Abstract
Duchenne and Becker dystrophinopathies (DMD and BMD) are X-linked recessive disorders caused by mutations in the dystrophin gene that lead to absent or reduced expression of dystrophin in both skeletal and heart muscles. DMD/BMD female carriers are usually asymptomatic, although about 8 % may exhibit muscle or cardiac symptoms. Several mechanisms leading to a reduced dystrophin have been hypothesized to explain the clinical manifestations and, in particular, the role of the skewed XCI is questioned. In this review, the mechanism of XCI and its involvement in the phenotype of BMD/DMD carriers with both a normal karyotype or with X;autosome translocations with breakpoints at Xp21 (locus of the DMD gene) will be analyzed. We have previously observed that DMD carriers with moderate/severe muscle involvement, exhibit a moderate or extremely skewed XCI, in particular if presenting with an early onset of symptoms, while DMD carriers with mild muscle involvement present a random XCI. Moreover, we found that among 87.1 % of the carriers with X;autosome translocations involving the locus Xp21 who developed signs and symptoms of dystrophinopathy such as proximal muscle weakness, difficulty to run, jump and climb stairs, 95.2 % had a skewed XCI pattern in lymphocytes. These data support the hypothesis that skewed XCI is involved in the onset of phenotype in DMD carriers, the X chromosome carrying the normal DMD gene being preferentially inactivated and leading to a moderate–severe muscle involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duchenne and Becker dystrophinopathies (DMD, OMIM #310200 and Becker, BMD, OMIM #300376) are X-linked recessive disorders that affect, respectively, 1 in 3500 or 1:18,500 male births (Emery 1991). Both diseases are caused by mutations in the dystrophin (DMD) gene located on Xp21.2. Dystrophin plays an important role in the muscle structure, as it links the cytoskeleton to the extracellular matrix. In particular, the amino terminus of the dystrophin binds to F-actin in the cytoskeleton, while the carboxyl terminus binds to the dystrophin-associated protein complex (dystroglycans, sarcoglycans, integrins and caveolin) in the extracellular matrix (Nowak and Davies 2004). Dystrophin is expressed in striated skeletal and smooth muscles, heart and in the brain. Deletions and duplications are the most frequent mutations in the DMD gene and account for 70–75 % and 5–10 % of cases, respectively (Emery et al. 1991); the remaining cases are determined by single point mutations or small rearrangements (Pikó et al. 2009). According to the Haldane theory, one-third of cases are de novo mutations (Davie and Emery 1978). Mutations not leading to the production of dystrophin determine Duchenne muscular dystrophy phenotype (DMD; OMIM 300377), while mutations leading to a reduced amount or an altered size of the dystrophin protein cause Becker muscular dystrophy (BMD; OMIM 300376). In both cases, a destabilization of the sarcolemma is determined due to reduction of the dystrophin-associated protein complex (Matsumura et al. 1993; Nigro et al. 1995). In DMD patients, the lack of dystrophin causes a progressive proximal muscle weakness associated with respiratory involvement and/or cardiomyopathy that represent the most common causes of death. In BMD patients, the maintenance of some amount of dystrophin causes a less severe muscle weakness and prolonged functional abilities, but cardiomyopathy is more frequent and represents the most common cause of death.
DMD/BMD female carriers are usually asymptomatic, although several studies report that about 2.5–7.8 % may manifest skeletal muscle symptoms. The age of onset of muscle symptoms is variable; however, an onset before 15 years usually leads to severe clinical manifestations, similar to those observed in males (Moser and Emery 1974; Nigro et al. 1995; Politano et al. 1996; Palmucci et al. 1999; Romero et al. 2001; Ceulemans et al. 2008). Moreover, BMD/DMD carriers may show myocardial involvement (18 %; 16 % in DMD and 7 % in BMD) and in particular dilated cardiomyopathy, characterized by significant left ventricular dilatation and decreased shortening fraction (7–8 % in DMD or 6.6 % in BMD carriers, (Politano et al. 1996; Hoogerwaard et al. 1999; Grain et al. 2001).
Myocardial involvement such as hypertrophy, arrhythmias and left ventricular dilatation increases with age and has the same occurrence in both DMD and BMD carriers, with a prevalence of dilated cardiomyopathy after the age of 50. (Hoogerwaard et al. 1999). DMD/BMD carriers can develop isolated muscle or cardiac involvement (Comi et al. 1992; Nigro et al. 1995; Politano et al. 1996; Nigro et al. 2004), or both. However, cardiac symptoms develop usually later than muscle symptoms (Politano et al. 1986). Several mechanisms have been hypothesized to explain the clinical manifestations in BMD/DMD carriers, such as: (1) DMD gene mutation on both Xp21 alleles (Fujii et al. 2009; Soltanzadeh et al. 2010); (2) loss of one X chromosome (Turner syndrome) (Chelly et al. 1986; Satre et al. 2004); (3) polymorphisms in other non-DMD genes, such as osteopontin, recently associated with an early loss of ambulation in DMD males (Kyriakides et al. 2011); (4) skewed X-chromosome inactivation (XCI), with preferential inactivation of the normal dystrophin allele (Azofeifa et al. 1995; Yoshioka et al. 1998; Soltanzadeh et al. 2010; Seemann et al. 2011; Viggiano et al. 2013a); (5) women carrying X;autosome translocations involving the DMD gene (Boyd et al. 1986; Viggiano et al. 2013b); (6) uniparental disomy (Quan et al. 1997). All these mechanisms determine as final effect a reduction of dystrophin expression in the skeletal and cardiac muscles. In the present review, we will analyze the mechanism of XCI and its involvement in determining the onset of symptoms in DMD carriers.
X-chromosome inactivation
XCI is an epigenetic mechanism that equalizes X-linked gene dosage between men and women through the inactivation of one X chromosome in women. At the end of the process, women are a mosaic of two cell types expressing the maternal or the paternal X chromosome.
A random XCI indicates that cells present an equal (50:50) inactivation of the maternal or the paternal X chromosome. On the other hand, a skewed XCI indicates an unequal (>50 %) inactivation of the maternal or the paternal X chromosome.
However, there is no agreement on the cutoff for XCI to be considered skewed, as some authors consider 65:35 (Orstavik et al. 1996; Satoh et al. 2008), while others 70:30 (Matthews et al. 1995; Amos-Landgraf et al. 2006) or 80:20 (Orstavik 2009). Usually, the term extremely skewed XCI indicates the preferential inactivation of one X chromosome in 90–95 % of cells (Orstavik 2009).
