Abstract
Starch synthesis is activated in the endosperm during seed development and also in rice suspension cells cultured with abscisic acid. In the anticipation that the mechanisms of starch synthesis are similar between the endosperm and the suspension cells cultured with abscisic acid, expression of genes involved in starch synthesis was evaluated in the suspension cells after abscisic acid treatment. However, it was found that the regulatory mechanism of starch synthesis in the suspension cells cultured with abscisic acid was different from that in developing seeds. Expression analyses of genes involved in oil bodies, which accumulate in the embryo and aleurone layer, and seed storage proteins, which accumulate mainly in the endosperm, showed that the former were activated in the suspension cells cultured with abscisic acid, but the latter were not. Master regulators for embryogenesis, OsVP1 (homologue of AtABI3) and OsLFL1 (homologue of AtFUS3 or AtLFL2), were expressed in the suspension cells at levels comparable to those in the embryo. From these results, it is suggested that interactions between regulators and abscisic acid control the synthesis of phytic acid and oil bodies in the cultured cells and embryo. We suggest that the system of suspension cells cultured with abscisic acid helps to reveal the mechanisms of phytic acid and oil body synthesis in embryo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch, proteins and lipids which are stored in plant seeds are used as nutrient and energy source for human beings. Phytic acid, which is a storage compound of phosphorus, is known as a source of phosphorus pollution in water environment. To control their amount, it is informative to understand regulatory mechanisms of their synthesis.
Starch accumulates in the starchy endosperm from approximately 5 days after flowering (DAF) in rice (Jeon et al. 2010; Zhu et al. 2011). The weight of starch increases in parallel with increasing grain weight and eventually accounts for approximately 75 % of grain weight (Zhu et al. 2011). Seed storage proteins accumulate from 4 DAF and are classified into insoluble glutelin, alcohol-soluble prolamin, salt-soluble globulin and water-soluble albumin (Yamagata et al. 1982; Luthe 1983). The protein content of milled rice is approximately 8 %, and most of it is glutelin in the starchy endosperm (Villareal and Juliano 1978; Shewry and Halford 2002; Agboola et al. 2005). Seed storage proteins also accumulate in the embryo and aleurone layer, but these proteins are different from endosperm proteins (Cagampang et al. 1966). Lipids are stored from approximately 5 DAF as oil bodies consisting of triacylglycerols (TAGs) and surface proteins, oleosin and caleosin, in the embryo and aleurone layer (Choudhury and Juliano 1980; Chuang et al. 1996; Frandsen et al. 2001; Ichihara et al. 2003; Chen et al. 2012). Phytic acid, myo-inositol-1,2,3,4,5,6-hexakisphosphate, is a storage form of phosphorus and accumulates in the embryo and aleurone layer (O’Dell et al. 1972; Ogawa et al. 1979; Iwai et al. 2012).
A previous study using an antibody to abscisic acid (ABA) for inactivating it showed that the synthesis of seed storage proteins and lipids during seed development is affected by ABA activity (Phillips et al. 1997). Moreover, ABA is involved in conferring desiccation tolerance, dormancy and suppression of viviparous phenotype in developing seeds and stress response in vegetative tissues (Finkelstein et al. 2002; Gutierrez et al. 2007; Nakashima and Yamaguchi-Shinozaki 2013). Interactions between ABA and VP1/ABI3 or FUS3 induce the expression of seed storage proteins in Arabidopsis (Suzuki et al. 2003; Kagaya et al. 2005). Accumulation of starch during seed development positively correlates with accumulation of ABA (Yang et al. 2006; Tang et al. 2009). The expression of sucrose synthase, which is considered as a key enzyme of starch synthesis in developing seeds, is regulated by ABA and sucrose (Tang et al. 2009).
Starch synthesis was activated in suspension cells of rice cultured with ABA and sucrose (Akihiro et al. 2005, 2006). Rice cells cultured with ABA also activate phytic acid synthesis similar to that in developing seeds as the expression of genes involved in phytic acid synthesis in developing seeds is also up-regulated in cells cultured with ABA (Matsuno and Fujimura 2014). These results suggest that the suspension cells react with ABA in the same manner as developing seeds.
In the present study, starch synthesis in the suspension cells was investigated in detail to test this hypothesis and showed that starch content in suspension-cultured cells increased with increased concentrations of ABA. The expression analysis of genes involved in starch synthesis in suspension cells cultured with ABA indicated that the regulation of starch synthesis in the suspension cells was different from that in the endosperm. The expression analysis of the gene involved in synthesis of lipids and storage proteins showed that the former was activated in the suspension cells cultured with ABA without activation of the latter. Higher expression levels of master regulators of embryogenesis was observed, suggesting interactions between the master regulators and ABA-activated phytic acid and storage oil synthesis.
