Abstract
The retromer coat complex is a vital component of the intracellular trafficking mechanism sorting cargo from the endosomes to the trans-Golgi network or to the cell surface. In recent years, genes encoding components of the retromer coat complex and members of the vacuolar protein sorting 10 (Vps10) family of receptors, which play pleiotropic functions in protein trafficking and intracellular/intercellular signaling in neuronal and non-neuronal cells and are primary cargos of the retromer complex, have been implicated as genetic risk factors for sporadic and autosomal dominant forms of several neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease and frontotemporal lobar degeneration. In addition to their functions in protein trafficking, the members of the Vps10 receptor family (sortilin, SorL1, SorCS1, SorCS2, and SorCS3) modulate neurotrophic signaling pathways. Both sortilin and SorCS2 act as cell surface receptors to mediate acute responses to proneurotrophins. In addition, sortilin can modulate the intracellular response to brain-derived neurotrophic factor (BDNF) by direct control of BDNF levels and regulating anterograde trafficking of Trk receptors to the synapse. This review article summarizes the emerging data from this rapidly growing field of intracellular trafficking signaling in the pathogenesis of neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent molecular and genomic studies suggest that misprocessing and missorting of intracellular proteins within endosomal–lysosomal pathways are key pathological mechanism in several age-related neurodegenerative diseases including Alzheimer’s disease (AD), frontotemporal lobar degeneration (FTLD) and Parkinson’s disease (PD). Numerous genome-wide association studies (GWAS) and biochemical studies have identified core components of the retromer (VPS35 and VPS26) regulating endosomal sorting, and members of the vacuolar protein sorting-10 (Vps10) family of receptors (i.e., SORT1, SORL1, SORCS1, SORCS2 and SORCS3) that are primary cargos of the retromer, as risk factors for neurodegenerative diseases highly prevalent in the elderly, consistent with the notion that dysfunction within these pathways is a major contributing factor to disease development and progression. This article reviews the physiological actions of the retromer complex and the Vps10 receptor family in intracellular sorting pathways and summarizes the rapidly growing evidence underlying the retromer-based pathogenesis of neurodegenerative disease.
The retromer coat complex
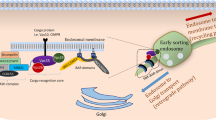
The retromer complex was first identified in the yeast Saccharomyces cerevisiae and was shown to mediate retrograde endosome-to-Golgi retrieval of the carboxy peptidase Y (CPY) receptor Vps10p. The complex, which sorts and traffics cargo from endosomes to the trans-Golgi network (TGN) or to the cell surface (Seaman 2012), comprises in yeast five proteins that are all encoded by vacuole protein sorting (VPS) genes: a trimeric core of VPS proteins, Vps35–Vps29–Vps26 binding a dimer of ‘sorting nexin’ (SNX) proteins Vps5 and Vps17 (Horazdovsky et al. 1997; Seaman et al. 1997, 1998). The Vps5 and Vps17 SNXs contain Bin/Amphiphysin/Rvs (BAR) domains (SNX-BAR proteins) that can induce and/or sense the formation of membrane tubules (Carlton et al. 2004; van Weering et al. 2012). The trimeric complex of Vps26, Vps29 and Vps35 does not have intrinsic membrane-binding activity and relies on association with the Rab7 ortholog, Ypt7, for recruitment to the vacuole membrane (Liu et al. 2012; Vardarajan et al. 2012; Harrison et al. 2014). In addition, binding of the core retromer to endosomes is also mediated through binding to SNX3 (Harterink et al. 2011).
While SNX proteins differ significantly between species, the Vps35–Vps29–Vps26 heterotrimer is highly conserved (Koumandou et al. 2011) and, therefore, considered to be the core functional component. It has been shown to recognize cargo proteins and is, therefore, named the cargo-selective complex (CSC) (Seaman et al. 1998). Human VPS35 is composed of 17 two-helix repeats folding into an α-solenoid typical of vesicle coat proteins (Hierro et al. 2007). Mammals express two Vps26 orthologs, VPS26A and VPS26B (Kerr et al. 2005) possessing an arrestin fold and binding to the highly conserved amino-terminal region of VPS35 (Shi et al. 2006; Collins et al. 2008) (Fig. 1). VPS29 possesses a non-functional metallophosphoesterase fold (Collins et al. 2005) and binds to the carboxy-terminal portion of VPS35 (Collins et al. 2008) where it is proposed to scaffold the helical solenoid of VPS35. Variably, the mammalian Vps35–Vps29–Vps26 core can form a complex with the homologues of Vps5p, namely SNX1 or SNX2 forming dimers with a Vps17p orthologue (either SNX5 or SNX6). In addition, the mammalian retromer complex binds other proteins or protein complexes, such as the Wiskott-Aldrich protein and SCAR homolog (WASH) complex (Gomez and Billadeau 2009; Harbour et al. 2010; Burd and Cullen 2014), which modifies its precise function in trafficking cargo out of endosomes (Fig. 1). Increasing retromer abundance suppresses degenerative phenotypes of mutations that affect the endolysosomal system (Wang et al. 2014).
