Abstract
S-RNase-based self-incompatibility is found in Solanaceae, Rosaceae, and Scrophulariaceae, and is the most widespread mechanism that prevents self-fertilization in plants. Although ‘Shatian’ pummelo (Citrus grandis), a traditional cultivated variety, possesses the self-incompatible trait, the role of S-RNases in the self-incompatibility of ‘Shatian’ pummelo is poorly understood. To identify genes associated with self-incompatibility in citrus, we identified 16 genes encoding homologs of ribonucleases in the genomes of sweet orange (Citrus sinensis) and clementine mandarin (Citrus clementine). We preliminarily distinguished S-RNases from S-like RNases with a phylogenetic analysis that classified these homologs into three groups, which is consistent with the previous reports. Expression analysis provided evidence that CsRNS1 and CsRNS6 are S-like RNase genes. The expression level of CsRNS1 was increased during fruit development. The expression of CsRNS6 was increased during the formation of embryogenic callus. In contrast, we found that CsRNS3 possessed several common characteristics of the pistil determinant of self-incompatibility: it has an alkaline isoelectric point (pI), harbors only one intron, and is specifically expressed in style. We obtained a cDNA encoding CgRNS3 from ‘Shatian’ pummelo and found that it is homolog to CsRNS3 and that CgRNS3 exhibited the same expression pattern as CsRNS3. In an in vitro culture system, the CgRNS3 protein significantly inhibited the growth of self-pollen tubes from ‘Shatian’ pummelo, but after a heat treatment, this protein did not significantly inhibit the elongation of self- or non-self-pollen tubes. In conclusion, an S-RNase gene, CgRNS3, was obtained by searching the genomes of sweet orange and clementine for genes exhibiting sequence similarity to ribonucleases followed by expression analyses. Using this approach, we identified a protein that significantly inhibited the growth of self-pollen tubes, which is the defining property of an S-RNase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-incompatibility is an important mechanism that inhibits fertilization by rejecting self-pollen and accepting non-self-pollen in bisexual flowering plants (De Nettancourt 2001). cDNA clones encoding the S-specific glycoprotein associated with self-incompatibility were first cloned from the gametophytic self-incompatible Nicotiana alata (Anderson et al. 1986). McClure et al. (1989) demonstrated that some of these glycoproteins share sequence similarity with the active sites of RNase T2 and RNase Rh. Thus, these glycoproteins are referred to as S-RNases (McClure et al. 1989). Around the same time, S-RNases were successfully identified in species from Rosaceae, such as Japanese pear (Sassa et al. 1992, 1993), apple (Broothaerts et al. 1995), almond (Tao et al. 1997), and sweet cherry (Tao et al. 1999). Citrus, one of the most important evergreen perennials worldwide, contains both self-incompatible and self-compatible species (Ngo 2001; Masashi et al. 2006). However, the mechanism of self-incompatibility in citrus remains largely unknown relative to species from Rosaceae.
In citrus, the combination of self-incompatibility and parthenocarpy yields seedlessness, a highly favorable commercial trait. Thus, an increasing number of studies have focused on the mechanism of self-incompatibility in citrus. Genes that contribute to self-incompatibility in citrus were identified using comparative transcriptome approaches (Caruso et al. 2012; Miao et al. 2015; Zhang et al. 2015). Genes were previously associated with self-incompatibility in citrus, such as CrWSKP1 and CrS1-3.15 from Citrus reticulate (Miao et al. 2011, 2013; Li et al. 2015), and CgSL2, SKP1-like, and F-box from C. grandis (Chai et al. 2010, 2011a, b). However, these genes are not essential for self-incompatibility, because they are not required for the rejection of S-allele-specific pollen. Many researchers suspect that the mechanism of self-incompatibility is similar in Citrus and Rosaceae in that the female determinant of self-incompatibility is an S-RNase gene (Miao et al. 2011; Chai et al. 2011b). However, no S-RNase genes that inhibit pollen tube growth have been discovered in citrus.
S-RNases belong to the RNase T2 family which catalyzes the cleavage of single-stranded RNA yielding oligonucleotides and mononucleotides. The RNase T2 family is divided into the S-RNases and S-like RNases subfamilies depending on whether the family members mediate the self-incompatibility response (Bariola and Green 1997). S-like RNases share structural features with S-RNases and are distributed throughout the kingdoms of life. Naturally, S-like RNases are associated with a broad range biological function, such as phosphate scavenging, antimicrobial defense, tRNA cleavage, and the enhancement of stress tolerance (Luhtala and Parker 2010). The expression of RNS2 in Arabidopsis is induced under phosphate starvation. In addition, Pi remobilizing and cell senescence affect the expression of the apparent ortholog of RNS2 from Antirrhinum, AhSL28 (Taylor et al. 1993; Liang et al. 2002). The expression of several stress-induced S-like RNases associated with virus infection, drought, or mechanical wounding is induced to remarkably high levels in response to stress (Perez-Amador et al. 2002; Salekdeh et al. 2002; Ohno and Ehara 2005; Wei et al. 2006). Consistent with the evidently different roles of the S-RNases and S-like RNases in the evolution of plants, S-RNases and S-like RNases are classified into three groups based on the number of their introns and phylogenetic analyses (Igic and Kohn 2001). S-like RNases were assigned to groups I and II. S-RNases were assigned to group III.
In contrast to S-like RNases, S-RNases are specifically expressed in the style and inhibit the growth of pollen tubes as demonstrated by the genetic transformation of Petunia inflata and Nicotiana (Lee et al. 1994; Murfett et al. 1994). However, similar experiments are impractical for woody perennial species because of their long juvenile period. Therefore, testing whether candidate S-RNases possess S-RNase activity and surveying the downstream reactions induced by candidate S-RNases using an in vitro has become a common approach for identifying S-RNases from such species (Hiratsuka et al. 2001; Wang et al. 2009). The ribonuclease activity of S-RNase proteins is essential for self-incompatibility (Huang et al. 1994). After S-RNases are taken up by pollen tubes, they use the 26S proteasome pathway to specifically degrade the RNA of self-pollen tubes, which destroys the self-pollen tubes (McCubbin and Kao 2000; Takayama and Isogai 2005; Hua et al. 2008; Cui et al. 2014).
‘Shatian’ pummelo is an ancient species of the Citrus genus that exhibits self-incompatibility. We tested whether we could identify RNase T2 candidates in pummelo (C. grandis) using the genomic databases of sweet orange (C. sinensis) and clementine mandarin (C. clementine), because the genomic database of ‘Shatian’ pummelo is not available. To obtain candidate S genes associated with self-incompatibility, gene structure, sequence phylogeny, promoter elements, and expression patterns were analyzed to distinguish S-RNases from S-like RNases. Based on this analysis, we identified a candidate S-RNase and used the in vitro system to test the self-incompatible activity of this candidate S-RNase. Among the 16 RNase T2 homologs that we identified, one gene, CgRNS3, exhibited all of the characteristics of an S-RNase. CgRNS3 was specifically expressed in pistil, and the protein encoded CgRNS3 was found to specifically inhibit the growth of self-pollen tubes in an in vitro culture system.
