Abstract
Malondialdehyde (MDA) is a naturally occurring product of lipid peroxidation and the level of MDA in plant is often used as a parameter to evaluate the damage to plants’ cells due to stress. Plant with lower amounts of MDA under drought conditions is generally considered as more tolerant to drought. In this study, a population of recombinant inbred lines was used to map the quantitative trait locus (QTLs) that controlled MDA content under well-watered condition (WW) and water deficit (WD) condition. A major QTL, designated as Qheb.mda-3B, was detected on the long arm of chromosome 3B. Based on interval mapping analysis, Qheb.mda-3B explained 31.5 and 39.0 % of the phenotypic variance under WW and WD conditions, respectively. Qheb.mda-3B was located in the same interval as a previously identified QTL (Qcrs.cpi-3B) that controlled resistance to Fusarium crown rot (FCR), a fungal disease caused by Fusarium species. Three pairs of near-isogenic lines (NILs) previously developed for Qcrs.cpi-3B were found to show significant differences in MDA content under WD condition. These results suggested that same set of genes is likely to be involved in drought tolerance and FCR resistance in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is a major stress that adversely affects wheat production in many regions in the world. It was estimated that 50 % of the wheat growing area in the developing and 70 % in the developed countries were affected by drought (Trethowan and Pfeiffer 2000). Wheat production could reduce to half or even lower in drought environments compared with those in well-watered areas (Rajaram et al. 1996). Till date, numerous studies have been conducted to identify the genes/QTLs contributing to drought tolerance in wheat (Quarrie et al. 2006; Kirigwi et al. 2007; Maccaferri et al. 2008; McIntyre et al. 2009; Zhang et al. 2014). Malondialdehyde (MDA) is a naturally occurring product of lipid peroxidation. It reacts with lipid, nucleic acid, glucose and protein and thus causes downstream reduction in the content of unsaturated fatty acid and the membrane resistance and fluidity, along with a rise in the quantity of electrolyte leakage. As a result, the function and structure of cytoplasmic membrane are damaged and a series of physiological metabolisms are changed (Scandalios 1993). MDA content is an indicator of oxidative damage in plant cell membrane induced by stress (Meloni et al. 2003; Verma and Mishra 2005). Many studies have used MDA content as a parameter to evaluate plant tolerance to drought (Chowdhury and Choudhuri 1985; Zhang and Kirkham 1994; Fu and Huang 2001; Wang et al. 2009; Yue et al. 2012; Tang et al. 2014). However, at present, most studies of MDA focus on physiological and biochemical aspects. There are only two reported studies about the QTL controlling MDA content in wheat so far. In those studies, the QTL controlling MDA content was mapped to wheat chromosome 1D, 2B, 3A, 4D, 5B, 6D, 7A, 7B, accounting for 8−30.18 % of the phenotypic variation (Jiang et al. 2013; Zhao et al. 2014).
FCR is a cereal disease caused by various Fusarium species. It is a serious wheat disease in Australia, as well as in the world (Burgess et al. 2001; Backhouse et al. 2004; Hogg et al. 2007; Ma et al. 2010; Poole et al. 2012; Zheng et al. 2014). Yield loss due to FCR has been documented to be from 9 to 89 %. It has been noticed for a long time that plants show a more severe symptom of FCR under drought conditions compared with those in normally watered conditions, both in field and glasshouse environments (Blaker and MacDonald 1981; Wiese 1987; Beddis and Burgess 1992; Smiley et al. 1996; Li et al. 2008). One possible reason for the interaction between drought and FCR resistance is that drought could affect crown rot resistance by modifying some morphological traits of plants. For example, plant height and heading date, the two traits that can be affected by drought were reported to have strong influences on FCR resistance in wheat and barley (Liu et al. 2010, 2012; Bai and Liu 2014). Another possibility is that the same genes may be involved in drought tolerance and FCR resistance. A study by Zhang et al. (2012) reported that transgenic wheat lines overexpressing a wheat myeloblastosis oncogenes TaPIMP1 displayed increased resistance to fungal pathogen disease and showed improved tolerance to drought. Alam et al. (2015) reported that a rice heme activator protein gene (OsHAP2E) conferred resistance to pathogen, salinity and drought. Similar results have been obtained in the studies in maize, tomato and Arabidopsis (Campo et al. 2012; Kamthan et al. 2012; Ramírez et al. 2009). It is known that besides drought, fungal disease infection could also cause the change of MDA content in plants (Wang et al. 2004; Chen et al. 2008). Therefore, it will be useful to explore the relationship between the QTL for MDA content and FCR resistance.