SNX-BAR-retromer-mediated cargo exit from lysosomal-mediated degradation. The VPS26:VPS29: VPS35 CSC engages cargo in a signal-dependent manner. Through WASH-mediated actin polymerization a cargo-enriched endosome subdomain is assembled thereby avoiding ESCRT-mediated sorting into forming intraluminal vesicles (Strochlic et al. 2008). Subsequent transport into recycling pathways back to the plasma membrane or retrograde transport to the TGN is mediated through retromer SNX-BAR-mediated tubule formation. Actin polymerization may aid further membrane remodeling and the efficiency of tubule scission to form a cargo-enriched tubular transport carrier. [ESCRT = endosomal sorting complexes required for transport (ESCRT) machinery]. Figure adapted from Burd et al. (2014), with permission from Society for Neuroscience
Cargo recognition
The mechanisms by which the CSC recognizes cargo are largely unclear. Some retromer cargoes possess at least one simple hydrophobic motif, F/W-L-M/V, required for retromer-dependent sorting (Seaman 2007). Vps35 has long been thought to provide the only site for cargo recognition. However, in recent years, VPS26 was shown to bind a FANSHY sorting signal in the cytoplasmic domain of sorLA, (Fjorback et al. 2012) and several additional proteins binding to CSC including SNX1, SNX2, SNX5 and SNX6 forming the SNX-BAR sub-complex as well as SNX3 and SNX27 have also been implicated in cargo recognition, (Parks et al. 2001; Heydorn et al. 2004; Strochlic et al. 2007, 2008; Harterink et al. 2011; Temkin et al. 2011; Steinberg et al. 2013) suggesting that there are various direct and indirect mechanisms by which CSC recognizes cargo.
The SNX-BAR sub-complex
Sorting nexins SNX1, SNX2, SNX5 and SNX6 forming the SNX-BAR sub-complex are recruited to cargo-containing endosomes through a phosphatidylinositol 3-monophosphate (PtdIns(3)P)-binding Phox homology (PX) domain, and use the carboxy-terminal Bin-amphiphysin-Rvs (BAR) domain to drive membrane deformation and to generate membrane tubules (Cullen 2008). In recruiting the cargo-selective sub-complex to the forming tubules, the SNX-BAR coat complex is thought to mediate retrograde transport between endosomes and the trans-Golgi network (TGN) through tubular-based endosomal trafficking (Cullen 2008).
Additional cargo-specific sub-complexes
Over the past 2 years, additional cargo-specific adaptors have been identified that mediate recycling by capitalizing on the SNX-BAR-retromer pathway. In yeast, the sorting nexin Grd19/Snx3p9 sub-complex recycles the Fet3p-Ftr1p reductive iron transporter through recognition of the cytoplasmic domain of Ftr1p and binding to the SNX-BAR-retromer allowing recycling of the Fet3p-Ftr1p back to the plasma membrane via the Golgi apparatus (Strochlic et al. 2007). In addition, two novel WD-40 domain proteins, Ere1 and Ere2 (Endosomal Recycling proteins) appear to function as adaptors for SNX-BAR-retromer-mediated sorting. In mammalian cells SNX27, a PDZ domain-containing SNX, functions as an adaptor for recycling of the β2-adrenergic receptor (β2AR) through SNX-BAR-retromer decorated tubules (Temkin et al. 2011). SNX27 associates with the SNX-BAR-retromer through binding to the WASH complex and mediates recycling of β2AR through a direct Rab4-dependent endosome-to-plasma membrane pathway (Lauffer et al. 2010; Temkin et al. 2011). SNX27 also regulates the endosomal sorting of other PDZ ligand-containing transmembrane spanning proteins including 5-hydroxytryptamine type 4 receptor (5-HT4R), (Joubert et al. 2004) the G protein-gated inward rectifying potassium (GIRK, or Kir3) channels, (Lunn et al. 2007; Balana et al. 2011) and the NMDA receptor subunit NR2C (Cai et al. 2011). As SNX27 also contains a FERM-like domain, it may further act as an adaptor for NPxY motif-containing cargoes.