Materials and methods
Plant materials
Fruit and embryogenic callus were used to test whether the RNase T2 genes might contribute to the development of fruit and embryos. We separately used two different genotypes of sweet orange (C. sinensis), because the late-ripening mutant and the embryonic callus from one genotype did not provide sufficient material for our experiments. The wild-type (WT) ‘Fengjie 72-1’ orange and its spontaneous late-ripening mutant (MT) ‘Fengwan’ were both cultivated in the Fengjie orchard in Chongqing City, China. The sample fruits were harvested at 150, 170, 190, 210, and 240 days after flowering (DAF) from three different trees (Wu et al. 2014b) and then sliced. The non-embryogenic callus (NEC) of sweet orange was induced following the protocol of Deng’s doctoral dissertation (Deng 1987). The embryonic callus was induced by culturing embryogenic callus (EC) on modified MT medium containing 2% (v/v) glycerol for 4 weeks (IEC4) (Liu and Deng 2002).
The tissues sampled from ‘Shatian’ pummelo (C. grandis) included young leaves and six different floral organs: petals, anthers, filaments, styles, ovaries, and pedicels, which were collected at the Citrus Research Institute (Guilin City, Guangxi, China) 10 days prior to anthesis (Liang et al. 2015). All of the samples were immediately frozen in liquid nitrogen and stored at −80 °C until further RNA and protein extractions. The anthers of ‘Shatian’ pummelo and Suan pummelo that were used for the in vitro pollen culture system were collected, dehisced, dried, and stored in a bottle containing desiccants at –40 °C.
In silico identification and sequence analysis of RNase T2 genes in citrus
The complete genomes and protein sequences of sweet orange (C. sinensis) and clementine mandarin (C. clementine) were separately downloaded from the C. sinensis genome database (http://www.citrus.hzau.edu.cn/orange/index.php) (Xu et al. 2013) and the phytozome database (http://www.phytozome.net/). The HMM profile of Ribonuclease T2 (PF00445_seed) was downloaded from the Pfam protein families database (http://www.pfam.xfam.org/), which was used as a query to identify the RNase T2 genes from sweet orange and clementine using HMMER 3.0 with an E-Value 1.0E−10. Each of the predicted genes was submitted to the SMART (http://www.smart.embl-heidelberg.de/) and Pfam (http://www.pfam.sanger.ac.uk/) databases to test whether they were bona fide members of the RNase T2 family.
The molecular weights (kDa) and isoelectric points (pI) of the proteins encoded by the predicted RNase T2 genes were calculated using the ProtParam tool at ExPASy (Gasteiger et al. 2005). The intron–exon structures were plotted using the Gene Structure Display Server (GSDS, http://www.gsds.cbi.pku.edu.cn/) based on the genomic sequences and the CDS sequences (Guo et al. 2007). Signal peptide regions were predicted using the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/).
Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignments for the amino-acid sequences of the 16 RNase T2 proteins were prepared using ClustalX (version 1.83). To distinguish the S-RNases from the S-like RNases, a phylogenetic analysis was performed with the 16 candidates and 74 RNase T2 enzymes (including 46 S-RNases and 28 S-like RNases) that were reported previously from diverse species and that were downloaded from the Uniprot database (http://www.uniprot.org/). The pertinent information for these 74 RNase T2 sequences is listed in Table S1. To avoid non-alignments caused by the excessive sequence divergence in the 5’ and 3’ ends, the regions between the first conserved position of the mature peptide and the last conserved cysteine residue were used for phylogenetic analyses (Igic and Kohn 2001). The phylogenetic tree was generated using MEGA5.0 (Tamura et al. 2011) using the neighbor-joining (NJ) method. The reliability test was carried out using 1000 bootstrap replications.
Promoter analysis of RNase T2 genes in citrus
To research the promoter sequences of the RNase T2 genes, 1,500 bp of genomic sequence that is upstream of the translational start site (ATG) was obtained from the sweet orange database. A draft containing all of the putative cis-acting motifs in these promoter-containing sequences was prepared by analyzing these sequences at the PlantCARE Website (http://www.intra.psb.ugent.be:8080/PlantCARE) (Lescot et al. 2002).
RNA extraction, semi-quantitative RT-PCR, and quantitative RT-PCR
Total RNA was extracted from fruit, embryogenic callus, leaves, and different floral organs as previously described by Liu and Liu. (Liu and Liu 2006). First-strand cDNA was synthesized from 2 μg of RNA using the RevertAid™ First-Strand cDNA Synthesis Kit (Fermentas, USA) according to the manufacturer’s instructions. The specific primer pairs of semi-quantitative RT-PCR and quantitative RT-PCR were designed in non-conserved region with the software Primer 3, and were listed in Table S2. For quantitative RT-PCR, expression levels were normalized to the expression of Actin gene.
Semi-quantitative RT-PCR was conducted using a volume of 20 µL. Each semi-quantitative RT-PCR contained 600 ng of cDNA, 0.5 µM of each primer, 200 µM of each dNTP, and 2.5 U of Fast Pfu DNA Polymerase in 1× reaction buffer. PCRs were performed with an MJ-PTC-200 thermal controller (MJ Research, Waltham Mass) with the following steps: 94 °C for 10 min followed by 32 cycles of 94 °C for 16 s, 55 °C for 20 s, 72 °C for 20 s, and a final step at 72 °C for 10 min. The amplified products were analyzed using 2% agarose gels.
Quantitative RT-PCR analysis was performed in 10 µL reaction volumes with the ABI 7900HT Fast Real-Time System with the SYBR Green PCR Master Mix. Each qRT-PCR contained 600 ng of cDNA and 0.5 µM of each primer in 1 × SYBR Green Mix. Quantitative RT-PCRs were performed with 384-well plates that included three biological replicates for each sample. The thermal cycling program began with an initial incubation at 50 °C for 2 min and an incubation at 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min.
In vivo pollinations
To test the self-incompatibility of ‘Shatian’ pummelo, a ‘Shatian’ pummelo plant was self-pollinated at the Citrus Research Institute. In most self-incompatible varieties, pollen tubes reach the ovary at early bud stages, but as the style develops, their ability to grow is gradually reduced (Chalivendra et al. 2013). After the anthers were removed, styles at different developmental stages (stage 1: 5 days before flowering, stage 2: 4 days before flowering, stage 3: 3 days before flowering, stage 4: 2 days before flowering, stage 5: 1 day before flowering) were pollinated with mature self-pollen that was collected in 2014. Pistils were collected after 3 days and were immediately fixed in formalin/acetic acid/alcohol (FAA). After fixing, the pistils were stained with the aniline blue fluorochrome as described by Lin et al. (Lin et al. 2015).