In this paper, we reported experiments carried out using RILs to identify the QTL controlling MDA content in wheat under WW and WD conditions. The relationship between the QTL for MDA content and QTL for FCR was investigated using three pairs of NILs previously developed for a major FCR QTL (Qcrs.cpi-3B) located on 3BL of wheat chromosome (Ma et al. 2010, 2012).
Materials and methods
Genetic materials
A population consisting 91 F8 RILs was used in this study. This population was derived from a cross between the Australian variety ‘Lang’ and a genotype ‘CSCR6’ which belongs to the taxon T. spelta. This population had been used to identify a major QTL controlling FCR resistance on the long arm of chromosome 3BL (Ma et al. 2010).
Three pairs of NILs (NIL_CR3BL_1A and NIL_CR3BL_1B; NIL_CR3BL_2A and NIL_CR3BL_2B; NIL_CR3BL_9A and NIL_CR3BL_9B), which were previously developed for the Qcrs.cpi-3B using heterogeneous inbred family method, were used to investigate the relationship between the QTL for MDA content and the QTL for crown rot (Tuinstra et al. 1997). These three pairs of NILs were derived from two different populations. The first two pairs of NILs (NIL 1A & 1B, NIL 2A & 2B) were derived from a backcross inbred lines between an Australia commercial variety ‘Janz’ and ‘CSCR6’ (‘Janz’*2/‘CSCR6’) and were at BC1F7. The NIL 9A & 9B pair was derived from the RILs used for QTL mapping in this study (‘Lang’/‘CSCR6’) and were at F9. The molecular marker that was used to develop the NILs was gwm181, which is about 2.2 cm away from the peak of Qcrs.cpi-3B (Ma et al. 2012).
Evaluation of MDA content in drought and normal water conditions
The measurement of MDA content of the mapping population and the two parents was conducted in the growth chamber in Baoding, Hebei agricultural university in China. The measurement of MDA content of the NILs was performed in the controlled environment facility (CEF) in CSIRO agriculture flagship, Brisbane in Australia. The settings for the growth chamber and CEF were: 25/15 (±1) °C day/night temperature and 65/85 (±5) % day/night relative humidity and a 14-h photoperiod (irradiance 500 μmol m−2 s−1).
Seeds were firstly selected by removing small and shrivelled kernels. The selected seeds were soaked in 70 % ethanol for 30 s to sterilize and then washed 2−3 times with distilled water. After that, the selected seeds were germinated in petri dishes under room temperature. After 1−2 days, the seedlings with coleoptiles about 0.5 cm were transferred to a container with 1/2 Hogland nutrient solutions in a growth chamber/CEF. When the seedlings were at two-leaf stage, 10 uniform seedlings of each line were selected, 5 seedlings for water deficit treatment in 1/2 Hogland nutrient solutions at –0.30 MPa induced by PEG6000 and the other 5 seedlings for well water treatment in normal 1/2 Hogland nutrient solutions. The solution containing PEG were changed every 5 days to keep the water potential stable. In total, three independent trials were conducted, each containing two replicates.
The leaf samples of the seedlings at four-leaf stage were collected, and were immediately frozen in liquid nitrogen and transferred to an ultra-freezer at –80 °C until the time of assay. For measurements of MDA content, ~0.5 g of seedlings was homogenized in 2 ml of a chilled reagent, which was composed of 0.25 % (w/v) TBA (thiobarbituric acid) in 10 % (w/v) TCA (trichloracetic acid), and then centrifuged at 10,000 rpm for 20 min using a Sigma 3−18 K centrifuge. The supernatant was heated at 95 °C for 30 min, quickly cooled on ice, and then centrifuged at 10,000 rpm for 20 min. The absorbances at 532 nm (A532), 600 nm (A600), and 450 nm (A450) were measured using a Beckman Coulter DU800 spectrophotometer. The MDA content was calculated with the equation: C (MDA content) = 6.45 × (A532−A600) − 0.56 × A450 (Wei et al. 2013).