Accessory proteins for SNX-BAR-retromer
In addition to cargo-specific adaptors, the CSC and SNX-BAR binding sites recruit a number of accessory proteins that further stabilize the growth and formation of retromer tubules. Among these is EHD1 belonging to the carboxy-terminal Eps15 homology domain (EHD) family (Gokool et al. 2007) showing similarities to dynamin GTPases (Daumke et al. 2007) and capable of assembling into oligomeric structures, inducing liposome tubulation in vitro, and hydrolyzing ATP (not GTP) (Naslavsky and Caplan 2011). Binding of EHD1 to SNX-BAR-retromer is required to stabilize tubule formation possibly by assisting further sculpturing of the maturing tubule (Gokool et al. 2007). In addition, ATP hydrolysis may induce conformation changes that lead to scission of SNX-BAR-retromer tubules (Daumke et al. 2007). As EHD proteins also associate with proteins containing the tripeptide NPF motif, EHD1 may recruit additional proteins required for further cargo capture and/or processing of the SNX-BAR-retromer tubules (Naslavsky and Caplan 2011).
For the SNX-BAR-retromer further membrane remodeling including membrane scission is connected to the underlying actin and microtubule cytoskeleton. SNX5 and SNX6 bind to the p150glued component of dynactin thereby coupling to the microtubule motor dynein (Hong et al. 2009; Wassmer et al. 2009). Through a decrease in the efficiency of tubule scission, uncoupling from dynein leads to extended SNX-BAR-retromer tubules (Wassmer et al. 2009).
A link with actin polymerization is provided through the association of the CSC to the WASH complex (Gomez and Billadeau 2009). WASH is a member of the WASP family regulating actin-nucleating properties of the Arp2/3 complex (Campellone and Welch 2010) and is associated with a regulatory complex composed of FAM21, SWIP, strumpellin and CCDC53. It interacts with the VPS26-VPS29-VPS35 CSC sub-complex through binding of VPS35 to FAM21 and additional interactions between VPS35 and SNX1/SNX2 to WASH and FAM21 (Gomez and Billadeau 2009). Knock down of WASH leads to the formation of elongated SNX-BAR-retromer tubules. The WASH complex also associates with the scission factor dynamin- II, and CAPZ, a capping protein for the barbed ends of actin filaments. This leads to the generation of longitudinal force by promoting branching (Gomez and Billadeau 2009; Jia et al. 2010). Thus, the SNX-BAR-retromer assembles a motor-dependent pulling force on the tubule and a pushing force on the endosomal vacuole generated by a localized burst of actin polymerization. These two opposing forces appear to combine to enhance the efficiency of membrane scission by increasing membrane tension. For fusion with the recipient compartment, the carrier must undergo uncoating. In the SNX-BAR-retromer pathway, this may be achieved by binding of VPS26-VPS29-VPS35 to TBC1D5 (Seaman et al. 2009).
The role of retromer in neurodegenerative disease
Over the past couple of years evidence accumulated that disturbed CSC function is causally involved in several neurodegenerative disorders highly prevalent in the elderly. The retromer was first linked to neurodegenerative disease by model-guided microarray analysis of the dentate gyrus and entorhinal cortex from AD tissue (Small et al. 2005) which showed that protein levels of the CSC components Vps35 and Vps26 are reduced within the entorhinal cortex subregion which is particularly vulnerable to AD (Small et al. 2005). In addition, a recent study suggests SNX1, SNX3 and RAB7A, which are essential for membrane association of the retromer, as possible AD risk genes and a role of SNX3 and RAB7A in membrane association of the retromer through interaction with the CSC (Vardarajan et al. 2012). There is evidence from some in vitro and in vivo models that the retromer negatively regulates Aβ production, with Vps35-deficient mice exhibiting increased Aβ40 and Aβ42 production (Muhammad et al. 2008; Wen et al. 2011). However, other models have suggested that retrograde trafficking is required for efficient Aβ40 production (Sullivan et al. 2011; Choy et al. 2012). Interestingly, Sullivan et al. (2011) observed increased secretion of APP CTFs via exosomes, suggesting that retromer dysfunction might redirect trafficking of APP CTFs into exosomes leading to an alternative pathway for secretion of APP fragments and a potential source of extracellular Aβ. Several mutations in retromer assembly and associated genes have also been associated with late-onset PD. A mutation in VPS35 (D620 N (c.1858G > A)) showing incomplete, age-associated penetrance disrupts the trafficking of cathepsin D, a CI-M6PR ligand and protease responsible for degradation of α-synuclein, (Follett et al. 2014) and leads to poor association with the WASH complex impairing WASH recruitment to endosomes leading to abnormal trafficking of the autophagy protein ATG9A and thereby autophagy dysfunction (Zavodszky et al. 2014). In a viral-mediated gene transfer rat model, (Tsika et al. 2014) the expression of VPS35 D620N induced the marked degeneration of substantia nigra dopaminergic neurons and axonal pathology, cardinal pathological hallmarks of PD. A recent experimental study further demonstrated that loss of SNX27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome (Wang et al. 2013). A mutation in the receptor-mediated endocytosis 8 gene (RME8), DNAJC13 (p.Asn855Ser) regulating the dynamics of clathrin coats on early endosomes confers a toxic gain-of-function and impairs endosomal transport (Vilarino-Guell et al. 2014). PD-associated mutations in RAB7L1 or LRRK2 lead to endolysosomal and Golgi apparatus sorting defects and deficiency of the VPS35 component of the retromer complex (MacLeod et al. 2013). Expression of wild-type VPS35, but not a familial PD-associated mutant form, rescues these defects. Additional mutations that have been reported, but are at present not considered established risk or susceptibility variants for late-onset PD include R524W, P316S and p.E787 K in VPS35 (Nuytemans et al. 2013), p.K93E in VPS26A and p.N72H in VPS29 (Table 1) (Vilarino-Guell et al. 2011; Zimprich et al. 2011; Lesage et al. 2012; Chen et al. 2013; Shannon et al. 2014). The locus for human VPS26A has also been genetically associated with type 2 diabetes (T2D) in South Asians (Kooner et al. 2011). T2D is a confirmed risk factor for AD (Ott et al. 1996; Luchsinger et al. 2001).