Antibody generation, protein extraction, and western blot analysis
A cDNA clone derived from CsRNS3 mRNA was obtained by PCR-mediated amplification of cDNA prepared from the styles of ‘Shatian’ pummelo. This cDNA sequence of CgRNS3 is available from genbank (accession number KX894564). The coding sequence of CgRNS3, without the signal peptide, was inserted into the pEASY blunt E1 expression vector (TransGen). The recombinant protein was expressed in Escherichia coli strain BL21 (DE3) (TransGen), purified, and used to develop anti-CgRNS3 antisera in rabbits. Total protein was extracted from the young leaves and six floral organs (petals, anthers, filaments, styles, ovaries, and pedicels) from ‘Shatian’ pummelo as described previously (Isaacson et al. 2006; Pan et al. 2009). The concentration of protein in these extracts was determined with a modified Bradford protein assay kit (Sangon Biotech) using bovine serum albumin (BSA) as standard. Equal amounts of protein (20 µg) were analyzed using SDS-PAGE. The 10% gels were analyzed by immunoblotting using polyvinylidene difluoride (PVDF) membranes, as previously described (Cao et al. 2012). The anti-CsRNS3 antisera were diluted 1:1000; the anti-actin antibodies (ABclonal) were diluted 1:5000. The appropriate secondary antibodies were diluted 1:2500. The immunoreactive bands were detected using the ECL plus western blot detection kit (GE Healthcare).
Recombinant protein expression and RNase activity assay
To obtain massive quantities of the recombinant CgRNS3 protein, the mature protein (i.e., lacking the signal peptide) was expressed as a GST-fusion protein using the pGEX-6P-1 expression vector, because GST tags enhance the expression level (Waugh 2005). The GST-CgRNS3 fusion protein was expressed in Escherichia coli strain BL21 (DE3) (TransGen). The strain was grown at 37 °C in LB medium containing ampicillin at a concentration of 100 µg/mL. When the OD600 of the suspension reached 0.6, the bacteria were transferred to fresh LB medium containing ampicillin at a concentration of 100 µg/mL with a 1:100 dilution. The expression of the GST-CgRNS3 fusion protein was induced with 0.25 mM IPTG at 16 °C for 20 h. The cells were lysed using sonication in binding buffer (containing 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 2 mM DTT, pH 7.3). The fusion protein was purified using glutathione Sepharose 4B beads (GE Healthcare) according to the manufacturer’s protocol. Protein concentrations were estimated using the Bradford assay using BSA as a standard. The RNase activity of the recombinant protein was assayed using torula yeast RNA (Sigma) as substrate as previously described (Nishimura et al. 2014). Sodium citrate buffers with pH values ranging from 3 to 9 were used to test the pH optimum. To test the subcellular localization of CgRNS3, a cDNA encoding the full-length CgRNS3 protein was inserted into pM999-GFP and transiently expressed in protoplasts that were isolated from citrus leaves according to the protocol of Yoo et al. (Yoo et al. 2007). GFP fluorescence was imaged using a confocal laser scanning microscope.
In vitro pollen culture and S-RNase inhibition assay
Pollen grains were cultured in liquid germination medium (GM) which contained 0.02% MgSO4, 0.01% KNO3, 0.03% Ca(NO3)2, 0.01% H3BO3, 15% PEG-4000, and 20% sucrose at pH 6.0. After the recombinant GST-CgRNS3 was added to the basal GM at concentrations of 25, 50, and 100 μg/mL, pollen was uniformly scattered onto the GM and incubated in a dark and humid chamber for 9 h at 28 °C. Three independent experiments were performed for each treatment. Images of the pollen were acquired using light microscopy. At least 150 pollen tubes were measured for each treatment using the image pro-plus software.
Results
In silico identification and characterization of RNase T2 genes
To identify the RNase T2 genes in the genomes of citrus, we used the Hidden Markov Model (HMM) profile of the Pfam RNase T2 domain as a query in a BLASTP search of the available sweet orange and clementine mandarin genomes. We identified 9 and 7 RNase T2 homologs from sweet orange and clementine mandarin, respectively, which we named CsRNS1 through CsRNS9 and ClRNS1 through ClRNS7 (Tables 1, 2). The calculated molecular weights for the proteins encoded by these 16 putative RNase T2 genes mostly ranged from 25 to 32 kDa, which is typical for RNase T2 enzymes (Deshpande and Shankar 2002). The calculated molecular weights of the proteins encoded by CsRNS8 and ClRNS7 were outside of this range (14.3 and 16.3 kDa, respectively). We calculated acidic pI values for 7 and basic pI values for eight of these putative RNase T2 enzymes. We calculated a nearly neutral pI value for only the RNase T2 enzyme encoded by CsRNS8 (Tables 1, 2).
Multiple sequence alignments of the full-length amino-acid sequences of these putative RNase T2 proteins indicated that all of these proteins, with the exception of CsRNS8, ClRNS1, and ClRNS7, contained two conserved active site (CAS I and CAS II) histidine residues that are essential for RNase T2 activity (Kawata et al. 1990) (Fig. S1). The coding sequences of CsRNS8 and ClRNS7 were short (approximately 400 bp) relative to the CsRNS and ClRNS sequences that contained both CAS I and CAS II. In CsRNS8 and ClRNS7, the CAS I motif was lost. The sequence identity between CsRNS1 and ClRNS1 is 84%. However, the deletion of a short sequence close to the CAS II motif was observed in ClRNS1 (Fig. S1). All the proteins also had half-cystine residues with different levels of conservation, which form disulfide bridges to promote the proper folding of protein tertiary structure (Irie 1999).
Phylogenetic analysis of RNase T2 genes in citrus
A phylogenetic tree was constructed with the 16 CsRNS and ClRNS genes using the MEGA program and the neighbor-joining (NJ) method. This analysis separated these genes into three groups (Fig. 1a). To further understand the relationships among these RNase T2 genes, the GSDS program was used to determine their intron–exon structures. We found that the genes that clustered into the same subgroups shared similar intron–exon structures. Genes that clustered into group I have only one intron, with the exceptions of CsRNS8 and ClRNS7, which have no intron. CsRNS1 and ClRNS1 have seven and six introns, respectively, and formed an independent cluster. The members of group III, namely CsRNS5, CsRNS6, ClRNS2, ClRNS3, and ClRNS4, each have three introns. Our phylogenetic tree and our findings on the intron–exon structures of these genes are consistent with the findings of the previous publication (Igic and Kohn 2001).
Phylogenetic analysis of 16 RNase T2 genes from citrus. a The phylogenetic tree and gene structures were generated using MEGA5.0 and GSDS, respectively. All of the RNase T2 genes tested were clustered into three distinct groups. Exons and introns are depicted by filled green boxes and single lines, respectively. b Phylogenetic relationships among 16 RNase T2 and 74 RNase T2 enzymes from diverse species. The tree was constructed using the neighbor-joining algorithm using the MEGA5.0 program. The reliability of this tree was inferred from a bootstrap analysis of 1000 replicates. The RNase T2 genes identified in this study are indicated with filled red circles
Next, we analyzed the phylogenetic relationships among the 16 putative RNase T2 genes and the other homologs to determine whether these putative RNase T2 genes encode S-RNases or S-like RNases. Thus, 46 S-RNase and 28 S-like RNase protein sequences were randomly collected from the UniProt database and a phylogenetic tree was constructed with the amino-acid sequences of the predicted mature proteins and the amino-acid sequences encoded by the 16 putative RNase T2 genes described above (Fig. 1b). We found that the S-RNases from Rosaceae and Solanaceae and some of the predicted RNase T2 enzymes clustered together and that the RNase T2 genes from citrus were more closely related to the S-RNases from Solanaceae than to the S-RNases from Rosaceae. Another branch contained CsRNS1, CsRNS5, CsRNS6, and ClRNS1, through ClRNS4, and the 28 S-like RNase sequences that we analyzed from various species (Fig. 1b). This phylogenetic analysis provides evidence that some of these putative RNase T2 genes might serve as S-RNases that contribute to the self-incompatibility reaction. In addition, we found that each putative RNase T2 gene from clementine mandarin and one RNase T2 gene from sweet orange comprised one subgroup (Fig. 1a, b). These data provide evidence that these putative RNase T2 genes from clementine and sweet orange perform the same reaction in vivo. On the other hand, pummelo share greater sequence identity with sweet orange than that with clementine, because sweet orange is a hybrid between pummelo and mandarin (Xu et al. 2013; Velasco and Licciardello 2014; Wu et al. 2014a). Hence, we decided to test the possible biological functions of CsRNS1 through CsRNS9.