Data analysis, linkage map and QTL mapping
Statistical analyses were performed using GenStat for Windows, 13th edition (copyright Lawes Agricultural Trust, Rothamsted Experimental Station, UK) and the SPSS statistics 17.0 for Windows statistical software package (SPSS Inc., Chicago). For each trial, the following model of mixed effects was used: Y ij = m + r i + g j + w ij , where Y ij trait value on the jth genotype in the ith replication; m general mean; r i effect due to ith replication; g j effect due to the jth genotype; w ij error or genotype by replication interaction, where genotype was treated as a fixed effect and that of replicate as random. The effects of replicate and genotype for each trait were determined using ANOVA. Homogeneity of variance was tested using Bartlett’s test to determine whether the data could be combined across trials for further analyses. The MDA content was calculated within each trial and a general mean across all the trials were calculated and used in the study. Student’s t test was used to test if the difference between the NILs pairs were significant or not.
A linkage map previously constructed for this population (Ma et al. 2010) had been updated by adding more SSR markers and then was used to identify the QTL controlling MDA content in the mapping population. The updated linkage map includes 881 DArT and 69 SSR markers. Linkage analysis was carried out using the computer package JoinMap (version 4.0 Van Ooijen 2006). MapQTL® 5.0 (Van Ooijen 2004) was used for QTL analysis. The Kruskal–Wallis test was used in a preliminary testing of associations between markers and MDA content. IM was then used to identify major QTL. Automatic cofactor selection was used to fit the multiple QTL model (MQM) and to select significantly associated markers as cofactors. For each trial, a test of 1000 permutations was performed to identify the LOD threshold corresponding to a genome-wide false discovery rate of 5 % (p < 0.05). Based on the permutation test, a threshold LOD value was used to declare the presence of a QTL. A linkage map showing the QTL positions was drawn using MAPCHART (Voorrips 2002).
Results
Characterization of MDA content in the mapping population
The two parents for the RILs showed a significant difference for MDA content under the two water conditions. The MDA content of Lang is 3.49 under WW conditions and 4.49 under WD condition, respectively. In contrast, the MDA content of CSCR6 was lower, with 3.22 and 3.28 under WW and WD conditions, respectively. Strong transgressing segregations were observed in the RILs, ranging from 2.51 to 5.41 under WW condition and 1.96 to 6.08 under WD condition (Table 1).
QTL detection under two water conditions
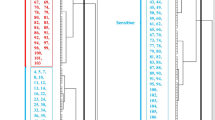
A major QTL for MDA content was detected on the long arm of chromosome 3B under both WW and WD conditions. We have designated the QTL as Qheb.mda-3B, where ‘MDA’ represents ‘MDA content’ and ‘heb’ represents ‘Agricultural University of Hebei’. Based on IM analysis, Qheb.mda-3B explained 31.5 % of the phenotypic variance under WW condition with an LOD value of 6.48 and 39.0 % of the phenotypic variance under WD condition with an LOD value of 8.49 (Fig. 1). The allele for the low MDA content is from CSCR6. Based on MQM analysis, markers flanking the Qheb.mda-3B locus were wPt10349 and wPt1977 under WW conditions and gwm181 and wPt1977 under WD conditions, respectively (Table 2).
QTL conferring MDA content detected on chromosome 3B in the population of Lang/CSCR6. Marker positions are shown to the left of the linkage map. The LOD values from each centimorgan of the chromosome were plotted against the chromosome, and the vertical dotted line indicates the average significance threshold (LOD = 3.4) derived from permutation tests. WW well-watered condition, WD water deficit condition
Relationship between QTL for MDA content and QTL for FCR resistance
Qheb.mda-3B was mapped to the same interval as a previously reported QTL controlling FCR resistance from the same parents (Qcrs.cpi-3B). The two markers flanking the Qcrs.cpi-3B were wPt10505 and wPt2277 (Ma et al. 2010). This interval overlapped with the interval where Qheb.mda-3B was located (between gwm181 and wPt1977). Besides, the closest linked SSR marker to Qheb.mda-3B was gwm181, which was also the closest linked SSR marker for Qcrs.cpi-3B and was used to develop the NILs candidates for Qcrs.cpi-3B (Ma et al. 2012).