Materials and methods
Cultured cells
Rice (Oryza sativa L. ssp. japonica cv. Nipponbare) suspension cells were induced and cultured as described previously (Matsuno and Fujimura 2014). Rice seeds were sterilised by immersion in 70 % ethanol for 1 min and then in sodium hydrochloride (5 % available chloride) for 20 min. After five rinses with sterile deionised water, the seeds were placed on a solid N6 medium (Chu et al. 1975) supplemented with sucrose (30 g/L), proline (10 mM), casein hydrolysate (300 mg/L) and 2,4-dichlorophenoxyacetic acid (2 mg/L) and were incubated at 28 °C for 3 weeks. Friable calli formed were transferred to 15 mL of a liquid N6 medium in a 100-mL flask. The calli were cultured in the dark at 28 °C on a reciprocal shaker (115 strokes per min), and the resulting suspension-cultured cells were subcultured every 5 days.
Cells cultured were passed through a 1.5-mm mesh to remove larger cell masses and then cultured in a fresh medium for 2 days. They were then transferred to a fresh medium after another sieving through a 1.75-mm mesh and cultured for 24 h to minimise the transfer effect. ABA was then added to the medium, and the culture was harvested after various intervals by filtration under reduced pressure. The cells were desiccated at 80 °C to measure starch contents or frozen in liquid nitrogen and stored at −80 °C for gene expression analyses.
Plant materials
Rice plants were grown in the field at the Agricultural and Forestry Research Center (University of Tsukuba, Ibaraki, Japan) under natural environmental conditions from May to September, 2012. Seeds were harvested 7 DAF, immersed in RNAlater (Ambion®) and kept at 4 °C. Dehulled seeds were separated into three parts: embryo, starchy endosperm and aleurone layer. The embryo was first isolated with a pair of tweezers. The seed coat, its inner part rinsed with RNAlater, was harvested as the aleurone layer. The rinse containing the inner part of the seed coat was harvested as the starchy endosperm.
Another rice plant was cultivated hydroponically with Yoshida nutrient solution (Yoshida et al. 1976) in a growth chamber (16 h light at 27 °C). At the six-leaf stage, the fifth leaf was harvested and stored at −80 °C for gene expression analyses.
Quantitative and semi-quantitative RT-PCR
Total RNA was extracted from the suspension cells with the Isogen® reagent (Nippon Gene), from the embryo, the aleurone layer or fifth leaf with the RNeasy Plant Mini Kit (Qiagen) or from the endosperm with the Isogen® reagent and High salt precipitation solution (Nippon Gene). The RNAs were then reverse transcribed using the PrimeScript® RT Master Mix (Takara Bio) following the manufacturer’s instructions after treatment with DNase I.
Expression levels of genes (Tables 1, 2) were quantified by real-time PCR (RT-PCR) using the SYBR® Premix Ex Taq™ II (Takara Bio) and the Thermal Cycler Dice® Real-Time System (Takara Bio). The standard curve method or the 2−ΔΔCT method (Livak and Schmittgen 2001) was applied, and the rice actin 1 gene (OsACT1, AK100267) or ubiquitin 5 gene (OsUBQ5, AK061988) (Jain et al. 2006) was amplified with 5′-CCCAAGGCCAATCGTGAGAAG-3′ and 5′-ACCATCACCAGAGTCCAACACAA-3′ or 5′-AAGGAAGGAGGAGGAAATCG-3′ and 5′-GGGCATCACAATCTTCACAG-3′, respectively, and used as an internal control. Semi-quantitative RT-PCR was performed with Tks Gflex® DNA Polymerase (Takara Bio) for 35 cycles. OsACT1 and OsUBQ5 were used as internal controls.
Measurement of starch content
An aliquot of desiccated and ground cells was immersed in 80 % (v/v) ethanol at 82 °C for 5 min, and then the supernatant was discarded to remove soluble sugars. The treatment was repeated twice. The total starch content of the cells was determined with a total starch assay kit (Megazyme International Ireland Ltd.) by the KOH method following the manufacturer’s instructions.