Stress-relevant cis-elements in the promoters of RNase T2 genes
S-like RNases often contribute to stress tolerance in plants. To identify the possible stress-related cis-elements in the promoter regions, we analyzed the 1500 bp located upstream of the translational start sites of CsRNS1 through CsRNS9 using the PlantCARE database. All the identified elements are displayed in Fig. S2. CAAT-boxes and TATA-boxes were highly conserved among the nine genes. As shown in Table 3, 15 types of stress-related cis-acting motifs were detected, and these motifs included the anaerobic induction element (ARE), fungal elicitor element (Box-W1), heat stress responsive element (HSE), drought inducibility element (MBS), low-temperature responsive element (LTR), defense and stress responsive element (TC-rich repeats), and multiple cis-acting elements that respond to hormones. Among all the different cis-elements that we identified, the MBS and TC-rich repeats were the most abundant, accounting for 32% of all the identified elements. The promoter of each putative RNase-encoding gene that we tested had at least one MBS element. The majority of the promoters among the putative RNase T2 genes possessed ten or more elements. We identified 19 cis-elements in CsRNS1, which was the maximum number of elements that we identified in any of the genes that we tested. Almost half of the elements (8/19 cis-elements) that we identified in the promoter of CsRNS1 were reported to respond to MeJA (Table 3).
Expression analysis of RNases under diverse biological processes
To obtain evidence for the biological functions of these RNase T2 genes, we tested their expression levels during embryogenesis and in different developmental stages of fruit. Morphological changes could be clearly observed among non-embryogenic callus (NEC), callus induced for 4 weeks (IEC4) and embryogenic callus (EC) (Fig. 2). Results from semi-quantitative PCR experiments revealed that only CsRNS3 and CsRNS6 were expressed during embryo development CsRNS3 was detected in the NEC and EC stages. There was no significant difference in the levels of expression of CsRNS3 during these two stages of development. CsRNS6 showed a prominent band at the EC stage but undetectable or weak expression at the NEC stage. In addition, quantitative RT-PCR was performed on RNA extracted from fruits harvested 150, 170, 190, 210, and 240 DAF in a spontaneous late-ripening orange mutant (MT) and its wild type (WT) (Fig. 3). In MT, the expression levels of 4 RNases (CsRNS1, CsRNS2, CsRNS3, CsRNS7, and CsRNS8) in MT significantly increased at 240 DAF and were obviously higher than in WT. It is noteworthy that the expression levels of CsRNS1 were continuously up-regulated during fruit ripening in MT and the WT and that in contrast, no difference were observed in the expression levels of CsRNS4, CsRNS5, CsRNS6, and CsRNS9 during fruit ripening in MT and the WT.
Expression patterns of CsRNS1 through CsRNS9 in three different callus tissues from sweet orange. Total RNA was extracted from embryogenic callus (EC), non-embryogenic callus (NEC), and embryogenic callus induced for 4 weeks (EC4). Gene expression was evaluated by semi-quantitative RT-PCR. Amplification of actin was used as a loading control
Expression patterns of CsRNS1 through CsRNS9 in the fruit of a spontaneous late-ripening orange mutant (MT) and the relevant wild type (WT). The developmental stages of fruit ripening that were tested included 150, 170, 190, 210, and 240 day after flowering. Quantitative RT-PCR was used to quantify mRNA levels. Three biological replicates were analyzed for the MT and the WT. Error bars indicate standard deviation
Tissue specific analysis of RNases
The defining characteristics of S-RNases are (1) they are specifically expressed in the style and (2) they participate in the self-incompatible reaction in Solanaceae, Rosaceae, and Scrophulariaceae (McClure et al. 1989). As shown in Fig. 4a, the length of self-pollen tubes was gradually shortened during the development of stigma and ultimately the tube stopped growing at the 1/3 position of the style. These data are consistent with ‘Shatian’ pummelo possessing a self-incompatibility system. To test whether some of the predicted RNases in our study might contribute to self-incompatibility, the levels of their expression were estimated in the leaves and in six floral organs of ‘Shatian’ pummelo using semi-quantitative PCR (Fig. 4b). CsRNS1 was highly expressed in all the sampled organs. In contrast, the expression levels of CsRNS4 and CsRNS9 were below the limits of detection in all of the tissues tested. CsRNS5, CsRNS6, CsRNS7, and CsRNS8 were expressed in multiple tissues. In contrast, CsRNS3 was specifically expressed in style, and CsRNS2 was specifically expressed in the pistil.
Expression patterns of CsRNS1 through CsRNS9 in ‘Shatian’ pummelo (Citrus grandis). a Aniline blue staining of self-pollinated ‘Shatian’ pummelo pistils at different developmental stages. The position of pollen tube inhibition is indicated with an arrow. Bar 1 cm. b Semi-quantitative RT-PCR in seven different tissues from ‘Shatian’ pummelo. Leaves and six flower tissues dissected from mature flowers (ovary, petal, anther, peduncle, filament, and style) were analyzed. Amplification of actin was used as a loading control
Cloning and function of CgRNS3
Based on the results of our phylogenetic and expression analyses, we concluded that CsRNS3 is potentially an S-RNase that contributes to self-incompatibility in citrus. A cDNA encoding CgRNS3 (C. grandis RNase 3) synthesized from mRNA extracted from the styles of ‘Shatian’ pummelo was cloned and sequenced. There was 99% sequence identity between CgRNS3 and CsRNS3. The signal peptide of CgRNS3 was followed by a special structural domain found in type T2 RNases and a single intron was inserted into the hypervariable region (Fig. 5). The CgRNS3 protein shares the conserved regions C1, C2, C3, and C5, but the C4 region was different from the S-RNases from Solanaceae and Rosaceae. CgRNS3 is similar to Rosaceae S-RNases, which have only one hypervariable region named RHV, and is distinct from Solanaceae S-RNases in that Solanaceae S-RNases have two hypervariable regions (HVa and HVb) (Fig. 5).