For the NILs experiments, a remarkable high level of MDA was detected in all of the plants under WD condition when compared with plants under WW condition. Under WW condition, the MDA content in the resistant and susceptible lines of each pair of NILs were similar. In contrast, under WD conditions, the MDA content in the resistant lines of the three pairs of NILs were all lower compared with their corresponding susceptible lines, indicating that the FCR resistant lines experienced less lipid peroxidation and membrane injury than the susceptible lines under drought treatment and thus they were more tolerant to drought (Fig. 2). The biggest difference was found between 1A and IB NIL pair. Under WW condition, the amounts of MDA were 6.17 and 7.90 in the NIL 1A and NIL 1B, respectively. Under WD condition, the amounts of MDA in the NIL 1A increased to 13.48, whereas the MDA content in NIL 1B increased to 18.97. Significantly different MDA content between the resistant and susceptible lines was also found in NIL 2A/2B pair and NIL 9A/9B pair under WD condition.
MDA content analysis of three pairs of near-isogenic lines developed for Qcrs.cpi-3B under well-watered and water deficit conditions. 1R/1S: NIL_CR3BL_1A/1B; 2R/2S: NIL_CR3BL_2A/2B; 9R/9S: NIL_CR3BL_9A/9B. Data are mean ± standard deviation of three experiments. Means denoted by the same letter do not significantly differ at P < 0.05 as determined by Duncan’s multiple range test
Discussion
MDA content is often adopted as a suitable physiological index to reflect the degree of lipid peroxidation and drought stress tolerance in plants. However, at present, most studies of MDA focus on physiological and biochemical aspects. Till date, there are only two reported studies of the QTLs for MDA content in wheat. Jiang et al. (2013) identified QTL on wheat chromosome 2B, 5B, 7A, 7B and 7D that controlled MDA content during different seed germination time. These QTL explained 8.00−30.18 % of the phenotypic variation. Zhao et al. (2014) identified QTL on chromosome 1D, 3A, 4D, 6D for MDA content in wheat under potassium-deficiency conditions, accounting for 6.48–11.27 % of the phenotypic variation. In both of these QTL mapping studies, commercial varieties of T. aestivum were used to develop the mapping populations. In our study, however, we used a RIL population derived from an Australia local variety ‘Lang’ and a genotype ‘CSCR6’ which belongs to taxon T. spelta-cultivated wheat that was rarely grown nowadays since 1900s. We expected that T. spelta genotype could provide new genes for MDA content that are different from those identified from T. aestivum in the previous studies. As expected, we firstly reported a major QTL controlling MDA content on the distal end of chromosome 3B under drought and normal water conditions. This QTL explained 31.5 % of the phenotypic variance in the population with a LOD value at 6.48 under WW conditions. Under WD conditions, this QTL explained 39.0 % of the phenotypic variance in the population with a LOD value at 8.49 (Table 2). The allele for low MDA content was from CSCR6. There are no known barriers in generating fully fertile progeny and in transferring genes between T. spelta and T. aestivum. Thus, it would be feasible to incorporate Qheb.mda-3B from T. spelta into breeding programmes of bread wheat to increase drought tolerance of released cultivars.