Mechanism of XCI
The mechanism of XCI is not completely known. Several studies in mice demonstrated that the X-chromosome inactivation center (Xic)—a single cis-acting control locus at Xq13.2—is necessary for: (1) initiation of XCI by counting the number of X chromosomes and choosing one X chromosome to be inactivated; (2) spreading the inactivation in cis the X chromosome; and (3) maintaining the inactive state (Gartler et al. 1992). The non-coding X inactive-specific transcript (Xist)—a non-coding RNA (ncRNA)—is transcribed by the Xic (Kind and van Steensel 2010). Xist has different functions: (1) gene silencing in cis with Xic (Brown et al. 1991; Brockdorff et al. 1992; Brown et al. 1992); (2) spatial reorganization of X chromosome inactivated (Xi) (Splinter et al. 2011; Nora et al. 2012); (3) initiation of chromatin changes (Csankovszki et al. 2001; Plath et al. 2003). The mechanism induced by Xist to silence genes is not clear. It is suggested that the upregulation of Xist in the Xi, forms a sub-nuclear compartment or domain where the RNA polymerase II and transcription factors are sequestered, so that the genes in this region are not transcribed (Okamoto et al. 2004; Chaumeil et al. 2006; Dixon et al. 2012). This domain presents dense regions of interspersed nuclear elements (LINE) suggested to be involved in the silencing and/or silencing maintenance (Chaumeil et al. 2006). A recent work on mouse embryonic fibroblasts, embryoid bodies and trophoblast stem cells shows that genes and LINES-dense regions are present in separate nuclear territories, in particular adjacent to the Xist gene, suggesting a role of the LINE dense regions in Xist mediating silencing, but not a colocalization between genes inactivated and LINES (Calabrese et al. 2012). The spatial reorganization of Xi consists in modifications of the interaction between genes in cis and/or in trans with genes on autosome chromosomes. In particular, the active X chromosome (Xa) presents more gene interactions both in cis and in trans, whereas Xist upregulation determines in the Xi few gene interactions in cis but not in trans. Only genes that escape the inactivation show interaction in cis or in trans with other active genes (Splinter et al. 2011; Nora et al. 2012). Moreover, Xic presents several regulatory elements of Xist, in particular down-regulator elements (Tsix, Linx, Dxpas34 and Xite, Oct4, Sox2, Nanog and Rex1) and up-regulator elements (Ftx, Jpx, Rnf12) (Debrand et al. 1999; Lee 2005; Cohen et al. 2007; Barakat et al. 2011; Chureau et al. 2011; Masui et al. 2011; Gontan et al. 2012). About 15 % of human X-linked genes escape the silencing, and an additional 10 % of genes present a variable pattern of inactivation (Carrel and Willard 2005). Some of these genes are pseudo-autosomal regions (PARs), homologous sequences of nucleotides on the X and Y chromosome (Lyon 1961). In particular, all genes of the PAR1 escape the XCI, while only some genes of PAR2 are silenced (Ciccodicola et al. 2000). Alu repetitive elements and ncRNAs are enriched around genes that escape XCI (Wang et al. 2006a, b; Reinius et al. 2010). Few mechanisms have been suggested to explain skewed XCI and its correlation with age:
-
1.
The stochastic mechanism (Gale et al. 1997), according to which the XCI pattern depends on the pattern of stem cell expressing the maternal or paternal X chromosome at the time of lyonization. With age, there is a stochastic reduction of stem cells as some of them will be lost through the terminal differentiation of both daughter cells.
-
2.
The genetic mechanisms (Vickers et al. 2001; Kristiansen et al. 2005) Previous studies reported cases of familial skewed XCI, leading to severe symptoms (Bicocchi et al. 2005; Renault et al. 2007) or cases of discordant phenotype (Orstavik et al. 1999; Tanner et al. 1999). Furthermore, a mutation in the XIST was reported that causes a familial skewed XCI (Plenge et al. 1997), and linkage analysis studies on XCI phenotype in normal human families found a linkage to the Xq13 trait that contains the XIC locus including the XIST gene. The authors hypothesized two types of XIC alleles—strong and weak—and suggested that “strong” XIC alleles have a higher probability of staying active. In turn, only subjects carrying alleles of different ‘strength’ will present skewed XCI (Naumova et al. 1998). It is also postulated that some polymorphic X-linked genes can influence cell division, growth or apoptosis, and in turn the selection of cell population expressing the maternal or the paternal X chromosome. Furthermore, cells with high turnover, such as hematopoietic or skin cells, seem to have a higher probability of skewed XCI compared with cells with lower mitotic activity (Knudsen et al. 2007).
-
3.
The selection mechanism, according to which skewed XCI derives in elderly women from a growth or survival advantage conferred by one of the parental X chromosomes. In general, selection will favor cells expressing the normal allele (Migeon 2006), thus explaining the limited number of symptomatic carriers of X-linked diseases. However, in some cases, as X;autosome translocations, the cells can also express the mutant allele.
Skewed XCI in normal women
Several studies analyzed the prevalence of skewed XCI in unaffected women in the general population with controversial results, probably due to the different (a) methods of analysis (Gale et al. 1991; Fey et al. 1992), (b) age of women, or (c) type of tissue analyzed. Amos-Landgraf et al. (2006) reported a normal distribution of XCI pattern in the vast majority of the general population of women and an extremely skewed XCI in only 5 % of them, data confirmed by other authors (Migeon 2007; Bolduc et al. 2008). A correlation of skewed XCI with age (Fey et al. 1994) and type of tissue (Azofeifa et al. 1995; Gale et al. 1997) has also been reported. An increase of extremely skewed XCI with age up to 100 years (Lanasa et al. 2001; Kristiansen et al. 2005) has been reported by studies on blood, using the PCR-based androgen receptor gene (AR) analysis. Skewed XCI occurs in about 16–37 % of women >60 years and in 49 % of centenarians, while it occurs in 14 % of women aged ≤25 years and in 4.9–14.2 % of newborns (Kristiansen et al. 2005; Christensen et al. 2000; Bolduc et al. 2008). An extremely skewed XCI occurs in about 16–27 % of women ≥60 years and in 18 % of centenarians (Busque et al. 1996; Christensen et al. 2000; Sharp et al. 2002), while it occurs in 7 % of women ≤25 years and in 0.7–2.7 % of newborns (Busque et al. 1996; Lanasa et al. 2001; Bolduc et al. 2008). However, a higher percentage of skewing (27.9 %) and extreme skewing (4.9 %) XCI has been reported in mothers compared to their newborns, suggesting that hematopoietic cells suffer from age-associated skewing in early adulthood (Bolduc et al. 2008). Moreover, XCI pattern correlates with age-associated skewing occurrence in both hematopoietic and non-hematopoietic cells (e.g., buccal epithelial cells or urine samples) (Knudsen et al. 2007; Bolduc et al. 2008). However, this correlation is lost after the age of 60 years, suggesting that while the occurrence of skewing in hematopoietic cells continues to increase, in non-hematopoietic cells there is a plateau.
Moreover, there is no agreement among researchers on the correlation of the XCI pattern in different tissues. In fact, some authors report a good correlation between blood and epithelial tissue of the same individual (Gale et al. 1991; Sharp et al. 2002; Knudsen et al. 2007; Bolduc et al. 2008), and others between thyroid gland and muscle, or leucocytes and muscle, suggesting that tissues deriving from the same embryogenic layer have the same XCI pattern (Azofeifa et al. 1995). However, Bittel et al. (2008) reported a good correlation also between haematopoietic (blood and/or spleen) tissue and tissues deriving from different embryogenic layers, brain, skin, heart, lungs, muscles, kidneys, and gastrointestinal tract included.