Amino-acid sequence alignment of the S-RNases from citrus and other species. The conserved regions and the hypervariable regions of the S-RNases from Solanaceae and Rosaceae are indicated under the corresponding sequences. The position of the intron is inserted in the gene encoding CgRNS3 which is marked with an arrow and the signal peptide is indicated with a straight line. The reported S-RNase sequences are from Nicotiana alata, Petunia hybrid, Malus domestica, Pyrus pyrifolia, and Prunus armeniaca
We used quantitative RT-PCR and western blot analyses to test the expression levels of CgRNS3 in the different tissues of ‘Shatian’ pummelo, and found that CgRNS3 was expressed at high levels in the style (Fig. 6). To test whether CgRNS3 participates in the self-incompatible reaction, we first prepared recombinant CgRNS3 protein by fusing the full-length cDNA without the signal peptide to GST in pGEX-6P-1 and expressed the fusion protein in E. coli (Fig. 7a). The ribonuclease activity and pH optimum of the recombinant CgRNS3 were tested using torula yeast RNA as the substrate. RNase activity was quantified at several different pH values ranging from 3 to 9. The activity of RNase A was also tested in these assays, for a comparison. RNase A exhibited the highest activity at an acidic pH value. We found that the recombinant CgRNS3 protein possessed ribonuclease activity. In contrast to RNase A, the recombinant CgRNS3 exhibited optimum activity at pH 8 (Fig. 7b). Moreover, the subcellular localization of CgRNS3 indicated that it was a cytoplasm protein (Fig. 7c).
RNase activity assay and the subcellular localization of CgRNS3. a Purification of the GST-CgRNS3 fusion protein. The crude lysate and the purified fusion protein were analyzed using SDS-PAGE. Lane 1, crude lysate; lane 2, purified recombinant protein. M, protein marker (labeled in kDa); CK, pGEX-6P-1 recombinant protein. b pH optimum of CgRNS3. The RNase assay was performed at several different pH values that ranged from 3 to 9. (filled square) recombinant protein. RNase A (circle). The mean values from three replicates are shown. Error bars indicate standard deviation (SD). c Subcellular localization of the full-length CgRNS3 fused to green fluorescent protein (GFP). Bar 10 μm
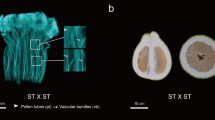
To confirm whether CgRNS3 was functionally involved in the self-incompatibility response, we added CgRNS3 to the in vitro culture system at three different concentrations: 25, 50, and 100 μg/mL. In production, the pollen from Suan pummelo is always used to pollinate ‘Shatian’ pummelo because of the self-incompatibility problem in ‘Shatian’ pummelo. Thus, we reasoned that in theory, the pollen from Suan pummelo should develop normally in an in vitro culture system programmed with pistils from ‘Shatian’ pummelo. Indeed, we found that the phenotype of the non-self-pollen tube appeared relatively normal in the in vitro culture system (Fig. 8a). In addition, we observed that most of the self-pollen tubes exhibited a curved apex when CgRNS3 was present at 25 μg/mL. This abnormal phenotype was enhanced when the concentration of CgRNS3 was increased. However, the phenotype of curved apex was almost no detection in non-self-pollen tube (Fig. 8a). Based on measurements of pollen tube length, the inhibitory effect on the growth of the pollen tubes was not obvious for both self- and non-self-pollen tubes when the CgRNS3 protein concentration was 25 μg/mL. However, the CgRSN3 protein strongly inhibited the growth of both types of pollen tubes when the protein concentration exceeded 25 μg/mL. Interestingly, when the CgRNS3 protein was present at 100 μg/mL, we observed a 59% reduction in the length of the tubes of ‘Shatian’ pummelo. In contrast, when the CgRNS3 protein was present at 100 μg/mL, we observed a 47% reduction in the tubes of Suan pummelo. After the protein was heated, it no longer induced the curved apex phenotype in the self-pollen tubes and the inhibition of pollen tube growth was alleviated (Fig. 8a, b). In summary, CgRNS3 appears to be an S-RNase, because CgRNS3 has the characteristic structural and expression pattern of an S-RNase and similar to other reported S-RNases CgRNS3 possessed ribonuclease activity and inhibited the growth of self-pollen in the in vitro culture system.
Inhibitory effects of CgRNS3 on pollen tube growth in vitro. a Phenotypes of self (‘Shatian’ pummelo) and non-self (Suan pummelo) pollen tubes treated with CgRNS3. Control: untreated (CK) pollen. Bar 200 μm. b Effect of CgRNS3 and heated CgRNS3 on the growth of self (gray bars) and non-self (stippled bars) pollen tubes. The average values from three independent replicates are shown. Error bars indicate standard deviation (SD)
Discussion
Identification and classification of RNase T2 in citrus
RNase T2 genes perform a variety of functions in diverse prokaryotic and eukaryotic organisms. Recently, the publication of abundant genetic and genomic information for sweet orange and clementine mandarin have facilitated a better understanding of many biological traits in citrus (Xu et al. 2013; Wu et al. 2014a). Here, we report on the identification of 9 and 7 RNase T2 homologs from the sweet orange and clementine mandarin databases, respectively (Tables 1, 2). Based on our phylogenetic analysis, we conclude that each of the putative RNase T2 genes from clementine mandarin is closely related to one of the putative RNase T2 genes from sweet orange (Fig. 1a, b). Plant RNases T2 genes were classified into three major groups based on the previous phylogenetic analyses and the number of introns in these genes (Igic and Kohn 2001). The S-like RNases are in groups I and II. The genes in group I have two or three introns. The genes in group II have seven or more introns. The S-RNases are in group III and have only one intron (Lee et al. 1992; Igic and Kohn 2001; Roalson and McCubbin 2003; Banović et al. 2009). Our phylogenetic analysis indicated that all of the candidate RNases T2 genes that we identified in this study were also classified into three groups. The numbers of introns in the genes from these groups are consistent with the previous findings (Fig. 1a). The genes with three introns (CsRNS5 and CsRNS6) and the single gene with seven introns (CsRNS1) formed a single cluster. The CsRNS2/3/4/7/8/9 genes have only one intron or no introns (CsRNS8) formed a distinct cluster. Moreover, we obtained the same results with the phylogenetic tree constructed with 74 RNase T2 homologs and also found that CsRNS1, CsRNS5, and CsRNS6 clustered with the 28 S-like RNases. The other predicted RNase T2 genes in sweet orange clustered with the S-RNases from Rosaceae and Solanaceae (Fig. 1b). Thus, our analysis appeared to distinguish S-RNases and S-like RNases.
Function and expression analysis of S-like RNases in citrus
It has been reported that the S-like RNases from plants are responsive to various stresses, such as virus infection, drought, and mechanical wounding (Perez-Amador et al. 2002; Salekdeh et al. 2002; Ohno and Ehara 2005). In this study, we found that the expression of CsRNS1 was continuously up-regulated during fruit development and that at 240 DAF, its expression in a late-ripening orange mutant was remarkably higher than in wild type (Fig. 3). Furthermore, we found eight MeJA-responsive cis-elements in the promoter region of CsRNS1 (Table 3). There are a large number of publications indicating that MeJA induces senescence (Woo et al. 2001; Schommer et al. 2008). Consistent with S-like RNases contributing to senescence, Chai et al. (2011b) reported that the expression of an S-like RNase gene (CgSL2) from ‘Zigui shatian’ pummelois up-regulated during the senescence of ovaries. There is a 95% sequence identity between CsRNS1 and CgSL2. These data provide evidence that CsRNS1 is an S-like RNase gene that probably contributes to senescence or fruit development. More evidence that S-like RNases contribute to the development of embryos comes from RNase Dre1 and Dre2 from Zebrafish. The expression of these two S-like RNase genes was gradually increased when embryos were induced (Hillwig et al. 2009). We found that the expression of CsRNS6 was only detectable in embryogenic callus (Fig. 2). These data imply that CsRNS6 may act as an S-like RNase gene and involve in embryo development (Hillwig et al. 2009; Ambrosio et al. 2014).
