tube formation. Frem2 is expressed during development and the loss of Frem2 in mouse model leads to NTD features such as exencephaly (Timmer et al. 2005). Three patients carried variants in FREM2, consistent with a significant role of this gene in neural tube fusion (Timmer et al. 2005). Thus, FREM2 variants are overrepresented in our cohort as compared to frequencies given by gnomAD in the general population, with a frequency of 6%. Altogether, these observations involve FREM2 in the etiology of NTD in humans.
One important result of this study is the implication of the SHH pathway in NTDs. Variants in genes belonging to this pathway such as DISP1 and PTCH1 were identified in our cohort. The pattern of neural plate bending is regulated by ventral influence from the notochord, with Shh playing a role (Nikolopolou et al. 2017). Although DISP1 has never been directly implicated in NTDs, it has been shown to control secretion of SHH in relation to holoprosencephaly (Mouden et al. 2016; Roessler et al. 2009). In mice model, mutations in Ptch1 could affect neural tube closure and lead to exencephaly (Juriloff et al. 2001). Recently, a new gene GPR161 coding a ciliary G-protein-coupled receptor that regulates Sonic Hedgehog (SHH) signaling, has been implicated in NTD patients (Kim et al. 2018). Further and consistent with our findings, Wang et al. (2013) described a higher risk of spina bifida for patients with PTCH1 variants in the Chinese population. These observations altogether support the involvement of the SHH pathway in NTDs.
In our cohort, three patients presented a combination of variants in two genes (CELSR1/FREM2; PTK7/SCRIB; VANGL1/PTCH1). Recently, human neural tube defects patients carrying digenic variants of planar cell polarity genes were reported in six NTD cases: three of them associated with variants in CELSR1 and SCRIB, one anencephaly case with variants in CELSR1 and DVL3 and one with variants in PTK7 and SCRIB (Wang et al. 2018). The same association of variants in PTK7 and SCRIB was observed in our cohort. Further, digenic cases involving PCP genes are also reported in mouse models (Murdoch et al. 2014). CELSR1 and VANGL1 are core components of the PCP pathway, whereas PTK7 and SCRIB are associated PCP components. The role of each of these genes in PCP signaling is consistent with a cumulative effect of these variants in patients.
Although digenic cases are not observed in the majority of the cases, they could very well account for the low penetrance observed in those families where the variants are inherited from asymptomatic parents.
These digenic cases we report are in agreement with the multigenic mode of inheritance that has been previously suggested in the literature for neural tube defects. A multifactorial threshold model has been proposed to explain the complex pattern of inheritance observed in humans (Harris et al. 2007; Ishida et al. 2018; Zohn 2012). In this model, multiple genetic and environment factors interact in an additive and synergistic fashion, thereby perturbing neurulation and triggering the apparition of neural tube defects (Zohn 2012). Indeed, given the numerous examples found in the literature, it becomes obvious that many developmental genetic disorders have a non-Mendelian mode of transmission, implying that several variants in different genes are required to explain the appearance of the phenotype (Cornec-Le Gall et al. 2018; Gao et al. 2017; Kim et al. 2019; Dubourg et al. 2018). These variants can involve genes either in the same or in different pathways. These genes could also act in a particular environmental context, as it is well documented that folic acid deficiency has a crucial influence on NTD incidence in humans, while folic acid supplementation can reduce it. The actual frequency of this mode of transmission is difficult to evaluate based on the moderate size of our cohort.
Our results highlight the large number of genes involved in NTDs, strengthen the involvement of the PCP pathway genes in their etiology and implicate for the first time genes belonging to the SHH pathway. Undoubtedly, the accumulation of NGS data will help to refine the knowledge about genetic bases of this complex disease and to improve genetic counseling for and neural tube defect disorders.