Results
The accumulation of starch in the suspension cells cultured with ABA
The suspension cells cultured without ABA contained smaller amounts of starch. The starch content increased with increasing concentrations of ABA 24 h after ABA treatment (Fig. 1) by 1.4-, 2.7- and 2.9-fold at 0.17, 17 and 50 µM ABA, respectively, compared with that without ABA. Given that starch accumulation differed little between 17 and 50 µM ABA, 17 µM ABA was used for subsequent experiments.
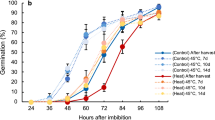
Starch contents in the suspension cells cultured with 17 µM ABA increased 3.1-fold from their initial value 24 h after adding ABA (Fig. 2a) and increased to 7.1-fold by 96 h (Fig. 2a) with a temporary decrease at 90 min (Fig. 2b).
Expression analysis of genes involved in starch synthesis after adding ABA
Expression levels of genes involved in starch synthesis (Table 1) reported by Ohdan et al. (2005) were evaluated in cells cultured with 17 µM ABA. OsGBSSI expression in the suspension cells was not detected by qPCR. Eighteen genes, OsAGPL1, OsAGPL3, OsAGPS1, OsAGPS2a, OsAGPS2b, OsISA2, OsISA3, OsPUL, OsGBSSII, OsBEIIa, OsBEIIb, OsDPE1, OsDPE2, OsSSI, OsSSIIa, OsSSIIb, OsSSIIc and OsSSIVa responded to the addition of ABA (Fig. 3). Ten of these, OsAGPL1, OsAGPL3, OsAGPS1, OsAGPS2a, OsISA2, OsISA3, OsBEIIa, OsDPE1, OsSSI and OsSSIIa, maintained higher expression levels than the control for 24 h after adding ABA, seven, OsABS2b, OsPUL, OsGBSSII, OsBEIIb, OsDPE2, OsSSIIc and OsSSIVa, increased temporarily and OsSSIIb decreased temporarily.
Expression analysis of genes involved in starch synthesis in suspension-cultured cells after addition of ABA. The expression levels of genes were evaluated by qRT-PCR in the suspension cells cultured with 17 μM ABA (represented by triangles and solid lines) or without ABA (represented by diamonds and dotted lines). The expression levels were calculated by the standard curve method. OsACT1 was used as an internal control, and the 0-h value was used as the reference value. Values shown are means and standard deviations of each qPCR (n = 3). Cultivation and sampling of cells were performed in two independent experiments for each treatment
Comparison of the expression of genes involved in starch synthesis in rice suspension-cultured cells with ABA in the endosperm and in silico
Expression patterns of genes involved in starch synthesis were clustered based on the data set RXP_0001 (accession number GEO21396, Gene Expression Omnibus) and were analysed with RiceXPro (Sato et al. 2011, 2013) (NIAS, http://ricexpro.dna.affrc.go.jp/). OsSSIIIa, OsBEIIb, OsBEI, OsAGPS2a/b, OsSSIIa, OsAGPL2, OsGBSSI, OsPUL and OsISA1 constituted a cluster, and the expression of these genes increased rapidly at 5 DAF in the ovary and occurred predominantly in the endosperm (Fig. 4, clade I). The expression pattern was spatially and temporally consistent with starch synthesis in the rice endosperm. Expression levels of those genes were evaluated with ad hoc qRT-PCR and proved higher in the endosperm than the embryo, except for AGPS2a (Fig. 5). Thus, it was inferred that they may play roles in starch synthesis in the endosperm. In the rice suspension cells, 10 genes, OsAGPL1, OsAGPL3, OsAGPS1, OsAGPS2a, OsISA2, OsISA3, OsBEIIa, OsDPE1, OsSSI and OsSSIIa, increased for 24 h after adding ABA (Fig. 3), suggesting that these genes play roles in activated starch synthesis in cells. Only two of these genes, OsAGPS2a and OsSSIIa, were included in clade I and the other eight were excluded from it. These results showed that the set of genes involved in starch synthesis activated in the cells cultured with ABA was different from the set of genes involved in starch synthesis in the endosperm.