X chromosome inactivation in X;autosome translocations
Studies in animal models demonstrated that the spread of inactivation in X;autosome translocations is discontinuous, so that the autosomal genes in cis to the Xic gene are inactivated less efficiently than the normal X-linked genes (Russell 1963; Cattanach 1974). This suggests that autosomal chromatin lacks important signals in the spread and/or maintenance of X inactivation (Bailey et al. 2000; Ross et al. 2005). The spread of inactivation on the autosome is high near the breakpoint, although some inactivation is also observed distant from it while the spread across the centromere is more limited than in euchromatic regions (Cotton et al. 2014).
It is suggested that the LINEs frequency at the breakpoint may influence the degree of inactivation spread in the autosomal chromosome (Stankiewicz et al. 2006).
Skewed XCI in DMD carriers
Previous studies demonstrated a correlation between skewed XCI and onset of symptoms in carriers of X-linked diseases such as hemophilia B (Espinós et al. 2000; Okumura et al. 2008), dyskeratosis congenita (Devriendt et al. 1997), Wiskott–Aldrich syndrome (Wenger et al. 1992), and focal dermal hypoplasia (Gorski 1991). No agreement exists on DMD muscular dystrophies, as some AA report a good correlation between symptomatic carriers and skewed XCI (Azofeifa et al. 1995; Satoh et al. 2008; Jonàs Juan-Mateu 2012; Sandra Mercier 2013; Viggiano et al. 2013a) while others deny this correlation. (Sumita et al. 1998; Soltanzadeh et al. 2010; Brioschi et al. 2012). Table 1 shows the pattern of XCI in symptomatic DMD carriers. Although the number of subjects for each phenotype (mild, moderate and severe) is limited, skewed XCI seems to better correlate with severe rather then mild phenotype. However, further studies on a larger number of carriers are necessary to confirm this observation. Only one study analyzed XCI analysis in symptomatic carriers at muscular vs cardiac level and showed the highest degree of XCw inactivation in carriers manifesting at the muscular level. Moreover, the AA found a correlation between the degree of XCw inactivation and onset of symptoms, because all subjects presenting a severe muscle phenotype and a higher skewed XCI were younger (<40 years) compared with carriers showing cardiomyopathy (Viggiano et al. 2013a).
Possible explanations for the conflicting data on the correlation between skewed XCI and clinical symptoms in DMD carriers are:
-
1.
To have analyzed as a sole group not homogeneous BMD and DMD carriers, or symptomatic and asymptomatic carriers (Bushby et al. 1993; Sumita et al. 1998).
-
2.
Patient age: to have considered only the younger carriers that may manifest symptoms later and neglected that XCI increases with age (Sharp et al. 2002).
-
3.
The failure in identifying the allele that carries the wild or the mutant DMD gene (Bushby et al. 1993; Matthews et al. 1995; Seemann et al. 2011) and in turn which X chromosome is highly inactivated.
-
4.
The recombination between the androgen receptor locus (used for the STR analysis) and the DMD loci that confuses the linkage analysis for the identification of the mutant allele.
-
5.
The different cutoff value to define a skewed XCI: >65:35 (Yoon et al. 2011), >70:30 (Bushby et al. 1993; Pegoraro et al. 1994; Matthews et al. 1995; Azofeifa et al. 1995), or >80:20 (Soltanzadeh et al. 2010; Seemann et al. 2011; Brioschi et al. 2012; Viggiano et al. 2013a).
-
6.
The presence of alleles not located on the X chromosome that may modify the phenotype (modifiers alleles): in this respect, only one study analyzed the polymorphism in the osteopontin promoter (SPP1) that is involved in a more rapid progression of DMD (Pegoraro et al. 1995; Kyriakides et al. 2011), but both authors did not find any statistically significant difference.
-
7.
The type of mutation: only few studies on XCI analysis in DMD carriers mentioned the type of causative mutation; furthermore, the only study that performed a statistical analysis was unable to show significant results (Soltanzadeh et al. 2010).
-
8.
The type of tissue analyzed: some researchers suggest that the XCI analyzed in blood does not reflect the XCI pattern observed in the muscle, and in turn the XCI performed in the blood does not correlate with the phenotype. However, only few studies analyzed the pattern of XCI in muscle cells, because muscle biopsy is not a diagnostic test for BMD/DMD carriers. Moreover, a concordance of the XCI pattern between lymphocytes and muscles, tissues of same embryonic origin (Fialkow 1973; Azofeifa et al. 1995), has been proven. In addition, symptoms in BMD/DMD carriers seem to correlate better with the XCI pattern in blood than in the muscle cells (Matthews et al. 1995; Pegoraro et al. 1995; Brioschi et al. 2012). Finally, the XCI analyzed in cells from a single muscle does not reflect the XCI pattern of the total muscles, due to the mosaic pattern observed in carriers. In fact, studies in heterozygous mice for a null mutation of dystrophin gene (mdx/+) demonstrated a variable pattern of dystrophin expression between samples from different skeletal muscles, and between cardiac and skeletal muscles, suggesting a different pattern of XCI (Weller et al. 1991; Bittner et al. 1997). This result could rely on the mechanism of (a) biochemical and (b) genetic normalization. The first consists in the production of dystrophin in the area near a dystrophin-negative membrane, by nearby dystrophin-positive myonuclei (Watkins et al. 1989; Karpati et al. 1990); the second consists in the regeneration of myofibers by the activation of satellite dystrophin-positive cells (Moss and Leblond 1971; Segalés et al. 2014).
We hypothesize that mild symptomatic carriers present both biochemical and genetic normalization, whereas carriers with moderate–severe muscle phenotype present only genetic normalization that leads to failure of dystrophin production in muscles, as previously reported (Pegoraro et al. 1995). This is also supported by the evidence that muscle normalization increases with age (Karpati et al. 1990; Bittner et al. 1997), and that carriers with random XCI usually do not show symptoms worsening with age or onset of a severe phenotype in old ages.
-
9.
Finally, it is impossible to exclude that carriers with extremely XCI and a severe phenotype present a X;autosome chromosome translocation, because the karyotypes were not analyzed in all studies. In some papers, in fact, DMD carriers exhibit muscle involvement in addition to other symptoms (e.g., speech delay, behavioral anomalies, mental retardation, aggressiveness) (Pegoraro et al. 1994; Jonàs Juan-Mateu 2012) not common in DMD, suggesting that other genes or chromosome abnormalities could be involved.
Skewed XCI in women with balanced Xp21;autosome translocations
X;autosome translocations occur in about 1/30,000 live births and may show different breakpoints on the X chromosome (Gupta et al. 2006). The estimated risk for developing a clinical phenotype in cases with X;autosome translocations is approximately 6 % (Genesio et al. 2011).
In balanced X;autosome translocations, the normal X chromosome is usually, though not always, inactivated to prevent deleterious monosomy of autosomal genes (Mattei et al. 1981, 1982; Sharp et al. 2002). In unbalanced X;autosome translocations, there is a preferential inactivation of the derivative X chromosome that causes the lack of expression of the extra-autosomal genes and prevents the occurrence of deleterious trisomies of autosomal genes (Hall et al. 2002; Gupta et al. 2006). However, several cases of a preferential inactivation of the normal X have been reported in unbalanced X;autosome translocations, probably to confer a survival advantage to the fetus (Palmer et al. 1980; Gupta et al. 2006). In the balanced X;autosome translocations disrupting the DMD gene, this is usually split into two parts and joined with a segment of the autosome; as a consequence, no functional dystrophin can be produced by the derivative X chromosome. Previous studies suggested that the part of the X chromosome on the autosome, separated from the Xic, will not be silenced. In addition, the inactivation of the derivative X chromosome will spread into the translocated autosomal sequences resulting in a silencing of the autosomal genes. The derivative monosomy of the autosome genes determines the apoptosis of the cells with X-derivative inactivated in early embryonic stages, resulting in the selection of the cells: only cells with the normal X chromosome inactivated survive.