Involvement of CgRNS3 in the self-incompatibility of ‘Shatian’ pummelo
S-RNases accumulate it the style and act as female determinants of self-incompatibility in Solanaceae, Rosaceae, and Scrophulariaceae (Bredemeijer and Blaas 1981; Uchida et al. 2012). To screen for genes encoding S-RNases in citrus, we investigated the expression patterns of CsRNS1 through CsRNS9 by measuring the expression levels of these genes in the diverse tissues of the self-incompatible cultivar ‘Shatian’ pummelo. We observed style-specific expression only for CsRNS3 (Fig. 4b). In addition, the molecular weight (25.7 kDa) and the highly basic pI (9.37) of CsRNS3 are similar to the S-RNases that were identified in many other species (Anderson et al. 1986; Sassa et al. 1993; Asquini et al. 2011). Thus, we cloned the cDNA of CgRNS3 from ‘Shatian’ pummelo and found only a few differences in the amino-acid sequences relative to CsRNS3. Results from quantitative RT-PCR and western blotting experiments indicate that CgRNS3 is also expressed in the style (Fig. 6). Although the amino-acid sequences of S-RNases diverge substantially, their protein topology is highly conserved. For instance, S-RNases from Solanaceae have five conserved regions (C1 to C5). These conserved regions, except for C4 conserved region, are also present in the S-RNases from Rosaceae. Interestingly, the C4 region of CgRNS3 was distinguished from that of Rosaceae and Solanaceae. Two hypervariable regions are always observed in S-RNases from Solanaceae (HVa and HVb). In contrast, only one hypervariable region (RHV) is observed in S-RNases from Rosaceae (De Franceschi et al. 2012; Wu et al. 2013). The single hypervariable region of CgRNS3 is distinct relative to the hypervariable regions of the S-RNases from Rosaceae. Indeed, the amino-acid sequence of CgRNS3 is approximately 30% similar to all known S-RNases in NCBI database. Thus, we suspect that CgRNS3 is a novel S allele relative to the S alleles from Rosaceae and Solanaceae.
The enzyme activity of CgRNS3 was also tested. Consistent with CgRNS3 serving as an S-RNase and in contrast to most RNase T2 enzymes, we found that the recombinant CgRNS3 that we purified from E. coli had a basic pH optimum (Irie 1999). The cytoplasmic localization is also consistent with CgRNS3 serving as an S-RNase (Li et al. 2016). Demonstrating S-RNase activity in an in vitro culture system is essential for establishing whether a candidate S-RNase contributes to self-incompatibility (Jahnen et al. 1989; Hiratsuka et al. 2001). Consistent with CgRNS3 serving as an S-RNase, the addition of the recombinant CgRNS3 protein to an in vitro culture system disturbed the growth of self-pollen tubes. Indeed, the CgRNS3-treated self-pollen tubes were abnormally shaped and were remarkably shortened in length relative to the untreated control. In contrast, the CgRNS3-treated non-self-pollen tubes grew normally without curved apex. Although the elongation of the non-self-pollen tubes was inhibited to some extent, the inhibition was less than the self-pollen tubes. We also tested whether CgRNS3 might also contribute to self-incompatibility in Suan pummelo by attempting to clone a cDNA encoding CgRNS3 from the styles of Suan pummelo. Indeed, the cDNA clone encoding CgRNS3 that we cloned from Suan pummelo is nearly identical to the cDNA clone encoding CgRNS3 that we cloned from ‘Shatian’ pummelo. Based on these data, we suggest that ‘Shatian’ and Suan pummelo have an S allele in common, which result in the inhibition of non-self-pollen tubes. We also found that the difference in the inhibition of growth caused by CgRNS3 in the self- and non-self-pollen tubes was reduced when the concentration of CgRNS3 was increased. Based on these data, we suggest that if the CgRNS3 protein was to accumulate to high levels in vivo, CgRNS3 would inhibit the growth of both self- and non-self-pollen tubes, which is consistent with the previous work (Meng et al. 2014). Furthermore, the heated protein without RNase activity alleviated the inhibition on the growth of pollen tubes. Although the previous studies have indicated that heating S-RNases from N. alata can dramatically increase its inhibitory activity and cause non-specific inhibition of pollen tube growth (Jahnen et al. 1989; Gray et al. 1991), we found that heat treating CgRNS3 dramatically reduced its ability to inhibit the growth of pollen tubes. Differences in the pollen of C. grandis and N. alata may explain these results. Another possible explanation is different source of S-RNase protein. Instead of testing whether a purified recombinant protein could inhibit pollen tube growth, the previous studies tested whether a crude pistil extracts could inhibit pollen tube growth. A crude pistil extract might contain unknown factors that stimulate the growth of pollen tubes. The most parsimonious interpretation of our data is that after CgRNS3 enters pollen tubes, it accumulates in the cytoplasm and causes cytotoxic effects that depend on its RNase activity and that these cytotoxic effects disrupt the growth of self-pollen tubes in ‘Shatian’ pummelo.
In summary, we separately identified 9 and 7 RNase T2 homologs from sweet orange and clementine mandarin genomes. Based on phylogenetic comparisons and intron number comparisons, these putative RNase T2 enzymes were grouped into three clusters. This clustering allowed us to distinguish S-RNases from S-like RNases. We conclude that CsRNS1 and CsRNS6 are S-like RNases that are separately involved in senescence and embryo development. We conclude that CgRNS3 is an S-RNase that is specifically expressed in the style and is required for self-incompatibility in ‘Shatian’ pummelo. We also conclude that CgRNS3 is a novel S-RNase and that this new information will lead to deeper understanding of the mechanism of self-incompatibility in citrus.