Expression levels of genes involved in starch synthesis. Twenty-eight genes were subjected to clustering by RiceXPro (http://ricexpro.dna.affrc.go.jp/) based on dataset RXP_0001 to generate a dendrogram (a). Given that OsAGPS2a and OsAGPS2b, transcripts of the locus Os08g0345800, were not distinguishable by probes used in the microarray analysis, they are described as OsAGPS2a/b. Genes in clusters labelled I, II, III and IV were expressed mainly in the ovary and endosperm, ovary and embryo, embryo and leaf in vegetative stage, respectively. Genes with plus sign (+) in column (b) showed abnormal features in the endosperm when they were mutated (Sano et al. 1986; Kubo et al. 1999; Nishi et al. 2001; Umemoto et al. 2002; Satoh et al. 2003; Lee et al. 2007). Genes with plus sign (+) in column (c) were considered to function in starch synthesis in the endosperm (Ohdan et al. 2005), and genes with plus sign (+) in column (d) were up-regulated in the cultured cells by ABA treatment for 24 h
qRT-PCR analysis of the expression of genes involved in starch synthesis. Immature seeds at 7 DAF were harvested and dissected into three parts: embryo (black bars), aleurone layer (white bars) and endosperm (grey bars). RNAs extracted were analysed by qRT-PCR with primer sets for the same genes as in Fig. 4. The expression levels were calculated by the 2−ΔΔCt method with the references of the embryo tissue and the internal control of OsUBQ5. Values shown are means and standard deviations of each qPCR (n = 3)
RPBF (Kawakatsu et al. 2009), OsbZIP58 (Wang et al. 2013) and RSR1 (Fu and Xue 2010) have been reported as regulators of starch synthesis in the endosperm. To investigate the effects of these regulators on the activation of starch synthesis in the suspension cells cultured with ABA, their expression levels were evaluated (Fig. 6a). OsbZIP58 was expressed at a low level in the cells, but no expression of RPBF was detected. OsbZIP58 and RPBF were accordingly considered to be of little relevance to the activation of starch synthesis in the suspension cells. RSR1, which is a negative regulator, was expressed to some degree in the suspension cells, but the level decreased after ABA addition (Fig. 6a). This result raised the possibility that RSR1 functions in the activation of starch synthesis in the suspension cells.
Expression levels of regulatory genes in three parts of immature seeds and the suspension cells. Seeds at 7 DAF were harvested and dissected into three parts: embryo, aleurone layer and endosperm. RNAs were extracted from them and from the suspension cells cultured with (+) or without (−) 17 μM ABA for 12 h. Expression levels of regulators of starch synthesis (a), seed storage proteins (b), seed oil bodies (c), master regulators (d) and internal control (e) were evaluated by RT-PCR. Numbers of PCR cycles were 26 for OsUBQ5, 28 for OsACT1 and 35 for others. Samples from seed parts were triplicated and from cells were duplicated
Expression of genes involved in seed storage proteins and lipids
As starch and phytic acid were accumulated in the suspension cells cultured with ABA, similar to developing seeds (Akihiro et al. 2005; Matsuno and Fujimura 2014), synthesis of seed storage proteins and lipids in the suspension cells was also expected to be activated by addition of ABA. However, the expression of OsGlb-1 and OsGluB-1a, encoding globulin and glutelin, respectively, (Nakase et al. 1996; Kawakatsu et al. 2008) was not detected in the suspension cells with or without ABA (Fig. 6b). The expression of OsWRI1a/b, which is regarded as a regulator of fatty acid synthesis in rice, OsOLE16, OsOLE18 and OsCAL32, encoding 16 and 18 kDa oleosins composing oil bodies (Chuang et al. 1996) and encoding 32 kDa caleosin (Chen et al. 2012), respectively, were also analysed (Figs. 6c, 7). OsOLE16, OsOLE18 and OsCAL32 were expressed clearly in the suspension cells but weakly in leaves (Fig. 7). After ABA treatment, their expression levels were strongly up-regulated in the suspension cells, and the expression of OsWRI1a/b was up-regulated threefold compared with that of the control.
Expression levels of genes in suspension-cultured cells. RNAs were extracted from three parts of immature seeds at 7 DAF: embryo, aleurone layer and endosperm, suspension cells cultured with (+) or without (−) 17 μM ABA for 12 h and the fifth leaf of young rice plants. Expression levels were evaluated by qRT-PCRs using the 2−ΔΔCt method with the value of ABA (−) as reference. Values shown are means and standard deviations of each qPCR (n = 3)
Master regulators of seed development in the rice suspension cells
In Arabidopsis, four master regulators of embryogenesis, LEC1, LEC2, FUS3 and ABI3, have been well investigated and are known to be involved in the synthesis of seed storage substances (Meinke et al. 1994; Gutierrez et al. 2007). To investigate the ability of master regulators to function in rice cells, expression levels of OsLEC1A (Xie et al. 2008), OsLEC1B (Xie et al. 2008), OsLFL1 (Peng et al. 2007, 2008) and OsVP1 (Hattori et al. 1994), homologues of Arabidopsis master regulators, were evaluated. The expression of OsLEC1A was not detected in the suspension cells (Fig. 6d). OsLFL1 and OsVP1 were expressed at levels comparable with those in the embryo at 7 DAF and were not altered by ABA addition in the suspension cells (Fig. 7). OsLEC1B was expressed at a higher level in the ABA-free medium than in the embryo, and its expression was reduced in the suspension cells by ABA addition (Fig. 7).