In Table 2, we report 43 cases with X;autosome translocation involving the locus Xp21 so far published. All cases reported are de novo translocations. Thirty-four of them (79 %) showed a mild or moderate–severe DMD phenotype, while 5 (11 %) did not show symptoms and/or signs of muscle involvement. However, the age at reporting was <5 years in four of these cases. In five cases in which chromosomes 2, 3, 5 or 9 were involved in translocations, neurological symptoms, such as mental retardation and epilepsy, were also associated.
In the only one fetus with 46,X,t(X;l)(p21.2;p36) in which muscle biopsy was performed in utero, normal dystrophin immunostaining was found (Evans et al. 1993). The analysis of the autosomes involved in translocations shows that the highest percentage involved the chromosome 9 (16 %), followed by chromosome 4 and 22 (11.3 %), chromosomes 1, 2, 3 (9 %) and chromosomes 5, 6, 11 (6.8 %). Chromosomes 7, 8, 12, 15, 17 and 21 were involved in only one case, respectively, and never chromosomes 10, 13, 14, 16, 18 and 20.
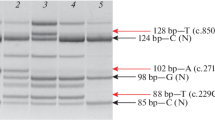
The analysis of XCI, performed on 20/34 symptomatic patients, on lymphocytes, showed a skewed XCI pattern in all cases. In particular, the XCI was extremely skewed in 15/20, near to extremely skewed (93:7) in 2 subjects and skewed in 3. XCI was analyzed in both muscle and lymphocytes in three symptomatic DMD carriers, and only in one the pattern was the same in the two tissues. In two out of five asymptomatic cases of X(p21);autosome translocations, in which the XCI was performed, this was skewed and extremely skewed, respectively. However, it is impossible to exclude that these subjects will present muscle symptoms later, because of their young age (under 4 years) at the time of investigation. A summary of the phenotype—DMD, Mild DMD, Absent, or Not reported—associated with a skewed or extremely skewed XCI, is shown in Table 3.
Discussion
The present review shows that skewed XCI is the main factor determining the appearance of symptoms in DMD carriers or in women with X;autosome translocations involving the locus Xp21, and in particular in women with an early onset of symptoms.
The data reported support the hypothesis that a random XCI can cause only mild symptoms (Pegoraro et al. 1995), while a skewed XCI plays an important role in the moderate–severe phenotypes. In the case of skewed XCI, the preferential inactivation of the X chromosome carrying the normal allele determines in muscles an aberrant dystrophin expression—likely, near to zero—avoiding any biochemical and/or genetic normalization and favoring the onset of a DMD-like phenotype. The mechanism that leads to a skewed XCI, including the cases of X;autosome translocations, is not completely clear. Several mechanisms have been suggested, including stochastic factors (Gale et al. 1997; Brown and Robinson 2000), genetic mechanisms (Vickers et al. 2001; Kristiansen et al. 2005) or developmental post-inactivation selection, particularly in cells with a high turnover (Knudsen et al. 2007). Though familial cases of skewed XCI presenting mutations in the XIST gene promoter have been reported (Plenge et al. 1997; Tomkins et al. 2002), however, the analysis of XIST promoter in DMD symptomatic carriers did not show any variant in this region (Jonàs Juan-Mateu 2012). Moreover, no correlation between the XCI pattern and mother–daughter pairs was found, suggesting that the pattern of XCI is not inherited (Abrams and Cotter 2004; Viggiano et al. 2013a, b). However, it is not possible to exclude a genetic control, because the paternal inheritance was not investigated. In cases of X;autosome translocations, the functional disomy for the segment of X chromosome translocated or the monosomy for the autosomal translocated segment play a crucial role; in fact only cells expressing the derivative X chromosome can survive (Schmidt and Du Sart 1992; Waters et al. 2001).
A limitation of this review is that the groups of DMD and BMD carriers in which the correlation between XCI and phenotype was analyzed in the different studies were not homogeneous. In fact, only few analyzed DMD versus BMD carriers, or symptomatic versus asymptomatic carriers, or identified correctly the mutant versus the wild allele and reported exactly the clinical symptoms. Moreover, the cases of X;autosome translocations so far published are usually symptomatic, and XCI analysis was performed only in 20 subjects, thus preventing to perform a statistical analysis to validate the data. However, the hypothesis that X;autosome translocation involving the Xp21 determines a skewed XCI associated with the clinical phenotype is supported by previous studies demonstrating that the normal X chromosome is preferentially inactivated in balanced X;autosome translocations to confer survival to the fetus (Emery 1991; Gupta et al. 2006; Giliberto et al. 2014). Our experience in the field of muscular dystrophies emphasizes the problem of a correct communication on the risk of a possible DMD phenotype in women prenatally diagnosed as DMD carriers or X;autosome translocated, with breakpoint at the Xp21 locus. In fact, such a possibility is usually underestimated in prenatal genetic counseling and families are not informed about this risk. In our opinion, information should be given, even in cases in which the mothers are asymptomatic carriers. However, further studies on a larger group of subjects are necessary to confirm this hypothesis.
In everyday clinical practice, XCI analysis should be recommended a) in the case of adult women with reporting muscle symptoms to exclude a skewed XCI and b) in young girls (<18 years of age)—symptomatic at the muscle level and related to DMD patients—to evaluate skewed XCI as the potential cause of their symptoms, without the use of genetic testing for carrier status.