The vps10 receptor family
Yeast VPS10p, the first retromer cargo identified, has five mammalian homologs. These five members of the Vps10 receptor family are type 1 transmembrane proteins characterized by a Vps10 homology domain within the N terminus acting as a site for ligand binding and canonical internalization, and sorting motifs within the cytoplasmic tails mediating rapid internalization and intracellular sorting of ligands (Jacobsen et al. 2001; Nielsen et al. 2001). They are abundantly expressed in the brain with differential distribution in hippocampal subregions, and are induced by neuronal activity (Hermey et al. 2001, 2004).
Sorl1
SORL1 was the first Vps10 receptor identified as genetically associated with sporadic late-onset AD (Table 2), (Rogaeva et al. 2007) and over the past 2 years SORL1 mutations have also been implicated in familial AD (Pottier et al. 2012). Numerous studies have replicated the association of SORL1 with AD in different datasets, and a recent comprehensive meta-analysis of the performed candidate gene studies (Reitz et al. 2011a) as well as a GWAS by the International Genomics of Alzheimer’s Project (IGAP) in over 75,000 subjects (Lambert et al. 2013) confirmed that multiple SORL1 variants are associated with AD. Consistent with this notion, SORL1 transcripts are decreased in the brains of patients with mild cognitive impairment (Sager et al. 2007) and AD, (Dodson et al. 2006) and genetic variants in SORL1 have also been associated with AD endophenotypes including age of onset of AD, white matter hyperintensities, hippocampal atrophy, CSF Aβ42 levels, cognitive function and SORL1 expression in the brain (Seshadri et al. 2007; Kolsch et al. 2008, 2009; Grear et al. 2009).
In vitro and in vivo studies have demonstrated that SorL1 is required for endosome-to-TGN trafficking of APP (Vieira et al. 2010; Fjorback et al. 2012) (Fig. 2). Disruption of the Vps26 binding motif within the SorL1 cytoplasmic tail results in increased localization of APP to endosomal compartments and increased amyloidogenic processing of APP to produce Aβ (Fjorback et al. 2012). SorL1 has also been demonstrated to regulate exit of APP from the TGN, (Schmidt et al. 2007) exit of APP from early endosomal compartments (Offe et al. 2006) and oligomerization of APP which regulates its affinity for the secretases (Lao et al. 2012; Schmidt et al. 2012).
APP sorting and processing. i From the TGN, APP is—dependent on the AP4 adaptor protein complex—either sorted to the plasma membrane (secretory pathway) or into clathrin-coated vesicles entering the endosomal pathway. Within the secretory pathway, APP is cleaved at the plasma membrane by the α-secretases into soluble APPα (sAPPα) and a membrane-bound αCTF. The αCTF is subsequently cleaved by γ-secretase generating the p3 fragment and the APP intracellular domain (AICD). ii At the plasma membrane, some unprocessed APP escapes α-secretase cleavage and is internalized into the endosomal pathway. Within low pH endosomal compartments, APP is cleaved by β-secretase resulting in generation of sAPPβ and the βCTF. Cleavage of the βCTF by γ-secretase generates Aβ peptides of varying lengths and the AICD. iii, iv. Via the retromer complex and its receptors, the Vps10 family members, APP is recycled from the early endosomal compartments to the TGN. Figure adapted from Lane et al. (2012) with permission from Society for Neuroscience
The neuronal retromer, while not present within long-range moving vesicles, is nonetheless required for long-range retrograde transport of APP I-containing vesicles (Bhalla et al. 2012). Using cultured hippocampal neurons, it has been demonstrated that the CSC components Vps35 and Vps26, partially colocalize with SorL1 and APP to distinct puncta that are positive for early endosome markers and are localized within neuronal processes (Bhalla et al. 2012). Under Vps35 knockdown conditions, APP long-range transport is reduced, resulting in a more static behavior of APP-positive vesicles, indicating that Vps35 may also be required for the regulated exit of APP from early endosomes in distal processes. This block of APP exit from early endosomes parallels an increase in endosomal size and Aβ production (Bhalla et al. 2012), potentially consistent with previous observations of enlarged endosomes during the earliest stages of AD before amyloid deposition (Cataldo et al. 1997, 2000, 2004) and in patient-derived stem cells (Qiang et al. 2011; Israel et al. 2012). In summary, disruption of the retromer-SorL1 interaction seems to impact APP metabolism and Aβ production by affecting endosome-to-TGN trafficking of APP, (Vieira et al. 2010; Fjorback et al. 2012) exit of APP from the TGN, exit of APP from early endosomal compartments and oligomerization of APP; (Muhammad et al. 2008; Vieira et al. 2010; Fjorback et al. 2012) and retromer dysfunction seems to increase clustering of APP and Aβ production in early endosomes.