Discussion
In the study, starch synthesis in the suspension cells was activated by the addition of ABA as previously reported (Akihiro et al. 2005). A positive correlation between added ABA concentration and starch content was observed in the suspension cells (Fig. 1) as rice kernels matured (Yang et al. 2006; Tang et al. 2009). These results suggest that ABA participates in starch accumulation in both the endosperm and suspension cells.
Developing seeds begin starch accumulation rapidly from approximately 5 DAF in the endosperm and reach the maximum at approximately 21 DAF (Jeon et al. 2010; Zhu et al. 2011). OsAGPL2 (Lee et al. 2007), OsAGPS2b (Lee et al. 2007), OsSSIIa (Umemoto et al. 2002), OsBEI (Satoh et al. 2003), OsBEIIb (Nishi et al. 2001), OsISA1 (Kubo et al. 1999) and OsGBSSI (Sano et al. 1986), which are known to be involved in starch synthesis in the endosperm, constituted a gene cluster in the dendrogram (Fig. 4, cluster I). Genes in the cluster were expressed at higher levels in the endosperm than in the embryo, except for AGPS2a (Fig. 5), and these findings support the assumption that these genes function in starch synthesis in the endosperm (Ohdan et al. 2005). Thus, the genes in the cluster are considered to be also involved in starch synthesis in the endosperm. In the suspension cells cultured with ABA, OsAGPL1, OsAGPL3, OsAGPS1, OsAGPS2a, OsISA2, OsISA3, OsBEIIa, OsDPE1, OsSSI and OsSSIIa were expressed at higher levels than in the control during the 24 h after the addition of ABA (Fig. 3), suggesting that these genes contributed to activated starch synthesis in the suspension cells cultured with ABA. OsbZIP58, which is known as a regulator of starch synthesis in the endosperm, controls gene expression involved in starch synthesis in the endosperm, and starch in the endosperm of an Osbzip58 mutant is altered quantitatively and qualitatively (Wang et al. 2013). OsbZIP58 is expressed specifically in the endosperm at maximum levels at 5–10 DAF (Sato et al. 2011; Wang et al. 2013). In the suspension cells, the expression of OsbZIP58 was low and not altered by ABA treatment (Fig. 6a), suggesting that increased starch synthesis in the suspension cells was not regulated by OsbZIP58. Taking these results together, it is indicated that the regulatory mechanism of starch synthesis in the suspension cells cultured with ABA is different from that in the endosperm.
Most seed storage proteins are classified into glutelin and stored in the endosperm (Villareal and Juliano 1978; Yamagata et al. 1982). OsGlb-1, encoding 26 kDa α-globlin, and OsGluB-1a, encoding glutelin, are expressed specifically in the endosperm and are up-regulated by RPBF and OsbZIP58 synergistically (Wu et al. 1998; Yamamoto et al. 2006; Kawakatsu et al. 2008). The expression analysis showed that the expression levels of OsGluB-1a, OsGlb-1 and RPBF were undetected and that OsbZIP58 expression was detected at a low level in the suspension cells (Fig. 6a, b), suggesting that the seed storage protein was not synthesised in the suspension cells.
Seed storage lipids are stored in the embryo and aleurone layer as oil bodies composed of TAGs surrounded by a single layer of phospholipids and proteins, oleosins and caleosin (Frandsen et al. 2001; Chen et al. 2012). A transcription factor, AtWri1, activates fatty acid synthesis and thereby activates TAG synthesis (Focks and Benning 1998; Cernac and Benning 2004; To et al. 2012). Two homologues of this factor are found in both rice and maize (Pouvreau et al. 2011). As ZmWRI1 activates fatty acid synthesis in maize (Shen et al. 2010), OsWRI1a/b, orthologue of ZmWRI, is presumed to activate fatty acid synthesis in rice. Two isoforms of oleosin, 16 and 18 kDa oleosins, have been detected in the embryo and aleurone layer, and 32 kDa caleosin has been detected in the embryo in rice (Chuang et al. 1996; Qu and Takaiwa 2004; Chen et al. 2012). Expression levels of OsOLE18, OsOLE16 and OsCAL32 were up-regulated by ABA treatment at levels comparable to those in the embryo (Figs. 6c, 7). As the expression of OsWRI1a/b was also up-regulated by ABA treatment (Figs. 6c, 7), oil body synthesis must have been activated in the suspension cells cultured with ABA.