References
Abbadi N, Philippe C, Chery M et al (1994) Additional case of female monozygotic twins discordant for the clinical manifestations of Duchenne muscular dystrophy due to opposite X-chromosome inactivation. Am J Med Genet 52:198–206. doi:10.1002/ajmg.1320520215
Abrams L, Cotter PD (2004) Prenatal diagnosis of de novo X;autosome translocations. Clin Genet 65:423–428. doi:10.1111/j.0009-9163.2004.00255.x
Allen RC (1993) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239
Amos-Landgraf JM, Cottle A, Plenge RM et al (2006) X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet 79:493–499
Azofeifa J, Voit T, Hübner C, Cremer M (1995) X-chromosome methylation in manifesting and healthy carriers of dystrophinopathies: concordance of activation ratios among first degree female relatives and skewed inactivation as cause of the affected phenotypes. Hum Genet 96:167–176
Bailey JA, Carrel L, Chakravarti A, Eichler EE (2000) Molecular evidence for a relationship between LINE-1 elements and X chromosome inactivation: the Lyon repeat hypothesis. Proc Natl Acad Sci USA 97:6634–6639
Barakat TS, Gunhanlar N, Pardo CG et al (2011) RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet 7:e1002001. doi:10.1371/journal.pgen.1002001
Bicocchi MP, Migeon BR, Pasino M, Lanza T, Bottini F, Boeri E et al (2005) Familial nonrandom inactivation linked to the X inactivation centre in heterozygotes manifesting haemophilia A. Eur J Hum Genet 13:635–640
Bittel DC, Theodoro MF, Kibiryeva N, Fischer W, Talebizadeh Z, Butler MG (2008) Comparison of X-chromosome inactivation patterns in multiple tissues from human females. J Med Genet 45:309–313
Bittner RE, Popoff I, Shorny S et al (1997) Dystrophin expression in heterozygous mdx/+ mice indicates imprinting of X chromosome inactivation by parent-of-origin-, tissue-, strain- and position-dependent factors. Anat Embryol 195:175–182
Bjerglund Nielsen L, Jacobsen BB, Nielsen IM, Tabor A (1983) X;autosome translocation in a girl with muscular dystrophy. Clin Genet 23:242
Bodrug SE, Roberson JR, Weiss L et al (1990) Prenatal identification of a girl with a t(X;4)(p21;q35) translocation: molecular characterisation, paternal origin, and association with muscular dystrophy. J Med Genet 27:426–432
Bolduc V, Chagnon P, Provost S, Dubé M-P, Belisle C, Gingras M et al (2008) No evidence that skewing of X chromosome inactivation patterns is transmitted to offspring in humans J Clin Invest 118:333–341
Boyd Y, Buckle V, Holt S et al (1986) Muscular dystrophy in girls with X;autosome translocations. J Med Genet 23:484–490
Brioschi S, Gualandi F, Scotton C et al (2012) Genetic characterization in symptomatic female DMD carriers: lack of relationship between X-inactivation, transcriptional DMD allele balancing and phenotype. BMC Med Genet 13:73. doi:10.1186/1471-2350-13-73
Brockdorff N, Ashworth A, Kay GF et al (1992) The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71:515–526
Brown CJ, Robinson WP (2000) The causes and consequences of random and non-random X chromosome inactivation in humans. Clin Genet 58:353–363
Brown CJ, Ballabio A, Rupert JL et al (1991) A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349:38–44. doi:10.1038/349038a0
Brown CJ, Hendrich BD, Rupert JL et al (1992) The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71:527–542
Bushby KM, Goodship JA, Nicholson LV, Johnson MA, Haggerty ID, Gardner-Medwin D, Bushby KM, Goodship JA (1993) Variability in clinical, genetic and protein abnormalities in manifesting carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord NMD 3:57–64. doi:10.1016/0960-8966(93)90042-I
Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y et al (1996) Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 88:59–65
Calabrese JM, Sun W, Song L et al (2012) Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell 151:951–963. doi:10.1016/j.cell.2012.10.037
Canki N, Dutrillaux B, Tivadar I (1979) Dystrophie musculaire de Duchenne chez une petite fille porteuse d’une translocation t(X;3)(p21;ql3) de novo. Annales de génétique 22:35–39
Carrel L, Willard HF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434:400–404. doi:10.1038/nature03479
Cattanach BM (1974) Position effect variegation in the mouse. Genet Res 23:291–306
Ceulemans BP, Storm K, Reyniers E Jr et al (2008) Muscle pain as the only presenting symptom in a girl with dystrophinopathy. Pediatr Neurol 38:64–66. doi:10.1016/j.pediatrneurol.2007.09.006
Chaumeil J, Le Baccon P, Wutz A, Heard E (2006) A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev 20:2223–2237. doi:10.1101/gad.380906
Chelly J, Marlhens F, Marec BL et al (1986) De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Hum Genet 74:193–196. doi:10.1007/BF00282093
Christensen K, Kristiansen M, Hagen-Larsen H, Skytthe A, Bathum L, Jeune B et al (2000) X-linked genetic factors regulate hematopoietic stem-cell kinetics in females. Blood 95:2449–2451
Chureau C, Chantalat S, Romito A et al (2011) Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet 20:705–718. doi:10.1093/hmg/ddq516
Ciccodicola A, D’Esposito M, Esposito T et al (2000) Differentially regulated and evolved genes in the fully sequenced Xq/Yq pseudoautosomal region. Hum Mol Genet 9:395–401
Cohen DE, Davidow LS, Erwin JA et al (2007) The DXPas34 repeat regulates random and imprinted X inactivation. Dev Cell 12:57–71. doi:10.1016/j.devcel.2006.11.014
Comi LI, Nigro G, Politano L, Petretta VR (1992) The cardiomyopathy of Duchenne/Becker consultants. Int J Cardiol 34:297–305
Cotton AM, Chen C-Y, Lam LL et al (2014) Spread of X-chromosome inactivation into autosomal sequences: role for DNA elements, chromatin features and chromosomal domains. Hum Mol Genet 23:1211–1223. doi:10.1093/hmg/ddt513
Csankovszki G, Nagy A, Jaenisch R (2001) Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J Cell Biol 153:773–784
Davie AM, Emery AE (1978) Estimation of proportion of new mutants among cases of Duchenne muscular dystrophy. J Med Genet 15:339–345
Debrand E, Chureau C, Arnaud D et al (1999) Functional analysis of the DXPas34 locus, a 3′ regulator of Xist expression. Mol Cell Biol 19:8513–8525
Devriendt K, Matthijs G, Legius E et al (1997) Skewed X-chromosome inactivation in female carriers of dyskeratosis congenita. Am J Hum Genet 60:581–587
Dixon JR, Selvaraj S, Yue F et al (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485:376–380. doi:10.1038/nature11082
Doriguzzi C, Palmucci L, Mongini T et al (1999) Variable histological expression of dystrophinopathy in two females. Acta Neuropathol 97:657–660
Dubowitz V (1986) X;autosome translocations in females with Duchenne or Becker muscular dystrophy. Nature 322:291–292. doi:10.1038/322291b0
Dubowitz V (1995) Muscle disorders in childhood. Saunders, London
Emanuel BS, Zackai EH, Tucker SH (1983) Further evidence for Xp21 location of Duchenne muscular dystrophy (DMD) locus: X;9 translocation in a female with DMD. J Med Genet 20:461–463