SorLA also binds glial cell line-derived neurotrophic factor (GDNF) in complex with its receptor GFRalpha1, mediates their uptake and subsequent intracellular sorting, and may therefore play a crucial role in the regulation of GDNF activity (Glerup et al. 2013).
Sorcs1
Also SORCS1 was identified as a potential risk factor for AD (Liang et al. 2009) and implicated in retromer-related regulation of APP metabolism and Aβ generation (Lane et al. 2010; Reitz et al. 2011c). Several groups reported genetic associations of SORCS1 SNPs with LOAD (Liang et al. 2009; Laumet et al. 2010; Reitz et al. 2011c; He et al. 2012; Wang et al. 2012; Xu et al. 2013) or changes in memory retention in various ethnic groups (Table 2) (Reitz et al. 2011b). In several of these studies, the associations were strongest in females. Complexes containing SorCS1, APP, Vps35 and SorL1 were isolated from mouse brain tissue, and Vps35 and SorL1 protein levels were reduced in the brains of Sorcs1-deficient mice, suggesting disruption of the retromer in the absence of SorCS1 (Lane et al. 2010). Analysis of APP metabolites and Aβ in the brains of female Sorcs1-deficient mice revealed increased processing of endogenous APP as shown by elevated levels of α/β CTF and Aβ production (Lane et al. 2010). In cultured cells, SorCS1 interacts with APP and regulates Aβ generation, (Lane et al. 2010; Reitz et al. 2011c) potentially through an impact on γ-secretase processing of APP (Reitz et al. 2011c).
Interestingly, SORCS1 was also identified as a quantitative trait locus for T2DM in rats and mice (Clee et al. 2006; Granhall et al. 2006) and identified in GWAS as a risk factor for type 1 diabetes (Paterson et al. 2010) and T2DM (Goodarzi et al. 2007). While the mechanism through which SORCS1 contributes to T2DM remains uncharacterized, the interaction with the retromer provides a potential point of junction between AD and T2DM, not only for SorCS1 but also for other Vps10 receptors including sortilin and SorL1. Sortilin is involved in trafficking of Glut4-containing, insulin-responsive vesicles (IRVs), and a screen to identify additional components of IRVs identified both SorL1 and Vps35 in rat adipocytes (Jedrychowski et al. 2010). Recycling of Glut4-containing IRVs from endosomal compartments to the TGN is dependent on retrograde trafficking pathways and is essential for correct Glut4 trafficking in response to insulin (Vassilopoulos et al. 2009). As described above, genetic data now also point toward the retromer in T2DM with VPS26a recently identified as a novel susceptibility locus (Kooner et al. 2011). Finally, genetic variation in SORCS1 has also been associated with attention deficit hyperactivity disorder (ADHD) (Lionel et al. 2011).
SORCS2 and SORCS3
There is evidence from candidate gene studies that also genetic variation in SORCS2 and SORCS3 is associated with AD (Table 2) (Rogaeva et al. 2007; Reitz et al. 2013). A recent study by Reitz et al. (2013) further suggests that there may be additive epistatic effects of genetic variants in SORCS1, SORCS2 and SORCS3 on AD risk. These observations are in line with the notion that knockdown of these genes using short hairpin RNAs in HEK293 cells causes a significant increase in APP processing (Reitz et al. 2013). Variation in SORCS2 has also been associated with changes in temporal brain structure, (Kohannim et al. 2012) bipolar disorder and schizophrenia (Christoforou et al. 2011) and body mass index, (Wei et al. 2012) and variation in SorCS3 is associated with ADHD (Lionel et al. 2011).