In the rice seed, the accumulation of glutelin is mostly observed in the endosperm along with starch accumulation, and the accumulation of seed storage lipids is observed in the embryo and aleurone layer along with phytic acid accumulation. The regulation of phytic acid synthesis in the suspension cells appeared similar to its regulation in developing seeds (Matsuno and Fujimura 2014). In contrast, with respect to starch synthesis, regulatory mechanisms seemed to be different in the suspension cells and in developing seeds as mentioned above. The combined results suggest that the suspension cells mimic embryo-specific expression and ABA response (Fig. 8). The suggestion would imply the possibility that the epigenetic regulation in the suspension cells was influenced by its origin since the suspension cells was derived from embryo.
Predicted regulation mechanisms for synthesis of storage substance in the immature rice seed and suspension cells. a Phytic acid and oil body synthesis in the rice embryo and suspension cells are presumed to be under the control of OsVP1 and OsLFL1. OsVP1 and OsLFL1 may interact with ABA signals and then regulate embryo-specific ABA responses including fatty acid synthesis and expression levels of oleosin and caleosin genes and phytic acid synthesis. b Starch and seed storage protein synthesis in the endosperm are under the control of RSR1, OsbZIP58 and RPBF. Solid lines represent demonstrated relationships (Suzuki et al. 2003; Yamamoto et al. 2006; Kawakatsu et al. 2008, 2009; Wang et al. 2013), and dotted lines have been assumed here
In the study, expression of OsVP1, a homologue of AtABI3, was observed at a level comparable to that in the embryo at 7 DAF (Fig. 7), a finding also reported by Nakagawa et al. (1996). VP1/ABI3 regulates gene expression and induces seed-specific ABA response (Suzuki et al. 2003). The expression of oleosin gene in the suspension cells of Brassica napus was activated by ABA treatment and overexpression of ABI3 (Crowe et al. 2000). In the rice suspension cells, it is likely that highly expressed OsVP1 (Fig. 7) enables the expression of oleosin genes as a seed-specific ABA response (Fig. 8). OsLEC1A and OsLEC1B are homologues of AtLEC1 (Xie et al. 2008). Microarray data [RiceXPro (Sato et al. 2011)] have shown that OsLEC1A and OsLEC1B were expressed specifically in the endosperm and embryo, respectively. Our results also showed that OsLEC1B was expressed specifically in the embryo (Figs. 6d, 7). Given that OsLEC1B was expressed at high levels in the suspension cells but OsLEC1A was not, the suspension cells resemble the rice embryo in expression specificity (Figs. 6d, 7). OsLFL1 has high similarity to AtFUS3 and AtLEC2 and is expressed in the embryo, callus, anther and pollen (Peng et al. 2007, 2008). Given that AtLEC2 and AtFUS3 regulates fatty acid metabolism via AtWri1 (Baud et al. 2007; Wang and Perry 2013), OsLFL1 also appears to regulate fatty acid metabolism through OsWri1a/b. The interaction of AtFUS3 with ABA regulates the expression of seed-specific or seed-preferential genes (Yamamoto et al. 2010). In addition to the interaction of OsVP1 and ABA, the interaction of OsLFL1 with ABA may enable the suspension cells to display a seed-specific ABA response (Fig. 8).
In the rice suspension cells, starch synthesis was activated by ABA treatment via regulatory mechanisms different from those in the endosperm. The synthesis of oil bodies was suggested to be activated by ABA treatment, but that of seed storage protein was not. The synthesis of phytic acid in the suspension cells has been reported to be activated by ABA (Matsuno and Fujimura 2014). OsVP1 and OsLFL1 were expressed at levels comparable to those in embryo at 7 DAF, and it was accordingly inferred that the suspension cells mimic embryos rather than the endosperm or aleurone layer in ABA response, including the synthesis of phytic acid and oil bodies. In developing seeds, a study focusing on the relationship between master regulators and synthesis of phytic acid or oil bodies is difficult because master regulators affect many aspects of early seed development including the process of embryogenesis, which is accompanied by morphological changes. Further studies using the suspension cells will clarify the regulatory mechanism of OsVP1 or OsLFL1 in the synthesis of phytic acid or oil bodies.