References
Ambrosio L, Morriss S, Riaz A, Bailey R, Ding J, MacIntosh GC (2014) Phylogenetic analyses and characterization of RNase X25 from Drosophila melanogaster suggest a conserved housekeeping role and additional functions for RNase T2 enzymes in protostomes. PLoS One 9:e105444
Anderson MA, Cornish EC, Mau SL, Williams EG, Hoggart R, Atkinson A, Bonig I, Grego B, Simpson R, Roche PJ, Haley JD, Penschow JD, Niall HD, Tregear GW, Coghlan JP, Crawford RJ, Clarke AE (1986) Cloning of cDNA for a stylar glycoprotein associated with expression of self incompatibility in Nicotiana alata. Nature 321:38–44
Asquini E, Gerdol M, Gasperini D, Igic B, Graziosi G, Pallavicini A (2011) S-RNase-like sequences in styles of Coffea (Rubiaceae). Evidence for S-RNase based gametophytic self-Incompatibility? Trop Plant Biol 4:237–249
Banović B, Šurbanovski N, Konstantinović M, Maksimović V (2009) Basic RNases of wild almond (Prunus webbii): cloning and characterization of six new S-RNase and one “non-S RNase” genes. J Plant Physiol 166:395–402
Bariola PA, Green PJ (1997) Plant ribonucleases. Ribonucleases: structures and functions. Academic Press, New York
Bredemeijer GMM, Blaas J (1981) S-specific proteins in styles of self-incompatible Nicotiana alata. Theor Appl Genet 59:185–190
Broothaerts W, Janssens GA, Proost P, Broekaert WF (1995) cDNA cloning and molecular analysis of two self-incompatibility alleles from apple. Plant Mol Biol 27:499–511
Cao H, Zhang J, Xu J, Ye J, Yun Z, Xu Q, Xu J, Deng X (2012) Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J Exp Bot 63:4403–4417
Caruso M, Merelo P, Distefano G, La Malfa S, Lo Piero AR, Tadeo FR, Talon M, Gentile A (2012) Comparative transcriptome analysis of stylar canal cells identifies novel candidate genes implicated in the self-incompatibility response of Citrus clementina. BMC Plant Biol 12:20
Chai L, Biswas MK, Ge X, Deng X (2010) Isolation, characterization, and expression analysis of an SKP1-like gene from ‘Shatian’ pummelo (Citrus grandis Osbeck). Plant Mol Biol Rep 28:569–577
Chai L, Ge X, Biswas MK, Deng X (2011a) Molecular analysis and expression of a floral organ-relative F-box gene isolated from ‘Zigui shatian’ pummelo (Citrus grandis Osbeck). Mol Biol Rep 38:4429–4436
Chai L, Ge X, Xu Q, Deng X (2011b) CgSL2, an S-like RNase gene in ‘Zigui shatian’ pummelo (Citrus grandis Osbeck), is involved in ovary senescence. Mol Biol Rep 38:1–8
Chalivendra SC, Lopez-Casado G, Kumar A, Kassenbrock AR, Royer S, Tovar-Mèndez A, Covey PA, Dempsey LA, Randle AM, Stack SM (2013) Developmental onset of reproductive barriers and associated proteome changes in stigma/styles of Solanum pennellii. J Exp Bot 64:265–279
Cui J, Wang H, Liu S, Zhu L, Qiu X, Jiang Z, Wang X, Liu Z (2014) SNP discovery from transcriptome of the Swimbladder of Takifugu rubripes. PLoS One 9:e92502
De Franceschi P, Dondini L, Sanzol J (2012) Molecular bases and evolutionary dynamics of self-incompatibility in the Pyrinae (Rosaceae). J Exp Bot 63:4015–4032
De Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Springer-Verlag, Berlin
Deng X (1987) Studies on the isolation, regeneration and fusion of protoplast in Citrus. Dissertation, Huazhong Agriculture University
Deshpande RA, Shankar V (2002) Ribonucleases from T2 family. Crit Rev Microbiol 28:79–122
Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In Walker JM (ed) The Proteomics Protocols Handbook. Humana Press, pp 571–607
Gray JE, Mcclure BA, Bönig I, Anderson MA, Clarke AE (1991) Action of the style product of the self-incompatibility gene of Nicotiana alata (S-RNase) on in vitro-grown pollen tubes. Plant Cell 3:271–283
Guo A, Zhu Q, Chen X, Luo J (2007) GSDS: a gene structure display server. Yi Chuan 29:1023–1026
Hillwig MS, Rizhsky L, Wang Y, Umanskaya A, Essner JJ, MacIntosh GC (2009) Zebrafish RNase T2 genes and the evolution of secretory ribonucleases in animals. BMC Evol Biol 9:170
Hiratsuka S, Zhang SL, Nakagawa E, Kawai Y (2001) Selective inhibition of the growth of incompatible pollen tube by S-protein in the Japanese pear. Sex Plant Reprod 13:209–215
Hua ZH, Fields A, Kao Th (2008) Biochemical models for S-RNase-based self-incompatibility. Mol Plant 1:575–585
Huang S, Lee HS, Karunanandaa B, Kao TH (1994) Ribonuclease activity of Petunia inflata S proteins is essential for rejection of self-pollen. Plant Cell 6:1021–1028
Igic B, Kohn JR (2001) Evolutionary relationships among self-incompatibility RNases. Proc Natl Acad Sci USA 98:13167–13171
Irie M (1999) Structure-function relationships of acid ribonucleases: lysosomal, vacuolar, and periplasmic enzymes. Pharmacol Ther 81:77–89
Isaacson T, Damasceno CM, Saravanan RS, He Y, Catalá C, Saladié M, Rose JK (2006) Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protoc 1:769–774
Jahnen W, Lush WM, Clarke AE (1989) Inhibition of in vitro pollen tube growth by isolated S-glycoproteins of Nicotiana alata. Plant Cell 1:501–510
Kawata Y, Sakiyama F, Hayashi F, Kyogoku Y (1990) Identification of two essential histidine residues of ribonuclease T2 from Aspergillus oryzae. Eur J Biochem 187:255–262
Lee HS, Singh A, Kao T (1992) RNase X2, a pistil-specific ribonuclease from Petunia inflata, shares sequence similarity with solanaceous S proteins. Plant Mol Biol 20:1131–1141
Lee HS, Huang S, Kao TH (1994) S proteins control rejection of incompatible pollen in petunia inflata. Nature 367:560–563
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Li P, Miao HX, Ma YW, Wang L, Hu GB, Ye ZX, Zhao JT, Qin YH (2015) CrWSKP1, an SKP1-like Gene, is involved in the self-Incompatibility reaction of “Wuzishatangju” (Citrus reticulata Blanco). Int J Mol Sci 16:21695–21710
Li J, Zhang Y, Song Y, Zhang H, Fan J, Li Q, Zhang D, Xue Y (2016) Electrostatic potentials of the S-locus F-box proteins contribute to the pollen S specificity in self-incompatibility in Petunia hybrida. Plant J. doi:10.1111/tpj.13318
Liang LZ, Lai Z, Ma WS, Zhang YS, Xue YB (2002) AhSL28, a senescence-and phosphate starvation-induced S-like RNase gene in Antirrhinum. Biochim Biophys Acta 1579:64–71
Liang M, Yang X, Li H, Su S, Yi H, Chai L, Deng X (2015) De novo transcriptome assembly of pummelo and molecular marker development. PLoS ONE 10:e0120615
Lin Z, Eaves DJ, Sanchez Moran E, Franklin FCH, Franklin Tong VE (2015) The Papaver rhoeas S determinants confer self-incompatibility to Arabidopsis thaliana in planta. Science 350:684–687
Liu J, Deng X (2002) Regeneration and analysis of citrus interspecific mixoploid hybrid plants from asymmetric somatic hybridization. Euphytica 125:13–20
Liu Y, Liu Q (2006) Efficient isolation of RNA from fruit peel and pulp of ripening navel orange (Citrus sinensis Osbeck). Dissertation, Journal of Huazhong Agricultural University