Emery AE (1991) Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1:19–29
Emery AE, Skinner R, Holloway S (1979) A study of possible heterogeneity in Duchenne muscular dystrophy. Clin Genet 15:444–449
Espinós C, Lorenzo JI, Casaña P et al (2000) Haemophilia B in a female caused by skewed inactivation of the normal X-chromosome. Haematologica 85:1092–1095
Evans MI, Farrell SA, Greb A et al (1993) In utero fetal muscle biopsy for the diagnosis of Duchenne muscular dystrophy in a female fetus “suddenly at risk”. Am J Med Genet 46:309–312. doi:10.1002/ajmg.1320460314
Fey MF, Peter HJ, Hinds HL, Zimmermann A, Liechti-Gallati S, Gerber H et al (1992) Clonal analysis of human tumors with M27 beta, a highly informative polymorphic X chromosomal probe. J Clin Invest 89:1438–1444
Fey MF, Liechti-Gallati S, von Rohr A, Borisch B, Theilkäs L, Schneider V et al (1994) Clonality and X-inactivation patterns in hematopoietic cell populations detected by the highly informative M27 beta DNA probe. Blood 83:931–938
Fialkow PJ (1973) Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet 37:39–48
Fidzianska A, Morrone A, Pegoraro E et al (1995) An X:autosome translocation stabilizes truncated dystrophin: implications for lack of truncated dystrophins in Duchenne muscular dystrophy. Neuropediatrics 26:163–167. doi:10.1055/s-2007-979747
Fujii K, Minami N, Hayashi Y et al (2009) Homozygous female Becker muscular dystrophy. Am J Med Genet 149A:1052–1055. doi:10.1002/ajmg.a.32808
Gaál M, László J (1977) X inactivation pattern in an unbalanced X-autosome translocation with gonadal dysgenesis. Hum Hered 27:396–402
Gale RE, Wheadon H, Linch DC (1991) X-chromosome inactivation patterns using HPRT and PGK polymorphisms in haematologically normal and post-chemotherapy females. Br J Haematol 79:193–197
Gale RE, Fielding AK, Harrison CN, Linch DC (1997) Acquired skewing of X-chromosome inactivation patterns in myeloid cells of the elderly suggests stochastic clonal loss with age. Br J Haematol 98:512–519
Gartler SM, Dyer KA, Goldman MA (1992) Mammalian X chromosome inactivation. Mol Genet Med 2:121–160
Genesio R, Melis D, Gatto S et al (2011) Variegated silencing through epigenetic modifications of a large Xq region in a case of balanced X;2 translocation with Incontinentia Pigmenti-like phenotype. Epigenetics 6:1242–1247. doi:10.4161/epi.6.10.17698
Giacalone JP, Francke U (1992) Common sequence motifs at the rearrangement sites of a constitutional X/autosome translocation and associated deletion. Am J Hum Genet 50:725–741
Giliberto F, Radic CP, Luce L et al (2014) Symptomatic female carriers of Duchenne muscular dystrophy (DMD): genetic and clinical characterization. J Neurol Sci 336(1–2):36–41. doi:10.1016/j.jns.2013.09.036
Gontan C, Achame EM, Demmers J et al (2012) RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature 485:386–390. doi:10.1038/nature11070
Gorski JL (1991) Father-to-daughter transmission of focal dermal hypoplasia associated with nonrandom X-inactivation: support for X-linked inheritance and paternal X chromosome mosaicism. Am J Med Genet 40:332–337. doi:10.1002/ajmg.1320400317
Grain L, Cortina-Borja M, Forfar C et al (2001) Cardiac abnormalities and skeletal muscle weakness in carriers of Duchenne and Becker muscular dystrophies and controls. Neuromuscul Disord 11:186–191
Greenstein RM, Reardon MP, Chan TS et al (1980) An (X;11) translocation in a girl with Duchenne muscular dystrophy. Repository identification No. GM1695. Cytogenet Cell Genet 27:268
Grzeschik K-H, Kim MA, Johannsmann R (1975) Late replicating bands of human chromosomes demonstrated by fluorochrome and Giemsa staining. Hum Genet 29:41–59. doi:10.1007/BF00273350
Gupta N, Goel H, Phadke SR (2006) Unbalanced X; autosome translocation. Indian J Pediatr 73:840–842
Hall LL, Clemson CM, Byron M et al (2002) Unbalanced X;autosome translocations provide evidence for sequence specificity in the association of XIST RNA with chromatin. Hum Mol Genet 11:3157–3165. doi:10.1093/hmg/11.25.3157
Hoffman EP, Pegoraro E, Scacheri P et al (1996) Genetic counseling of isolated carriers of Duchenne muscular dystrophy. Am J Med Genet 63:573–580. doi:10.1002/(SICI)1096-8628(19960628)63:4<573:AID-AJMG11>3.0.CO;2-F
Holden JJ, Smith A, MacLeod PM et al (1986) Xp21/autosome translocations. Case report and risk for Duchenne muscular dystrophy. Clin Genet 29:516–522
Hoogerwaard EM, van der Wouw PA, Wilde AA et al (1999) Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord 9:347–351
Jacobs PA, Hunt PA, Mayer M, Bart RD (1981) Duchenne muscular dystrophy (DMD) in a female with an X/autosome translocation: further evidence that the DMD locus is at Xp21. Am J Hum Genet 33:513–518
Jonàs Juan-Mateu MJR (2012) Prognostic value of X-chromosome inactivation in symptomatic female carriers of dystrophinopathy. Orphanet J Rare Dis 7:82. doi:10.1186/1750-1172-7-82
Kalz-Füller B (1999) Characterisation, phenotypic manifestations and X-inactivation pattern in 14 patients with X-autosome translocations. Clin Genet 55:363–367
Karpati G, Zubrzycka-Gaarn EE, Carpenter S et al (1990) Age-related conversion of dystrophin-negative to -positive fiber segments of skeletal but not cardiac muscle fibers in heterozygote mdx mice. J Neuropathol Exp Neurol 49:96–105
Kimura S, Mitsuda T, Misugi N et al (1986) Clinical features in a girl with Duchenne muscular dystrophy with an X-autosome translocation; (X;4)(p21;q26). Brain Dev 8:619–623
Kind J, van Steensel B (2010) Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 22:320–325. doi:10.1016/j.ceb.2010.04.002
Knudsen GPS, Pedersen J, Klingenberg O, Lygren I, Ørstavik KH (2007) Increased skewing of X chromosome inactivation with age in both blood and buccal cells. Cytogenet Genome Res 116:24–28
Kristiansen M, Knudsen GPS, Bathum L, Naumova AK, Sørensen TIA, Brix TH et al (2005) Twin study of genetic and aging effects on X chromosome inactivation. Eur J Hum Genet 13:599–606
Kyriakides T, Pegoraro E, Hoffman EP et al (2011) SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy: predicting the severity of Duchenne muscular dystrophy: implications for treatment. Neurology. 77:1858. doi:10.1212/WNL.0b013e318239b9ae (author reply 1858–9)
Lanasa MC, Hogge WA, Kubik CJ, Ness RB, Harger J, Nagel T et al (2001) A novel X chromosome-linked genetic cause of recurrent spontaneous abortion. Am J Obstet Gynecol 185:563–568
Laurent C, Biemont MC, Dutrillaux B (1975) Four new cases of X-autosome translocation in man (author’s transl). Humangenetik 26:35–46
Lee JT (2005) Regulation of X-chromosome counting by Tsix and Xite sequences. Science 309:768–771. doi:10.1126/science.1113673
Lindenbaum RH, Clarke G, Patel C et al (1979) Muscular dystrophy in an X; 1 translocation female suggests that Duchenne locus is on X chromosome short arm. J Med Genet 16:389–392