In the current study, Qheb.mda-3B and Qcrs.cpi-3B were mapped to the same interval on the distal end of the long arm of chromosome 3B and the reducing alleles were all from CSCR6. It is known that collocations of QTLs for different traits could indicate that the genes underlying the QTLs are related by linkage and/or pleiotropy (Lebreton et al. 1995; Agrama and Moussa 1996; Tuberosa et al. 2002; Yan et al. 2011; Chen et al. 2012). To further test the relationship between the genes responsible for the change of MDA content and the genes for FCR resistance, we measured the MDA content of three pairs of NILs which were originally developed for Qcrs.cpi-3B, under WW and WD conditions. These NIL pairs were known to show significant difference for FCR resistance but did not differ in most of other morphology traits (Ma et al. 2012). The results showed that the MDA content between the resistant and susceptible lines of the FCR NIL pairs were similar under WW condition. In contrast, under WD condition, the MDA content was significantly lower in the FCR resistant lines compared with the FCR susceptible lines. The existence of Qheb.mda-3B in these NILs was confirmed. In addition, considering that the three pairs of FCR NILs were developed using gwm181, which also was one of the flanking markers for Qheb.mda-3B, these FCR NILs could also be treated as NIL candidates for Qheb.mda-3B. Therefore, the segregation of Qheb.mda-3B in all of the three pairs of FCR NILs developed for Qcrs.cpi-3B did not only confirm the effects and position of Qheb.mda-3B that we identified in our study, but also indicated that Qheb.mda-3B and Qcrs.cpi-3B were strongly associated. A research conducted by Knight and Sutherland (2013) found that during infection, the FCR pathogen Fusarium pseudograminearum produced penetration structures which appeared as hyphal swellings or septate foot-shaped appressoria to penetrate or grow around the cell membranes. Terashima et al. (2000) reported a substantial up-regulation of fungal genes encoding cell wall depolymerises during infection. As discussed earlier, MDA could damage the plant cell membrane. Thus, it is possible that the genes controlling MDA content could make the plant cell membrane more stable from breakdown by reducing the MDA content produced in plant cell when plants are infected by FCR. As a result, the plants become more resistant to FCR. Together with the results from the QTL mapping and NILs analysis, there is a high possibility that same genes are responsible for FCR resistance and the change of MDA content.
It has been noticed for a long time that drought could greatly favour the spread of FCR. By now, the molecular mechanism of the interaction between drought and FCR is still unclear. As discussed above, the strong association between Qheb.mda-3B and Qcrs.cpi-3B suggested that the same genes are likely to be involved in drought tolerance and FCR resistance in wheat. This hypothesis is consistent with other researchers who also reported the identification of genes with pleiotropic effects on drought tolerance and pathogen disease resistance. For instance, the transgenic wheat lines overexpressing myeloblastosis oncogenes TaPIMP1 were found to display increased resistance to fungal pathogen compared with wild-type control (Zhang et al. 2012). In other crop and model species such as rice, maize, tomato and Arabidopsis, the genes controlling both drought tolerance and pathogen disease resistance were also identified (Alam et al. 2015; Campo et al. 2012; Kamthan et al. 2012; Ramírez et al. 2009). However, to finally determine if Qheb.mda-3B and Qcrs.cpi-3B are tightly linked genes or a single gene with pleiotropic effects, further work such as fine mapping is required. If Qheb.mda-3B and Qcrs.cpi-3B are two tightly linked genes, it is expected that they will be segregated in the fine mapping population.
Abbreviations
- CEF:
-
Controlled environment facility
- MDA:
-
Malondialdehyde
- QTL:
-
Quantitative trait locus
- RILs:
-
Recombinant inbred lines
- WW:
-
Well-watered conditions
- WD:
-
Water deficit conditions
- NILs:
-
Near-isogenic lines
- FCR:
-
Fusarium crown rot
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloracetic acid
- MQM:
-
Multiple QTL model
- IM:
-
Interval mapping
References
Agrama HAS, Moussa ME (1996) Mapping QTLs in breeding for drought tolerance in maize (Zea mays L.). Euphytica 91:89–97