The mechanisms by which SORCS2 and SORCS3 exert its effects remain poorly understood. Hippocampal expression of SorCS3 is induced by neuronal activity (Hermey et al. 2004, 2013). This activity-dependent transcriptional induction is transient and independent of new protein synthesis, a characteristic of immediate early genes (Loebrich and Nedivi 2009). Therefore, SorCS3 may play a role in plasticity-related events in the nervous system. This notion is supported by additional studies. Expression of a constitutively active form of cAMP response element-binding protein (CREB), a transcription factor with established function in synaptic plasticity, results in upregulation of Npas4 and SorCS3 transcripts (Benito et al. 2011). Moreover, SorCS3 expression is misregulated in the absence of Npas4, (Lin et al. 2008) a transcription factor that regulates the formation and maintenance of inhibitory γ-aminobutyric acid (GABA)ergic synapses in response to excitatory synaptic activity on hippocampal neurons (Lin et al. 2008; Coutellier et al. 2012). Recently, it has been reported that long-term depression (LTD), i.e., a longlasting reduction in synaptic transmission, is impaired in SorCS3 knockout mice and that these mice display defects in spatial learning and memory and show increased fear extinction (Breiderhoff et al. 2013).
Sortilin
Sortilin has been implicated in AD (Mufson et al. 2010; Finan et al. 2011), FTD (Hu et al. 2010), several psychiatric disorders including depression and bipolar disorder (Dube et al. 2011; Nykjaer and Willnow 2012) and cardiovascular (Musunuru et al. 2010) and aortic anorysmal (Saratzis and Bown 2014) disease. In the AD brain, increased sortilin expression (Finan et al. 2011) together with a positive correlation between temporal cortex sortilin levels and severity of pathology has been reported (Mufson et al. 2010). In vitro, sortilin interacts with BACE1 and appears to positively regulate BACE1 cleavage of APP and Aβ production. Deletion of the sortilin intracellular domain possessing the putative retromer binding domain results in increased endosomal localization of sortilin and BACE1 (Finan et al. 2011).
Sortilin is causally liked to FTD as a receptor for progranulin in neurons affecting the uptake and targeted delivery of progranulin to the endosomal–lysosomal pathway (Hu et al. 2010). In line with this notion, candidate gene and GWA studies identified SORT1 as a regulator of plasma progranulin levels (Carrasquillo et al. 2010; Nicholson et al. 2014b). Progranulin localizes extensively with sortilin at the plasma membrane with binding dependent on the sortilin β-propeller domain (Hu et al. 2010). In vitro and in vivo models demonstrated that sortilin regulates extracellular progranulin levels through endocytosis and lysosomal accumulation of progranulin (Hu et al. 2010). Deletion of sortilin results in a 2.5- to 5-fold increase in progranulin levels and reversal of progranulin deficiency in the GRN +/− FTD model.
Role of vps10 receptors in proneurotrophin signaling
In addition to their abovementioned actions, several of the VPS10 receptors are also involved in the regulation of proneurotrophin signaling. The outgrowth of dendrites and axons and thereby the formation of neuronal networks in the developing nervous system are influenced by several factors (McAllister et al. 1995; Cohen-Cory et al. 2010). Among these are neurotrophins, which include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and NT-4, which are required for neuronal survival and differentiative, synapse formation, and synaptic plasticity (Snider 1994). These secreted proteins act through two classes of receptors: the tropomyosin-related kinase (Trk) receptor and the p75 neurotrophin (p75NTR)–sortilin receptor. Neurotrophins including NGF and BDNF are synthesized as precursor proteins (proneurotrophins) that are cleaved of their prodomains during maturation. However, following injury, or in neurodegenerative disease, pro-NGF is induced. Numerous studies have indicated that pro-NTs (NGF and BDNF) bind the p75NTR–sortilin complex to signal proapoptotic pathways while mature NTs bind the Trk receptor complexes to signal growth cone tuning, extension, and neuronal survival (Teng et al. 2010). While sortilin functions directly in apoptotic signaling pathways when complexed with p75NTR, it is also involved in anterograde trafficking of the Trk receptors from the soma to the nerve terminal thereby positively regulating neurotrophin signaling and cell survival (Vaegter et al. 2011), and regulation of BDNF levels through influencing both anterograde and lysosomal trafficking (Chen et al. 2005; Evans et al. 2011). Disruptions in NGF and BDNF signaling have been demonstrated to contribute to AD pathology. Aberrant processing of pro-NGF and/or altered axonal trafficking resulting in an imbalance of pro-NGF to mature NGF has been implicated in disease progression and decreased BDNF has been reported in AD.
Also SorCS2 binds to pro-NGF, thereby mediating acute collapse of growth cones of hippocampal neurons (Deinhardt et al. 2011). This retraction is initiated by an interaction between the p75 neurotrophin receptor (p75NTR) and SorCS2. Binding of pro-NGF to the p75NTR–SorCS2 complex induces growth cone retraction by initiating the dissociation of the guanine nucleotide exchange factor Trio from the p75NTR–SorCS2 complex, resulting in decreased Rac activity and, consequently, growth cone collapse. The actin-bundling protein fascin is also inactivated, contributing to the destabilization and collapse of actin filaments. Collectively, these results suggest dual synchronized mechanisms by which pro-NGF mediates acute neuronal remodeling. This increase in p75NTR in injured neurons and the increase in pro-NGF in AD suggest that SorCS2/p75NTR may play a role in disease pathogenesis. SorCS3 has also been reported to bind NGF (Westergaard et al. 2005), but this interaction remains to be further characterized.