Lupski JR, Garcia CA, Zoghbi HY et al (1991) Discordance of muscular dystrophy in monozygotic female twins: evidence supporting asymmetric splitting of the inner cell mass in a manifesting carrier of Duchenne dystrophy. Am J Med Genet 40:354–364. doi:10.1002/ajmg.1320400323
Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190:372–373
MacLeod PM, Holden J, Masotti R (1983) Duchenne muscular dystrophy in a female with an X-autosome translocation. Int Congr Genetics, New Delhi
Masui O, Bonnet I, Le Baccon P et al (2011) Live-cell chromosome dynamics and outcome of X chromosome pairing events during ES cell differentiation. Cell 145:447–458. doi:10.1016/j.cell.2011.03.032
Matsumura K, Ohlendieck K, Ionasescu VV et al (1993) The role of the dystrophin–glycoprotein complex in the molecular pathogenesis of muscular dystrophies. Neuromuscul Disord 3:533–535
Mattei MG, Mattei JF, Vidal I, Girauld F (1981) Structural anomalies of the X chromosome and inactivation center. Hum Genet 56:401–408
Mattei MG, Mattei JF, Ayme S, Giraud F (1982) X-autosome translocations: cytogenetic characteristics and their consequences. Hum Genet 61:295–309
Matthews PM, Benjamin D, Van Bakel I et al (1995) Muscle X-inactivation patterns and dystrophin expression in Duchenne muscular dystrophy carriers. Neuromuscul Disord 5:209–220. doi:10.1016/0960-8966(94)00057-G
Meitinger T, Boyd Y, Anand R, Craig IW (1988) Mapping of Xp21 translocation breakpoints in and around the DMD gene by pulsed field gel electrophoresis. Genomics 3:315–322
Migeon BR (2006) The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA 295:1428–1433
Migeon BR (2007) Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med 4:97–105
Moser H, Emery AE (1974) The manifesting carrier in Duchenne muscular dystrophy. Clin Genet 5:271–284
Moss FP, Leblond CP (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170:421–435. doi:10.1002/ar.1091700405
Narazaki O, Hanai T, Ueki Y, Mitsudome A (1985) Duchenne muscular dystrophy in a female with an X-autosome translocation. Rinsho Shinkeigaku 25:432–436
Naumova AK, Olien L, Bird LM, Smith M, Verner AE, Leppert M, Morgan K, Sapienza C (1998) Genetic mapping of X-linked loci involved in skewing of X chromosome inactivation in the human. Eur J Hum Genet 6:552–562
Nevin NC, Hughes AE, Calwell M, Lim JH (1986) Duchenne muscular dystrophy in a female with a translocation involving Xp21. J Med Genet 23:171–173
Nigro G, Di Somma S, Comi LI et al (1995) Structural basis of cardiomyopathy in Duchenne/Becker carriers. Endomyocardial biopsy evaluation. Ann NY Acad Sci 752:108–110
Nigro G, Comi L, Politano L, Nigro Ge (2004) Cardiomyopathies associated with muscular dystrophies. In: Engel AG, Franzini-Armstrong C (eds) Myology, 3rd edn. McGraw-Hill, New York, pp 1239–1256
Nora EP, Lajoie BR, Schulz EG et al (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485:381–385. doi:10.1038/nature11049
Nowak KJ, Davies KE (2004) Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep 5:872–876. doi:10.1038/sj.embor.7400221
Obersztyn E, Szczaluba K, Smyk M et al (2008) Duchenne muscular dystrophy (DMD) in a girl with balanced translocation (X;3)(p21.1;p13) and psychomotor delay. Eur J Paediatr Neurol 12(Supplement 1):S54–S55. doi:10.1016/S1090-3798(08)70182-X
Okamoto I, Otte AP, Allis CD et al (2004) Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303:644–649. doi:10.1126/science.1092727
Okumura K, Fujimori Y, Takagi A et al (2008) Skewed X chromosome inactivation in fraternal female twins results in moderately severe and mild haemophilia B. Haemophilia 14:1088–1093. doi:10.1111/j.1365-2516.2008.01786.x
Orstavik KH (2009) X chromosome inactivation in clinical practice. Hum Genet 126:363–373. doi:10.1007/s00439-009-0670-5
Orstavik KH, Orstavik RE, Halse J, Knudtzon J (1996) X chromosome inactivation pattern in female carriers of X linked hypophosphataemic rickets. J Med Genet 33:700–703
Orstavik KH, Orstavik RE, Schwartz M (1999) Skewed X chromosome inactivation in a female with haemophilia B and in her non-carrier daughter: a genetic influence on X chromosome inactivation? J Med Genet 36:865–866
Palmer CG, Hubbard TW, Henry GW, Weaver DD (1980) Failure of X inactivation in the autosomal segment of an X/A translocation. Am J Hum Genet 32:179–187
Palmucci L, Doriguzzi C, Mongini T et al (1999) Unusual clinical expression of dystrophinopathy in a female, mimicking a congenital myopathy. Eur Neurol 42:221–224
Pegoraro E, Schimke RN, Arahata K et al (1994) Detection of new paternal dystrophin gene mutations in isolated cases of dystrophinopathy in females. Am J Hum Genet 54:989–1003
Pegoraro E, Schimke RN, Garcia C et al (1995) Genetic and biochemical normalization in female carriers of Duchenne muscular dystrophy: evidence for failure of dystrophin production in dystrophin-competent myonuclei. Neurology 45:677–690
Perez Vidal M, SarretGrau E, PratsViñas J et al (1983) Distrofia muscular tipo Duchenne.en una hembra con una translocacion equilibrada X/6. Revista de Neurologia 11:155–158
Pikó H, Vancsó V, Nagy B et al (2009) Dystrophin gene analysis in Hungarian Duchenne/Becker muscular dystrophy families—detection of carrier status in symptomatic and asymptomatic female relatives. Neuromuscul Disord 19:108–112. doi:10.1016/j.nmd.2008.10.011
Plath K, Fang J, Mlynarczyk-Evans SK et al (2003) Role of histone H3 lysine 27 methylation in X inactivation. Science 300:131–135. doi:10.1126/science.1084274
Plenge RM, Hendrich BD, Schwartz C, Arena JF, Naumova A, Sapienza C et al (1997) A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet 17:353–356
Politano L, Comi L, Nigro G (1986) Dystrophic cardiomyopathy in Duchenne definite carriers (electrocardiographic and echocardiographic findings). Cardiomyology 5:139–151
Politano L, Nigro V, Nigro G et al (1996) Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA 275:1335–1338
Quan F, Janas J, Toth-Fejel S, Johnson DB, Wolford JK, Popovich BW (1997) Uniparental disomy of the entire X chromosome in a female with Duchenne muscular dystrophy. Am J Hum Genet 60:160–165
Reinius B, Shi C, Hengshuo L et al (2010) Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genom 11:614. doi:10.1186/1471-2164-11-614
Renault NK, Dyack S, Dobson MJ, Costa T, Lam WL, Greer WL (2007) Heritable skewed X-chromosome inactivation leads to haemophilia A expression in heterozygous females. Eur J Hum Genet 15:628–637
Ribeiro MC, Melaragno MI, Schmidt B et al (1986) Duchenne muscular dystrophy in a girl with an (X;15) translocation. Am J Med Genet 25:231–236. doi:10.1002/ajmg.1320250205
Robinson DO, Boyd Y, Cockburn D et al (1990) The parental origin of de novo X-autosome translocations in females with Duchenne muscular dystrophy revealed by M27 beta methylation analysis. Genet Res 56:135–140