Luhtala N, Parker R (2010) T2 Family ribonucleases: ancient enzymes with diverse roles. Trends Biochem Sci 35:253–259
Masashi Y, Tatsuya K, Shigeto T (2006) Self-and cross-incompatibility of various citrus accessions. J Jpn Soc Hortic Sci 75:372–378
McClure BA, Haring V, Ebert PR, Anderson MA, Simpson RJ, Sakiyama F, Clarke AE (1989) Style self-incompatibility gene products of Nicotlana alata are ribonucleases. Nature 342:955–957
McCubbin AG, Kao Th (2000) Molecular recognition and response in pollen and pistil interactions. Annu Rev Cell Dev Biol 16:333–364
Meng D, Gu Z, Yuan H, Wang A, Li W, Yang Q, Zhu Y, Li T (2014) The microtubule cytoskeleton and pollen tube Golgi vesicle system are required for in vitro S-RNase internalization and gametic self-incompatibility in apple. Plant Cell Physiol 55:977–989
Miao HX, Qin YH, da Silva JAT, Ye ZX, Hu GB (2011) Cloning and expression analysis of S-RNase homologous gene in Citrus reticulata Blanco cv. Wuzishatangju. Plant Sci 180:358–367
Miao HX, Ye ZX, Qin YH, Hu GB (2013) Molecular characterization and expression analysis of S1 self-incompatibility locus-linked pollen 3.15 gene in Citrus reticulata. J Integr Plant Biol 55:443–452
Miao HX, Ye ZX, Hu GB, Qin YH (2015) Comparative transcript profiling of gene expression between self-incompatible and self-compatible mandarins by suppression subtractive hybridization and cDNA microarray. Mol Breed 35:1–15
Murfett J, Atherton TL, Mou B, Gassert CS, Mcclure BA (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367:563–566
Ngo B (2001) Study on the self-incompatibility in Citrus (Rutaceae) with special emphases on the pollen tube growth and allelic variation. Dissertation, Kyushu University
Nishimura E, Jumyo S, Arai N, Kanna K, Kume M, J-i Nishikawa, J-i Tanase, Ohyama T (2014) Structural and functional characteristics of S-like ribonucleases from carnivorous plants. Planta 240:147–159
Ohno H, Ehara Y (2005) Expression of ribonuclease gene in mechanically injured or virus-inoculated Nicotiana tabacum leaves. Tohoku J Agric Res 55:99–109
Pan Z, Liu Q, Yun Z, Guan R, Zeng W, Xu Q, Deng X (2009) Comparative proteomics of a lycopene-accumulating mutant reveals the important role of oxidative stress on carotenogenesis in sweet orange (Citrus sinensis [L.] osbeck). Proteomics 9:5455–5470
Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130:1454–1463
Roalson EH, McCubbin AG (2003) S-RNases and sexual incompatibility: structure, functions, and evolutionary perspectives. Mol Phylogenet Evol 29:490–506
Salekdeh GH, Siopongco J, Wade L, Ghareyazie B, Bennett J (2002) A proteomic approach to analyzing drought-and salt-responsiveness in rice. Field Crops Research 76:199–219
Sassa H, Hirano H, Ikehashi H (1992) Self-incompatibility-related RNases in styles of Japanese pear (Pyrus serotina Rehd.). Plant Cell Physiol 33:811–814
Sassa H, Hirano H, Ikehashi H (1993) Identification and characterization of stylar glycoproteins associated with self-incompatibility genes of Japanese pear, Pyrus serotina Rehd. Mol Gen Genet 241:17–25
Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE, Nath U, Weigel D (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6:e230
Takayama S, Isogai A (2005) Self-incompatibility in plants. Ann Rev Plant Biol 56:467–489
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tao R, Yamane H, Sassa H, Mori H, Gradziel TM, Dandekar AM, Sugiura A (1997) Identification of stylar RNases associated with gametophytic self-incompatibility in Almond (Prunus dulcis). Plant Cell Physiol 38:304–311
Tao R, Yamane H, Sugiura A, Murayama H, Sassa H, Mori H (1999) Molecular typing of S-alleles through identification, characterization and cDNA cloning for S-RNases in sweet cherry. J Am Soc Hortic Sci 124:224–233
Taylor CB, Bariola PA, Raines RT, Green PJ (1993) RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc Natl Acad Sci USA 90:5118–5122
Uchida A, Takenaka S, Sakakibara Y, Kurogi S, Honsho C, Sassa H, Suiko M, Kunitake H (2012) Comprehensive analysis of expressed proteins in the different stages of the style development of self-incompatible ‘Hyuganatsu’ (Citrus tamurana hort. ex Tanaka). J Jpn Soc Hortic Sci 81:150–158
Velasco R, Licciardello C (2014) A genealogy of the citrus family. Nat Biotechnol 32:640–642
Wang CL, Xu GH, Jiang XT, Chen G, Wu J, Wu HQ, Zhang SL (2009) S-RNase triggers mitochondrial alteration and DNA degradation in the incompatible pollen tube of Pyrus pyrifolia in vitro. Plant J 57:220–229
Waugh DS (2005) Making the most of affinity tags. Trends Biotechnol 23:316–320
Wei JY, Li AM, Li Y, Wang J, Liu XB, Liu LS, Xu ZF (2006) Cloning and characterization of an RNase-related protein gene preferentially expressed in rice stems. Biosci Biotechnol Biochem 70:1041–1045
Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG (2001) ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13:1779–1790
Wu J, Gu C, Khan MA, Wu J, Gao Y, Wang C, Korban SS, Zhang S (2013) Molecular determinants and mechanisms of gametophytic self-Incompatibility in fruit trees of Rosaceae. Crit Rev Plant Sci 32:53–68
Wu GA, Prochnik S, Jenkins J, Salse J, Hellsten U, Murat F, Perrier X, Ruiz M, Scalabrin S, Terol J (2014a) Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat Biotechnol 32:656
Wu J, Xu Z, Zhang Y, Chai L, Yi H, Deng X (2014b) An integrative analysis of the transcriptome and proteome of the pulp of a spontaneous late-ripening sweet orange mutant and its wild type improves our understanding of fruit ripening in citrus. J Exp Bot 65:1651–1671
Xu Q, Chen LL, Ruan X, Chen D, Zhu A, Chen C, Bertrand D, Jiao W-B, Hao B-H, Lyon MP (2013) The draft genome of sweet orange (Citrus sinensis). Nat Genet 45:59–66
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zhang SW, Ding F, He XH, Luo C, Huang GX, Hu Y (2015) Characterization of the ‘Xiangshui’ lemon transcriptome by de novo assembly to discover genes associated with self-incompatibility. Mol Genet Genomics 290:365–375
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Nos. 31301760, 31521092, 31460507), the Special Fund for Agro-scientific Research in the Public Interest (201303093), and Scientific Research and Technology Development Program of Guangxi (Gui Ke He1599005-2-15). We also thank Prof. Zuoxiong Liu and Prof. Robert M. Larkin for help with English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that none of the authors have a conflict of interest and that this article does not contain any studies with human participants or animals.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, M., Yang, W., Su, S. et al. Genome-wide identification and functional analysis of S-RNase involved in the self-incompatibility of citrus. Mol Genet Genomics 292, 325–341 (2017). https://doi.org/10.1007/s00438-016-1279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-016-1279-8