References
Albertson R, Doe CQ (2003) Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol 5:166–170. https://doi.org/10.1038/ncb922
Allache R, De Marco P, Merello E, Capra V, Kibar Z (2012) Role of the planar cell polarity gene CELSR1 in neural tube defects and caudal agenesis. Birth Defects Res A Clin Mol Teratol 94:176–181. https://doi.org/10.1002/bdra.23002
Alotaibi H, Basilicata MF, Shehwana H, Kosowan T, Schreck I, Braeutigam C, Konu O, Brabletz T, Stemmler MP (2015) Enhancer cooperativity as a novel mechanism underlying the transcriptional regulation of E-cadherin during mesenchymal to epithelial transition. Biochim Biophys Acta 1849:731–742. https://doi.org/10.1016/j.bbagrm.2015.01.005
Amorim MR, Lima MA, Castilla EE, Orioli IM (2007) Non-Latin European descent could be a requirement for association of NTDs and MTHFR variant 677C> T: a meta-analysis. Am J Med Genet A 143A:1726–1732. https://doi.org/10.1002/ajmg.a.31812
Bassuk AG, Kibar Z (2009) Genetic basis of neural tube defects. Semin Pediatr Neurol 16:101–110. https://doi.org/10.1016/j.spen.2009.06.001
Bosoi CM, Capra V, Allache R, Trinh VQ, De Marco P, Merello E, Drapeau P, Bassuk AG, Kibar Z (2011) Identification and characterization of novel rare mutations in the planar cell polarity gene PRICKLE1 in human neural tube defects. Hum Mutat 32:1371–1375. https://doi.org/10.1002/humu.21589
Boyles AL, Hammock P, Speer MC (2005) Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet 135C:9–23. https://doi.org/10.1002/ajmg.c.30048
Burren KA, Savery D, Massa V, Kok RM, Scott JM, Blom HJ, Copp AJ, Greene ND (2008) Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet 17:3675–3685. https://doi.org/10.1093/hmg/ddn262
Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, Campagne F, Weinstein H, Ross ME (2005) Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci USA 102:12843–12848. https://doi.org/10.1073/pnas.0501963102
Cecconi F (1999) Apaf1 and the apoptotic machinery. Cell Death Differ 6:1087–1098. https://doi.org/10.1038/sj.cdd.4400602
Chang S, Lu X, Wang S, Wang Z, Huo J, Huang J, Shangguan S, Li S, Zou J, Bao Y, Guo J, Wang F, Niu B, Zhang T, Qiu Z, Wu J, Wang L (2018) The effect of folic acid deficiency on FGF pathway via Brachyury regulation in neural tube defects. FASEB J. https://doi.org/10.1096/fj.201801536R
Chen Z, Lei Y, Cao X, Zheng Y, Wang F, Bao Y, Peng R, Finnell RH, Zhang T, Wang H (2018) Genetic analysis of Wnt/PCP genes in neural tube defects. BMC Med Genom 11:38. https://doi.org/10.1186/s12920-018-0355-9
Cherot E, Keren B, Dubourg C, Carre W, Fradin M, Lavillaureix A, Afenjar A, Burglen L, Whalen S, Charles P, Marey I, Heide S, Jacquette A, Heron D, Doummar D, Rodriguez D, Billette de Villemeur T, Moutard ML, Guet A, Xavier J, Perisse D, Cohen D, Demurger F, Quelin C, Depienne C, Odent S, Nava C, David V, Pasquier L, Mignot C (2017) Using medical exome sequencing to identify the causes of neurodevelopmental disorders: experience of 2 clinical units and 216 patients. Clin Genet. https://doi.org/10.1111/cge.13102
Cornec-Le Gall E, Chebib FT, Madsen CD, Senum SR, Heyer CM, Lanpher BC, Patterson MC, Albright RC, Yu AS, Torres VE, Investigators HPoPKDG, Harris PC (2018) The Value of genetic testing in polycystic kidney diseases illustrated by a family with PKD2 and COL4A1 mutations. Am J Kidney Dis 72:302–308. https://doi.org/10.1053/j.ajkd.2017.11.015
Dady A, Havis E, Escriou V, Catala M, Duband JL (2014) Junctional neurulation: a unique development program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci 34(39):13208–13221