References
Agboola S, Ng D, Mills D (2005) Characterisation and functional properties of Australian rice protein isolates. J Cereal Sci 41:283–290
Akihiro T, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46:937–946
Akihiro T, Umezawa T, Ueki C, Lobna BM, Mizuno K, Ohta M, Fujimura T (2006) Genome wide cDNA-AFLP analysis of genes rapidly induced by combined sucrose and ABA treatment in rice cultured cells. FEBS Lett 580:5947–5952
Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50:825–838
Cagampang GB, Cruz LJ, Espiritu SG, Santiage RG, Juliano BO (1966) Studies on the extraction and composition of rice proteins. Cereal Chem 43:145–155
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Chen DH, Chyan CL, Jiang PL, Chen CS, Tzen JTC (2012) The same oleosin isoforms are present in oil bodies of rice embryo and aleurone layer while caleosin exists only in those of the embryo. Plant Physiol Biochem 60:18–24
Choudhury NH, Juliano BO (1980) Lipids in developing and mature rice grain. Phytochem 19:1063–1069
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, Bi FY (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci China Math 18:659–668
Chuang RL, Chen JC, Chu J, Tzen JT (1996) Characterization of seed oil bodies and their surface oleosin isoforms from rice embryos. J Biochem 120:74–81
Crowe AJ, Abenes M, Plant A, Moloney MM (2000) The seed-specific transactivator, ABI3, induces oleosin gene expression. Plant Sci 151:171–181
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:S15–S45
Focks N, Benning C (1998) wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118:91–101
Frandsen GI, Mundy J, Tzen JTC (2001) Oil bodies and their associated proteins, oleosin and caleosin. Physiol Plant 112:301–307
Fu FF, Xue HW (2010) Coexpression analysis identifies rice starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol 154:927–938
Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C (2007) Combined networks regulating seed maturation. Trends Plant Sci 12:294–300
Hattori T, Terada T, Hamasuna ST (1994) Sequence and functional analyses of the rice gene homologous to the maize Vp1. Plant Mol Biol 24:805–810
Ichihara K, Kobayashi N, Saito K (2003) Lipid synthesis and acyl-CoA synthetase in developing rice seeds. Lipids 38:881–884
Iwai T, Takahashi M, Oda K, Terada Y, Yoshida KT (2012) Dynamic changes in the distribution of minerals in relation to phytic acid accumulation during rice seed development. Plant Physiol 160:2007–2014
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y (2010) Starch biosynthesis in cereal endosperm. Plant Physiol Biochem 48:383–392
Kagaya Y, Okuda R, Ban A, Toyoshima R, Tsutsumida K, Usui H, Yamamoto A, Hattori T (2005) Indirect ABA-dependent regulation of seed storage protein genes by FUSCA3 transcription factor in Arabidopsis. Plant Cell Physiol 46:300–311
Kawakatsu T, Yamamoto MP, Hirose S, Yano M, Takaiwa F (2008) Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. J Exp Bot 59:4233–4245
Kawakatsu T, Yamamoto MP, Touno SM, Yasuda H, Takaiwa F (2009) Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J 59:908–920
Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y (1999) The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol 121:399–410
Lee SK, Hwang SK, Han M et al (2007) Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol Biol 65:531–546
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Luthe DS (1983) Storage protein accumulation in developing rice (Oryza sativa L.) seeds. Plant Sci Lett 32:147–158
Matsuno K, Fujimura T (2014) Induction of phytic acid synthesis by abscisic acid in suspension-cultured cells of rice. Plant Sci 217–218:152–157
Meinke DW, Franzmann LH, Nickle TC, Yeung EC (1994) Leafy cotyledon mutants of Arabidopsis. Plant Cell 6:1049–1064
Nakagawa H, Ohkura E, Ohmiya K, Hattori T (1996) The seed-specific transcription factor VP1 (OSVP1) is expressed in rice suspension-cultured cells. Plant Cell Physiol 37:355–362
Nakase M, Hotta H, Adachi T, Aoki N, Nakamura R, Masumura T, Tanaka K, Matsuda T (1996) Cloning of the rice seed α-globulin-encoding gene: sequence similarity of the 5′-flanking region to those of the genes encoding wheat high-molecular-weight glutenin and barley D hordein. Gene 170:223–226
Nakashima K, Yamaguchi-Shinozaki K (2013) ABA signaling in stress-response and seed development. Plant Cell Rep 32:959–970
Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127:459–472
O’Dell BL, De Boland AR, Koirtyohann SR (1972) Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J Agric Food Chem 20:718–723