SorLA binds glial cell line-derived neurotrophic factor (GDNF) in complex with its receptor GFRalpha1, and mediates their uptake and subsequent intracellular sorting. It may, therefore, play a crucial role in the regulation of GDNF activity (Glerup et al. 2013).
Conclusions
Over the past few years, significant evidence from genetic and cell biology studies has accumulated implicating intracellular trafficking as a key mechanism in the generation of toxic protein species involved in a number of age-related neurodegenerative diseases. In addition to these “end-stage” phenotypes, genetic and cell biology studies have now also correlated retromer function and proteins involved in intracellular trafficking with intermediate disease endophenotypes highly prevalent in the elderly, including hippocampal atrophy, memory retention or white matter hyperintensities. Evidence suggests that the Vps10 family of receptors regulates trafficking of proteins central to several neurodegenerative diseases within endosomal–lysosomal compartments through their interaction with the retromer complex which itself is implicated in AD, PD, and T2DM. Importantly, several of the Vps10 family members additionally regulate neurotrophic survival and apoptotic signaling pathways.
In considering retromer assembly or accessory proteins as targets for drug discovery, the exact mechanisms by which these pathways lead to age-related neurodegeneration need to be clarified. While there are several potential scenarios, studies showing that increasing retromer levels and interaction between individual retromer proteins stabilize the retromer core complex and enhance retromer-mediated trafficking and transport (Small et al. 2005; MacLeod et al. 2013), suggest that retromer function is especially important in clearing ‘toxic proteins’ from the cell. In line with this notion, a recent study seeking to identify potential pharmacological chaperones stabilizing retromer to limit APP processing identified a molecule that stabilized retromer against thermal denaturation, increased the levels of retromer proteins, shifted APP away from the endosome, and decreased the pathogenic processing of APP (Mecozzi et al. 2014). These findings suggest that retromer stability affects its function and that pharmacological chaperones can stabilize this function and may have potential therapeutic implications (Mecozzi et al. 2014).
References
Alexopoulos P, Guo LH, Tsolakidou A, Kratzer M, Grimmer T, Westerteicher C, Jiang M, Bujo H, Diehl-Schmid J, Kurz A, Perneczky R (2012) Interrelations between CSF soluble AbetaPPbeta, amyloid-beta 1-42, SORL1, and tau levels in Alzheimer’s disease. J Alzheimers Dis 28:543–552
Ando M, Funayama M, Li Y, Kashihara K, Murakami Y, Ishizu N, Toyoda C, Noguchi K, Hashimoto T, Nakano N, Sasaki R, Kokubo Y, Kuzuhara S, Ogaki K, Yamashita C, Yoshino H, Hatano T, Tomiyama H, Hattori N (2012) VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov Disord 27:1413–1417
Balana B, Maslennikov I, Kwiatkowski W, Stern KM, Bahima L, Choe S, Slesinger PA (2011) Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc Natl Acad Sci USA 108:5831–5836
Benito E, Valor LM, Jimenez-Minchan M, Huber W, Barco A (2011) cAMP response element-binding protein is a primary hub of activity-driven neuronal gene expression. J Neurosci 31:18237–18250
Bettens K, Brouwers N, Engelborghs S, De Deyn PP, Van Broeckhoven C, Sleegers K (2008) SORL1 is genetically associated with increased risk for late-onset Alzheimer disease in the Belgian population. Hum Mutat 29:769–770
Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA (2012) The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol Dis 47:126–134
Breiderhoff T, Christiansen GB, Pallesen LT, Vaegter C, Nykjaer A, Holm MM, Glerup S, Willnow TE (2013) Sortilin-related receptor SORCS3 is a postsynaptic modulator of synaptic depression and fear extinction. PLoS One 8:e75006
Burd C, Cullen PJ (2014) Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol 6
Caglayan S, Bauerfeind A, Schmidt V, Carlo AS, Prabakaran T, Hubner N, Willnow TE (2012) Identification of Alzheimer disease risk genotype that predicts efficiency of SORL1 expression in the brain. Arch Neurol 69:373–379
Cai L, Loo LS, Atlashkin V, Hanson BJ, Hong W (2011) Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C). Mol Cell Biol 31:1734–1747
Campellone KG, Welch MD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11:237–251
Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ (2004) Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol 14:1791–1800
Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, DeJesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R (2010) Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet 87:890–897