De Marco P, Merello E, Rossi A, Piatelli G, Cama A, Kibar Z, Capra V (2012) FZD6 is a novel gene for human neural tube defects. Hum Mutat 33:384–390. https://doi.org/10.1002/humu.21643
De Summa S, Malerba G, Pinto R, Mori A, Mijatovic V, Tommasi S (2017) GATK hard filtering: tunable parameters to improve variant calling for next generation sequencing targeted gene panel data. BMC Bioinform 18:119. https://doi.org/10.1186/s12859-017-1537-8
Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC (2005) Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol 27:515–524. https://doi.org/10.1016/j.ntt.2004.12.007
Dubourg C, Kim A, Watrin E, de Tayrac M, Odent S, David V, Dupé V (2018) Recent advances in understanding inheritance of holoprosencephaly. Am J Genet C Semin Med Genet 178(2):258–269
Gao Y, Lee C, Song J, Li S, Cui Y, Liu Y, Wang J, Lu F, Chen H (2017) Digenic mutations on SCAP and AGXT2 predispose to premature myocardial infarction. Oncotarget 8:100141–100149. https://doi.org/10.18632/oncotarget.22045
Greene ND, Gerrelli D, Van Straaten HW, Copp AJ (1998) Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev 73:59–72
Gustavsson P, Copp AJ, Greene ND (2008) Grainyhead genes and mammalian neural tube closure. Birth Defects Res A Clin Mol Teratol 82:728–735. https://doi.org/10.1002/bdra.20494
Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A (2002) Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129:5827–5838
Harris MJ, Juriloff DM (2007) Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol 79:187–210. https://doi.org/10.1002/bdra.20333
Harris BS, Franz T, Ullrich S, Cook S, Bronson RT, Davisson MT (1997) Forebrain overgrowth (fog): a new mutation in the mouse affecting neural tube development. Teratology 55: 231–40. https://doi.org/10.1002/(SICI)1096-9926(199704)55:4%3C231::AID-TERA3%3E3.0.CO;2-3
Honarpour N, Gilbert SL, Lahn BT, Wang X, Herz J (2001) Apaf-1 deficiency and neural tube closure defects are found in fog mice. Proc Natl Acad Sci USA 98:9683–9687. https://doi.org/10.1073/pnas.171283198
Houtgast EJ, Sima VM, Bertels K, Al-Ars Z (2018) Hardware acceleration of BWA-MEM genomic short read mapping for longer read lengths. Comput Biol Chem 75:54–64. https://doi.org/10.1016/j.compbiolchem.2018.03.024
Humphries AC, Mlodzik M (2018) From instruction to output: Wnt/PCP signaling in development and cancer. Curr Opin Cell Biol 51:110–116. https://doi.org/10.1016/j.ceb.2017.12.005
Ishida M, Cullup T, Boustred C, James C, Docker J, English C, Lench N, Copp AJ, Moore GE, Greene NDE, Stanier P (2018) A targeted sequencing panel identifies rare damaging variants in multiple genes in the cranial neural tube defect, anencephaly. Clin Genet 93:870–879. https://doi.org/10.1111/cge.13189
Jung JW, Ji AR, Lee J, Kim UJ, Lee ST (2002) Organization of the human PTK7 gene encoding a receptor protein tyrosine kinase-like molecule and alternative splicing of its mRNA. Biochim Biophys Acta 1579:153–163
Juriloff DM, Gunn TM, Harris MJ, Mah DG, WU MK, Dewell SL (2001) Multifactorial genetics of exencephaly in SELH/Bc mice. Teratology 64(4):189–200
Kallay JM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT (2006) Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 99:647–664
Kharfallah F, Guyot MC, El Hassan AR, Allache R, Merello E, De Marco P, Di Cristo G, Capra V, Kibar Z (2017) Scribble1 plays an important role in the pathogenesis of neural tube defects through its mediating effect of Par-3 and Vangl1/2 localization. Hum Mol Genet 26:2307–2320. https://doi.org/10.1093/hmg/ddx122
Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P (2007) Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356:1432–1437. https://doi.org/10.1056/NEJMoa060651
Kim SE, Lei Y, Hwang SH, Wlodarczyk BJ, Mukhopadhyay S, Shaw GM, Elizabeth Ross M, Finnell RH (2018) Dominant negative GPR161 rare variants are risk factors of human spina bifida. Hum Mol Genet. https://doi.org/10.1093/hmg/ddy339
Kim A, Savary C, Dubourg C, Carré W, Mouden C, Hamdi-Rozé H, Guyodo H, Le Douce J, Pasquier L, FlorI E, Gonzales M, Bénéteau C, Boute O, Attié-Bitach T, Roume J, Goujon L, Akloul L, Watrin E, Dupé V, Odent S, de Tayrac M, David V (2019) Integrated clinical and omics approach to rare diseases: novel genes and oligogenic inheritance in holoprosencephaly. Brain 142(1):35–49. https://doi.org/10.1093/brain/awy290
Lei Y, Zhu H, Duhon C, Yang W, Ross ME, Shaw GM, Finnell RH (2013) Mutations in planar cell polarity gene SCRIB are associated with spina bifida. PLoS One 8:e69262. https://doi.org/10.1371/journal.pone.0069262
Lei Y, Zhu H, Yang W, Ross ME, Shaw GM, Finnell RH (2014) Identification of novel CELSR1 mutations in spina bifida. PLoS One 9:e92207. https://doi.org/10.1371/journal.pone.0092207
Lemay P, Guyot MC, Tremblay E, Dionne-Laporte A, Spiegelman D, Henrion E, Diallo O, De Marco P, Merello E, Massicotte C, Desilets V, Michaud JL, Rouleau GA, Capra V, Kibar Z (2015) Loss-of-function de novo mutations play an important role in severe human neural tube defects. J Med Genet 52:493–497. https://doi.org/10.1136/jmedgenet-2015-103027
Lemay P, De Marco P, Emond A, Spiegelman D, Dionne-Laporte A, Laurent S, Merello E, Accogli A, Rouleau GA, Capra V, Kibar Z (2017) Rare deleterious variants in GRHL3 are associated with human spina bifida. Hum Mutat 38:716–724. https://doi.org/10.1002/humu.23214
Leung KY, Pai YJ, Chen Q, Santos C, Calvani E, Sudiwala S, Savery D, Ralser M, Gross SS, Copp AJ, Greene NDE (2017) Partitioning of one-carbon units in folate and methionine metabolism is essential for neural tube closure. Cell Rep 21:1795–1808. https://doi.org/10.1016/j.celrep.2017.10.072
Liu X, Wu C, Li C, Boerwinkle E (2016) dbNSFP v3.0: a one-stop database of functional predictions and annotations for human nonsynonymous and splice-site SNVs. Hum Mutat 37:235–241. https://doi.org/10.1002/humu.22932
Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M (2004) PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430:93–98. https://doi.org/10.1038/nature02677
Mansfield CW, Carr BR, Faye-Petersen OM, Chen D, Xing Y, Rainey WE, Parker CR Jr (2011) Differential gene expression in the adrenals of normal and anencephalic fetuses and studies focused on the Fras-1-related extracellular matrix protein (FREM2) gene. Reprod Sci 18:1146–1153. https://doi.org/10.1177/1933719111408113
Mohd-Zin SW, Marwan AI, Abou Chaar MK, Ahmad-Annuar A, Abdul-Aziz NM (2017) Spina bifida: pathogenesis, mechanisms, and genes in mice and humans. Scientifica (Cairo). https://doi.org/10.1155/2017/5364827
Mouden C, Dubourg C, Carré W, Rose S, Quélin C, Akloul L, Hamdi-Rozé H, Viot G, Salhi H, Darnault P, Odent S, Dupé V, David V (2016) Complex mode of inheritance in holoprosencephaly revealed by whole exome sequencing. Clin Genet 89:659–668. https://doi.org/10.1111/cge.12722
Murdoch JN, Damrau C, Paudyal A, Bogani D, Wells S, Greene ND, Stanier P, Copp AJ (2014) Genetic interactions between planar cell polarity genes cause diverse neural tube defects in mice. Dis Model Mech 7:1153–1163. https://doi.org/10.1242/dmm.016758