Ogawa M, Tanaka K, Kasai Z (1979) Accumulation of phosphorus, magnesium and potassium in developing rice grains: followed by electron microprobe X-ray analysis focusing on the aleurone layer. Plant Cell Physiol 20:19–27
Ohdan T, Francisco PB Jr, Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2007) Ectopic expression of OsLFL1 in rice represses Ehd1 by binding on its promoter. Biochem Biophys Res Commun 360:251–256
Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL (2008) Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J Plant Physiol 165:876–885
Phillips J, Artsaenko O, Fiedler U, Horstmann C, Mock HP, Müntz K, Conrad U (1997) Seed-specific immunomodulation of abscisic acid activity induces a developmental switch. EMBO J 16:4489–4496
Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon JP, Rouster J, Paul W, Rogowsky PM (2011) Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol 156:674–686
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Sano Y, Katsumata M, Okuno K (1986) Genetic studies of speciation in cultivated rice. 5. Inter- and intraspecific differentiation in the waxy gene expression of rice. Euphytica 35:1–9
Sato Y, Antonio BA, Namiki N, Takehisa H, Minami H, Kamatsuki K, Sugimoto K, Shimizu Y, Hirochika H, Nagamura Y (2011) RiceXPro: a platform for monitoring gene expression in japonica rice grown under natural field conditions. Nucleic Acids Res 39:D1141–D1148
Sato Y, Takehisa H, Kamatsuki K, Minami H, Namiki N, Ikawa H, Ohyanagi H, Sugimoto K, Antonio BA, Nagamura Y (2013) RiceXPro version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res 41:D1206–D1213
Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y (2003) Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol 133:1111–1121
Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol 153:980–987
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958
Suzuki M, Ketterling MG, Li QB, McCarty DR (2003) Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol 132:1664–1677
Tang T, Xie H, Wang Y, Lü B, Liang J (2009) The effect of sucrose and abscisic acid interaction on sucrose synthase and its relationship to grain filling of rice (Oryza sativa L.). J Exp Bot 60:2641–2652
To A, Joubès J, Barthole G, Lécureuil A, Scagnelli A, Jasinski S, Lepiniec L, Baud S (2012) WRINKLED transcription factors orchestrate tissue-specific regulation of fatty acid biosynthesis in Arabidopsis. Plant Cell 24:5007–5023
Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y (2002) Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor Appl Genet 104:1–8
Villareal RM, Juliano BO (1978) Properties of glutelin from mature and developing rice grain. Phytochem 17:177–182
Wang F, Perry SE (2013) Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol 161:1251–1264
Wang JC, Xu H, Zhu Y, Liu QQ, Cai XL (2013) OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J Exp Bot 64:3453–3466
Wu C, Adachi T, Hatano T, Washida H, Suzuki A, Takaiwa F (1998) Promoters of rice seed storage protein genes direct endosperm-specific gene expression in transgenic rice. Plant Cell Physiol 39:885–889
Xie Z, Li X, Glover BJ, Bai S, Rao GY, Luo J, Yang J (2008) Duplication and functional diversification of HAP3 genes leading to the origin of the seed-developmental regulatory gene, LEAFY COTYLEDON1 (LEC1), in nonseed plant genomes. Mol Biol Evol 25:1581–1592
Yamagata H, Sugimoto T, Tanaka K, Kasai Z (1982) Biosynthesis of storage proteins in developing rice seeds. Plant Physiol 70:1094–1100
Yamamoto MP, Onodera Y, Touno SM, Takaiwa F (2006) Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes. Plant Physiol 141:1694–1707
Yamamoto A, Kagaya Y, Usui H, Hobo T, Takeda S, Hattori T (2010) Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis. Plant Cell Physiol 51:2031–2046
Yang J, Zhang J, Wang Z, Liu K, Wang P (2006) Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J Exp Bot 57:149–160
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Routine procedure for growing rice plants in culture solution. Laboratory manual for physiological studies of rice, 3rd edn. International Rice Research Institute, Los Baños, pp 61–66
Zhu G, Ye N, Yang J, Peng X, Zhang J (2011) Regulation of expression of starch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets. J Exp Bot 62:3907–3916
Acknowledgments
We thank Morio Kato for providing the developing rice seeds from Agricultural and Forestry Research Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. K. Tyagi.
Rights and permissions
About this article
Cite this article
Matsuno, K., Fujimura, T. Do rice suspension-cultured cells treated with abscisic acid mimic developing seeds?. Mol Genet Genomics 290, 1551–1562 (2015). https://doi.org/10.1007/s00438-015-1018-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1018-6