Alam M, Tanaka T, Nakamura H, Ichikawa H, Kobayashi K, Yaeno T et al (2015) Overexpression of a rice heme activator protein gene (OsHAP2E) confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number. Plant Biotech J 13:85–96
Backhouse D, Abubakar AA, Burgess LW, Dennis JI, Hollaway GJ, Wildermuth GB et al (2004) Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia. Australas Plant Path 33:255–261
Bai ZY, Liu CJ (2014) Histological evidence for different spread of Fusarium crown rot in Barley genotypes with different heights. J Phytopathol 163:91–97
Beddis A, Burgess LW (1992) The influence of plant water stress on infection and colonization of wheat seedlings by Fusarium graminearum group 1. Phytopathology 82:78–83
Blaker NS, MacDonald JD (1981) Predisposing effects of soil moisture extremes on the susceptibility of rhododendron to Phytophthora root and crown rot. Phytopathology 71:1–834
Burgess LW, Backhouse D, Summerell BA, Swan LJ (2001) Crown rot in wheat—Chapter 20. In: Summerell BA, Leslie JF, Backhouse D, Bryden WL, Burgess LW (eds) Fusarium—Paul E. Nelson Memorial Symposium. APS Press, The American Phytopathological Society, St Paul
Campo S, Peris PC, Montesinos L, Peñas G, Messeguer J, San SB (2012) Expression of the maize ZmGF14-6 gene in rice confers tolerance to drought stress while enhancing susceptibility to pathogen infection. J Exp Bot 63:983–999
Chen XR, Wang XL, Zhang ZG, Wang YC, Zheng XB (2008) Differences in the induction of the oxidative burst in compatible and incompatible interactions of soybean and Phytophthora sojae. Physiol Mol Plant 73:16–24
Chen GD, Li HB, Zheng Z, Wei YM, Zheng YL, McIntyre CL et al (2012) Characterization of a QTL affecting spike morphology on the long arm of chromosome 3H in barley (Hordeum vulgare L.) based on near isogenic lines and a NIL-derived population. Theor Appl Genet 125:1385–1392
Chowdhury SR, Choudhuri MA (1985) Hydrogen peroxide metabolism as an index of water stress tolerance in jute. Physiol Plant 65:476–480
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exper Bot 45:105–114
Hogg AC, Johnston RH, Dyer AT (2007) Applying real-time quantitative PCR to Fusarium crown rot of wheat. Plant Dis 91:1021–1028
Jiang P, Wan Z, Wang Z, Li S, Sun Q (2013) Dynamic QTL analysis for activity of antioxidant enzymes and malondialdehyde content in wheat seed during germination. Euphytica 190:75–85
Kamthan A, Kamthan M, Azam M, Chakraborty N, Chakraborty S, Datta A (2012) Expression of a fungal sterol desaturase improves tomato drought tolerance, pathogen resistance and nutritional quality. Sci Rep 2:951
Kirigwi FM, Van Ginkel M, Brown-Guedira G, Gill BS, Paulsen GM, Fritz AK (2007) Markers associated with a QTL for grain yield in wheat under drought. Mol Breed 20:401–413
Knight NL, Sutherland MW (2013) Histopathological assessment of wheat seedling tissues infected by Fusarium pseudograminearum. Plant Pathol 62:679–687
Lebreton C, Lazić-Jančić V, Steed A, Pekić S, Quarrie SA (1995) Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J Exp Bot 46:853–865
Li XM, Liu CJ, Chakraborty S, Manners JM, Kazan K (2008) A simple method for the assessment of crown rot disease severity in wheat seedlings inoculated with Fusarium pseudograminearum. J Phytopathol 156:751–754
Liu YX, Yang XM, Ma J, Wei YM, Zheng YL, Ma HX et al (2010) Plant height affects Fusarium crown rot severity in wheat. Phytopathol 100:1276–1281
Liu YX, Zheng YL, Wei YM, Zhou MX, Liu CJ (2012) Genotypic differences in resistance to crown rot caused by Fusarium pseudograminearum in barley (Hordeum vulgare L.). Plant Breed 131:728–732
Maccaferri M, Sanguineti MC, Corneti S, Ortega JLA, Salem MB, Bort J et al (2008) Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics 178:489–511
Ma J, Li HB, Zhang CY, Yang XM, Liu YX, Yan GJ, Liu CJ (2010) Identification and validation of a major QTL conferring crown rot resistance in hexaploid wheat. Theor Appl Genet 120:1119–1128
Ma J, Yan GJ, Liu CJ (2012) Development of near-isogenic lines for a major QTL on 3BL conferring Fusarium crown rot resistance in hexaploid wheat. Euphytica 183:147–152
McIntyre CL, Mathews KL, Rattey A, Chapman SC, Drenth J, Ghaderi M et al (2009) Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions. Theor Appl Genet 120:527–541
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Poole GJ, Smiley RW, Paulitz TC, Walker CA, Carter AH, See DR et al (2012) Identification of quantitative trait loci (QTL) for resistance to Fusarium crown rot (Fusarium pseudograminearum) in multiple assay environments in the Pacific Northwestern US. Theor Appl Genet 125:91–107