Narisawa A, Komatsuzaki S, Kikuchi A, Niihori T, Aoki Y, Fujiwara K, Tanemura M, Hata A, Suzuki Y, Relton CL, Grinham J, Leung KY, Partridge D, Robinson A, Stone V, Gustavsson P, Stanier P, Copp AJ, Greene ND, Tominaga T, Matsubara Y, Kure S (2012) Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum Mol Genet 21:1496–1503. https://doi.org/10.1093/hmg/ddr585
Nikolopolou E, Galea GL, Rolo A, Greene ND, Copp AJ (2017) Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144(4):552–566
Okazaki T, Murata M, Kai M, Adachi K, Nakagawa N, Kasagi N, Matsumura W, Maegaki Y, Nanba E (2016) Clinical diagnosis of mendelian disorders using a comprehensive gene-targeted panel test for next-generation sequencing. Yonago Acta Med 59:118–125
Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT, Copp AJ, Greene ND (2015) Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 6:6388. https://doi.org/10.1038/ncomms7388
Peyrard-Janvid M, Leslie EJ, Kousa YA, Smith TL, Dunnwald M, Magnusson M, Lentz BA, Unneberg P, Fransson I, Koillinen HK, Rautio J, Pegelow M, Karsten A, Basel-Vanagaite L, Gordon W, Andersen B, Svensson T, Murray JC, Cornell RA, Kere J, Schutte BC (2014) Dominant mutations in GRHL3 cause Van der Woude syndrome and disrupt oral periderm development. Am J Hum Genet 94:23–32. https://doi.org/10.1016/j.ajhg.2013.11.009
Postma AV, Alders M, Sylva M, Bilardo CM, Pajkrt E, van Rijn RR, Schulte-Merker S, Bulk S, Stefanovic S, Ilgun A, Barnett P, Mannens MM, Moorman AF, Oostra RJ, van Maarle MC (2014) Mutations in the T (brachyury) gene cause a novel syndrome consisting of sacral agenesis, abnormal ossification of the vertebral bodies and a persistent notochordal canal. J Med Genet 51:90–97. https://doi.org/10.1136/jmedgenet-2013-102001
Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, Greene ND, Copp AJ, Stanier P (2012) Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat 33:440–447. https://doi.org/10.1002/humu.21662
Roessler E, Ma Y, Ouspenskaia MV, Lacbawan F, Bendavid C, Dubourg C, Beachy PA, Muenke M (2009) Truncating loss-of-function mutations of DISP1 contribute to holoprosencephaly-like microform features in humans. Hum Genet 125:393–400. https://doi.org/10.1007/s00439-009-0628-7
Salgado D, Desvignes JP, Rai G, Blanchard A, Miltgen M, Pinard A, Levy N, Collod-Beroud G, Beroud C (2016) UMD-predictor: a high-throughput sequencing compliant system for pathogenicity prediction of any human cDNA substitution. Hum Mutat 37:439–446. https://doi.org/10.1002/humu.22965
Shah RH, Northrup H, Hixson JE, Morrison AC, Au KS (2016) Genetic association of the glycine cleavage system genes and myelomeningocele. Birth Defects Res A Clin Mol Teratol 106:847–853. https://doi.org/10.1002/bdra.23552
Shaheen R, Alshail E, Alaqeel A, Ansari S, Hindieh F, Alkuraya FS (2015) T (brachyury) is linked to a Mendelian form of neural tube defects in humans. Hum Genet 134:1139–1141. https://doi.org/10.1007/s00439-015-1589-7
Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, Carmichael SL, Barcellos LF, Lammer EJ, Finnell RH (2009) 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 10:49. https://doi.org/10.1186/1471-2350-10-49
Shi OY, Yang HY, Shen YM, Sun W, Cai CY, Cai CQ (2014) Polymorphisms in FZD3 and FZD6 genes and risk of neural tube defects in a northern Han Chinese population. Neurol Sci 35:1701–1706. https://doi.org/10.1007/s10072-014-1815-4
Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N (2009) Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proc Natl Acad Sci USA 106:14426–14431. https://doi.org/10.1073/pnas.0901332106
Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP (2005) Mouse Disp1 is required in sonic hedgehog expressing celles for paracrine activity of the cholesterol-modified ligand. Development 132(1):133–142
Timmer JR, Mak TW, Manova K, Anderson KV, Niswander L (2005) Tissue morphogenesis and vascular stability require the Frem2 protein, product of the mouse myelencephalic blebs gene. Proc Natl Acad Sci USA 102:11746–11750. https://doi.org/10.1073/pnas.0505404102
van Gool JD, Hirche H, Lax H, De Schaepdrijver L (2018) Folic acid and primary prevention of neural tube defects: a review. Reprod Toxicol 80:73–84. https://doi.org/10.1016/j.reprotox.2018.05.004
Wallingford JB, Harland RM (2002) Neural tube closure requires dishevelled-dependent convergent extension of the midline. Development 129:5815–5825
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164. https://doi.org/10.1093/nar/gkq603
Wang B, Sinha T, Jiao K, Serra R, Wang J (2011) Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet 20:271–285. https://doi.org/10.1093/hmg/ddq462
Wang Z, Wang L, Shangguan S, Lu X, Chang S, Wang J, Zou J, Wu L, Zhang T, Luo Y (2013) Association between PTCH1 polymorphisms and risk of neural tube defects in a Chinese population. Birth Defects Res A Clin Mol Teratol 97:409–415. https://doi.org/10.1002/bdra.23152
Wang M, De Marco P, Merello E, Drapeau P, Capra V, Kibar Z (2015) Role of the planar cell polarity gene protein tyrosine kinase 7 in neural tube defects in humans. Birth Defects Res A Clin Mol Teratol 103:1021–1027. https://doi.org/10.1002/bdra.23422
Wang L, Xiao Y, Tian T, Jin L, Lei Y, Finnell RH, Ren A (2018) Digenic variants of planar cell polarity genes in human neural tube defect patients. Mol Genet Metab 124:94–100. https://doi.org/10.1016/j.ymgme.2018.03.005
Yang T, Jia Z, Bryant-Pike W, Chandrasekhar A, Murray JC, Fritzsch B, Bassuk AG (2014) Analysis of PRICKLE1 in human cleft palate and mouse development demonstrates rare and common variants involved in human malformations. Mol Genet Genom Med 2:138–151. https://doi.org/10.1002/mgg3.53
Zeitler J, Hsu CP, Dionne H, Bilder D (2004) Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol 167:1137–1146. https://doi.org/10.1083/jcb.200407158
Zhang T, Lou J, Zhong R, Wu J, Zou L, Sun Y, Lu X, Liu L, Miao X, Xiong G (2013) Genetic variants in the folate pathway and the risk of neural tube defects: a meta-analysis of the published literature. PLoS One 8:e59570. https://doi.org/10.1371/journal.pone.0059570
Zohn IE (2012) Mouse as a model for multifactorial inheritance of neural tube defects. Birth Defects Res C Embryo Today 96:193–205. https://doi.org/10.1002/bdrc.21011
Acknowledgements
We thank the Committee of Clinical and Translational Research (CORECT) of the University Hospital of Rennes for funding this work, the members of the National Reference Center for Spina Bifida of Rennes, Artem Kim for carefully reading this manuscript and the French Exome Project (FREX) for providing a control cohort.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2019_1993_MOESM1_ESM.pdf
Supplementary material 1 Sanger electropherograms of the identified genetic variants. a patient, b control, f father, m mother (PDF 545 KB)
Rights and permissions
About this article
Cite this article
Beaumont, M., Akloul, L., Carré, W. et al. Targeted panel sequencing establishes the implication of planar cell polarity pathway and involves new candidate genes in neural tube defect disorders. Hum Genet 138, 363–374 (2019). https://doi.org/10.1007/s00439-019-01993-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-019-01993-y