Romero NB, De Lonlay P, Llense S et al (2001) Pseudo-metabolic presentation in a Duchenne muscular dystrophy symptomatic carrier with “de novo” duplication of dystrophin gene. Neuromuscul Disord 11:494–498
Ross MT, Grafham DV, Coffey AJ et al (2005) The DNA sequence of the human X chromosome. Nature 434:325–337. doi:10.1038/nature03440
Russell LB (1963) Mammalian X-chromosome action: inactivation limited in spread and region of origin. Science 140:976–978
Saito F, Tonomura A, Kimura S et al (1985) High-resolution banding study of an X/4 translocation in a female with Duchenne muscular dystrophy. Hum Genet 71:370–371
Sandra Mercier AT (2013) Genetic and clinical specificity of 26 symptomatic carriers for dystrophinopathies at pediatric age. Eur J Hum Genet EJHG 21:892. doi:10.1038/ejhg.2013.74
Satoh M, Ogikubo S, Yoshizawa-Ogasawara A (2008) Correlation between clinical phenotypes and X-inactivation patterns in six female carriers with heterozygote vasopressin type 2 receptor gene mutations. Endocr J 55:277–284
Satre V, Monnier N, Devillard F et al (2004) Prenatal diagnosis of DMD in a female foetus affected by Turner syndrome. Prenat Diagn 24:913–917. doi:10.1002/pd.1031
Schmidt M, Du Sart D (1992) Functional disomies of the X chromosome influence the cell selection and hence the X inactivation pattern in females with balanced X-autosome translocations: a review of 122 cases. Am J Med Genet 42:161–169. doi:10.1002/ajmg.1320420205
Seemann N, Selby K, McAdam L et al (2011) Symptomatic dystrophinopathies in female children. Neuromuscul Disord 21:172–177. doi:10.1016/j.nmd.2010.11.001
Segalés J, Perdiguero E, Muñoz-Cánoves P (2014) Epigenetic control of adult skeletal muscle stem cell functions. FEBS J. doi:10.1111/febs.13065
Sharp AJ, Spotswood HT, Robinson DO et al (2002) Molecular and cytogenetic analysis of the spreading of X inactivation in X;autosome translocations. Hum Mol Genet 11:3145–3156
Soltanzadeh P, Friez MJ, Dunn D et al (2010) Clinical and genetic characterization of manifesting carriers of DMD mutations. Neuromuscul Disord 20:499–504. doi:10.1016/j.nmd.2010.05.010
Splinter E, de Wit E, Nora EP et al (2011) The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA. Genes Dev 25:1371–1383. doi:10.1101/gad.633311
Stankiewicz P, Kuechler A, Eller CD et al (2006) Minimal phenotype in a girl with trisomy 15q due to t(X;15)(q22.3;q11.2) translocation. Am J Med Genet A 140:442–452. doi:10.1002/ajmg.a.31096
Sumita DR, Vainzof M, Campiotto S et al (1998) Absence of correlation between skewed X inactivation in blood and serum creatine-kinase levels in Duchenne/Becker female carriers. Am J Med Genet 80:356–361
Tanner SM, Orstavik KH, Kristiansen M, Lev D, Lerman-Sagie T, Sadeh M et al (1999) Skewed X-inactivation in a manifesting carrier of X-linked myotubular myopathy and in her non-manifesting carrier mother. Hum Genet 104:249–253
Tihy F, Vogt N, Recan D et al (1994) Skewed inactivation of an X chromosome deleted at the dystrophin gene in an asymptomatic mother and her affected daughter. Hum Genet 93:563–567
Tomkins DJ, McDonald HL, Farrell SA, Brown CJ (2002) Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet 10:44–51
Trippe H, Wieczorek S, Kötting J et al (2014) Xp21/A translocation: a rarely considered genetic cause for manifesting carriers of duchenne muscular dystrophy. Neuropediatrics 45:333–335. doi:10.1055/s-0034-1383824
van Bakel I, Holt S, Craig I, Boyd Y (1995) Sequence analysis of the breakpoint regions of an X;5 translocation in a female with Duchenne muscular dystrophy. Am J Hum Genet 57:329–336
Verellen-Dumoulin C, Freund M, Meyer RD et al (1984) Expression of an X-linked muscular dystrophy in a female due to translocation involving Xp21 and non-random inactivation of the normal X chromosome. Hum Genet 67:115–119. doi:10.1007/BF00270570
Vickers MA, McLeod E, Spector TD, Wilson I (2001) Assessment of mechanism of acquired skewed X inactivation by analysis of twins. Blood 97:1274–1281
Viggiano E, Picillo E, Cirillo A, Politano L (2013a) Comparison of X-chromosome inactivation in Duchenne muscle/myocardium-manifesting carriers, non-manifesting carriers and related daughters. Clin Genet 84:265–270. doi:10.1111/cge.12048
Viggiano E, Picillo E, Politano L (2013b) DMD phenotype in girls with a de novo balanced X;3 autosome translocation: a case report. J Genet Syndr Gene Ther 4:132. doi:10.4172/2157-7412.1000132
Wang Z, Willard HF, Mukherjee S, Furey TS (2006a) Evidence of influence of genomic DNA sequence on human X chromosome inactivation. PLoS Comput Biol 2:e113. doi:10.1371/journal.pcbi.0020113
Wang Z, Willard HF, Mukherjee S, Furey TS (2006b) Evidence of influence of genomic DNA sequence on human X chromosome inactivation. PLoS Comput Biol 2:e113. doi:10.1371/journal.pcbi.0020113
Waters JJ, Campbell PL, Crocker AJ, Campbell CM (2001) Phenotypic effects of balanced X-autosome translocations in females: a retrospective survey of 104 cases reported from UK laboratories. Hum Genet 108:318–327
Watkins SC, Hoffman EP, Slayter HS, Kunkel LM (1989) Dystrophin distribution in heterozygote MDX mice. Muscle Nerve 12:861–868. doi:10.1002/mus.880121013
Weller B, Karpati G, Lehnert S et al (1991) Inhibition of myosatellite cell proliferation by gamma irradiation does not prevent the age-related increase of the number of dystrophin-positive fibers in soleus muscles of mdx female heterozygote mice. Am J Pathol 138:1497–1502
Wenger SL, Steele MW, Hoffman EP et al (1992) X inactivation and dystrophin studies in a t(X;12) female: evidence for biochemical normalization in Duchenne muscular dystrophy carriers. Am J Med Genet 43:1012–1015. doi:10.1002/ajmg.1320430619
Willard HF, Latt SA (1976) Analysis of deoxyribonucleic acid replication in human X chromosomes by fluorescence microscopy. Am J Hum Genet 28:213–227
Yoon J, Kim SH, Ki CS et al (2011) Carrier woman of Duchenne muscular dystrophy mimicking inflammatory myositis. J Korean Med Sci 26:587–591. doi:10.3346/jkms.2011.26.4.587
Yoshioka M, Yorifuji T, Mituyoshi I (1998) Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clin Genet 53:102–107
Zatz M, Vianna-Morgante AM, Campos P, Diament AJ (1981) Translocation (X;6) in a female with Duchenne muscular dystrophy: implications for the localisation of the DMD locus. J Med Genet 18:442–447
Acknowledgments
We thank the patients and families for their cooperation. The samples analyzed were derived from the Naples Human Genetic BioBank that is part of Telethon Network of Genetic Biobanks and EuroBioBank. The financial support of Telethon (Project GTB12001H to LP) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viggiano, E., Ergoli, M., Picillo, E. et al. Determining the role of skewed X-chromosome inactivation in developing muscle symptoms in carriers of Duchenne muscular dystrophy. Hum Genet 135, 685–698 (2016). https://doi.org/10.1007/s00439-016-1666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-016-1666-6