Quarrie SA, Quarrie SP, Radosevic R, Rancic D, Kaminska A, Barnes JD et al (2006) Dissecting a wheat QTL for yield present in a range of environments: from the QTL to candidate genes. Environ Exper Bot 57:2627–2637
Rajaram S, Braun HJ, Ginkel M (1996) CIMMYT’s approach to breed for drought tolerance. Euphytica 92:147–153
Ramírez V, Coego A, López A, Agorio A, Flors V, Vera P (2009) Drought tolerance in Arabidopsis is controlled by the OCP3 disease resistance regulator. Plant J 58:578–591
Scandalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:7–12
Smiley RW, Collins HP, Rasmussen PE (1996) Diseases of wheat in long-term agronomic experiments at Pendleton, Oregon. Plant Dis 80:813–820
Tang L, Cai H, Zhai H, Luo X, Wang Z, Cui L, Bai X (2014) Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Cell Tissue Organ Cult 118:1–10
Terashima H, Yabuki N, Arisawa M, Hamada K, Kitada K (2000) Up-regulation of genes encoding glycosylphosphatidylinositol (GPI)-attached proteins in response to cell wall damage caused by disruption of FKS1 in Saccharomyces cerevisiae. Mol Genet Genomics 264:64–74
Trethowan RM, Pfeiffer WH (2000) Challenges and future strategies in breeding wheat for adaptation to drought stressed environments: A CIMMYT wheat program perspective. In: Ribaut JM, Poland D (eds) Molecular approaches for the genetic improvement of cereals for stable production in water-limited environments. A strategic planning workshop held at CIMMYT El Batan, Mexico, 21–25 June 1999. CIMMYT, Mexico DF, pp 45–48
Tuberosa R, Sanguineti MC, Landi P, Giuliani MM, Salvi S, Conti S (2002) Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol Biol 48:697–712
Tuinstra MR, Ejeta G, Goldsbrough PB (1997) Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95:1005–1011
Van Ooijen JW (2004) MapQTL version 5.0, software for the mapping of quantitative trait loci in experimental populations. Kyazma BV, Wageningen
Van Ooijen JW (2006) JointMap 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. Plant Physiol 162:669–677
Voorrips RE (2002) MAPCHART: software for the graphical presentation of linkage maps and QTLs. Heredity 93:77–78
Wang YS, Tian SP, Xu Y, Qin GZ, Yao H (2004) Changes in the activities of pro-and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20° C. Postharvest Biol Technol 34:21–28
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wei J, Li C, Li Y, Jiang G, Cheng G, Zheng Y (2013) Effects of external potassium (K) supply on drought tolerances of two contrasting winter wheat cultivars. PLoS ONE 8:e69737
Wiese MV (1987) Compendium of wheat diseases, 2nd edn. APS Press, The American Phytopathological Society, St Paul
Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR et al (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4:319–330
Yue Y, Zhang M, Zhang J, Tian X, Duan L et al (2012) Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton. J Exp Bot 63:3741–3748
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X (2012) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense and stress-related genes. New Phytol 196:1155–1170
Zhang H, Cui F, Wang H (2014) Detection of quantitative trait loci (QTLs) for seedling traits and drought tolerance in wheat using three related recombinant inbred line (RIL) populations. Euphytica 196:313–330
Zhao Y, Li XY, Zhang SH, Wang J, Yang XF, Tian JC et al (2014) Mapping QTLs for potassium-deficiency tolerance at the seedling stage in wheat (Triticum aestivum L.). Euphytica 198:185–198
Zheng Z, Kilian A, Yan G, Liu CJ (2014) QTL Conferring Fusarium Crown Rot Resistance in the Elite Bread Wheat Variety EGA Wylie. PLoS ONE 9:e96011
Acknowledgments
The authors are grateful to Drs. Chunji Liu and Guijun Yan for their valuable suggestions during the preparation of the manuscript. This research was partially funded by an Australian Research Council (ARC) grant LP120200830.
Conflict of interest
J. Ma, GY. Du, XH. Li, CY. Zhang and JK. Guo declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by L. Xiong.
J. Ma and G. Du equally contributed to this work.
Rights and permissions
About this article
Cite this article
Ma, J., Du, G., Li, X. et al. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol Genet Genomics 290, 1955–1962 (2015). https://doi.org/10.1007/s00438-015-1053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1053-3