Cataldo AM, Barnett JL, Pieroni C, Nixon RA (1997) Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci 17:6142–6151
Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 157:277–286
Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA (2004) Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging 25:1263–1272
Cellini E, Tedde A, Bagnoli S, Pradella S, Piacentini S, Sorbi S, Nacmias B (2009) Implication of sex and SORL1 variants in italian patients with Alzheimer disease. Arch Neurol 66:1260–1266
Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25:6156–6166
Chen Y, Chen K, Song W, Chen X, Cao B, Huang R, Zhao B, Guo X, Burgunder J, Li J, Shang HF (2013) VPS35 Asp620Asn and EIF4G1 Arg1205His mutations are rare in Parkinson disease from southwest China. Neurobiol Aging 34(1709):e1707–e1708
Choy RW, Cheng Z, Schekman R (2012) Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc Natl Acad Sci USA 109:E2077–E2082
Christoforou A, McGhee KA, Morris SW, Thomson PA, Anderson S, McLean A, Torrance HS, Le Hellard S, Pickard BS, StClair D, Muir WJ, Blackwood DH, Porteous DJ, Evans KL (2011) Convergence of linkage, association and GWAS findings for a candidate region for bipolar disorder and schizophrenia on chromosome 4p. Mol Psychiatry 16:240–242
Clee SM, Yandell BS, Schueler KM, Rabaglia ME, Richards OC, Raines SM, Kabara EA, Klass DM, Mui ET, Stapleton DS, Gray-Keller MP, Young MB, Stoehr JP, Lan H, Boronenkov I, Raess PW, Flowers MT, Attie AD (2006) Positional cloning of Sorcs1, a type 2 diabetes quantitative trait locus. Nat Genet 38:688–693
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Collins BM, Skinner CF, Watson PJ, Seaman MN, Owen DJ (2005) Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat Struct Mol Biol 12:594–602
Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ (2008) Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 9:366–379
Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M (2012) Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS One 7:e46604
Cullen PJ (2008) Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 9:574–582
Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT (2007) Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449:923–927
Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, Chao MV, Hempstead BL (2011) Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci Signal 4:ra82
Dodson SE, Gearing M, Lippa CF, Montine TJ, Levey AI, Lah JJ (2006) LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J Neuropathol Exp Neurol 65:866–872
Dube JB, Johansen CT, Hegele RA (2011) Sortilin: an unusual suspect in cholesterol metabolism: from GWAS identification to in vivo biochemical analyses, sortilin has been identified as a novel mediator of human lipoprotein metabolism. BioEssays 33:430–437
Elias-Sonnenschein LS, Helisalmi S, Natunen T, Hall A, Paajanen T, Herukka SK, Laitinen M, Remes AM, Koivisto AM, Mattila KM, Lehtimaki T, Verhey FR, Visser PJ, Soininen H, Hiltunen M (2013) Genetic loci associated with Alzheimer’s disease and cerebrospinal fluid biomarkers in a Finnish case-control cohort. PLoS One 8:e59676
Evans SF, Irmady K, Ostrow K, Kim T, Nykjaer A, Saftig P, Blobel C, Hempstead BL (2011) Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J Biol Chem 286:29556–29567
Finan GM, Okada H, Kim TW (2011) BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin. J Biol Chem 286:12602–12616
Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, Willnow TE, Christensen EI, Mobley WB, Nykjaer A, Andersen OM (2012) Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci 32:1467–1480
Follett J, Norwood SJ, Hamilton NA, Mohan M, Kovtun O, Tay S, Zhe Y, Wood SA, Mellick GD, Silburn PA, Collins BM, Bugarcic A, Teasdale RD (2014) The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic 15:230–244
Gagliardi M, Annesi G, Tarantino P, Nicoletti G, Quattrone A (2014) Frequency of the ASP620ASN mutation in VPS35 and Arg1205His mutation in EIF4G1 in familial Parkinson’s disease from South Italy. Neurobiol Aging
Glerup S, Lume M, Olsen D, Nyengaard JR, Vaegter CB, Gustafsen C, Christensen EI, Kjolby M, Hay-Schmidt A, Bender D, Madsen P, Saarma M, Nykjaer A, Petersen CM (2013) SorLA controls neurotrophic activity by sorting of GDNF and its receptors GFRalpha1 and RET. Cell Rep 3:186–199
Gokool S, Tattersall D, Seaman MN (2007) EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic 8:1873–1886
Gomez TS, Billadeau DD (2009) A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17:699–711
Goodarzi MO, Lehman DM, Taylor KD, Guo X, Cui J, Quinones MJ, Clee SM, Yandell BS, Blangero J, Hsueh WA, Attie AD, Stern MP, Rotter JI (2007) SORCS1: a novel human type 2 diabetes susceptibility gene suggested by the mouse. Diabetes 56:1922–1929
Granhall C, Park HB, Fakhrai-Rad H, Luthman H (2006) High-resolution quantitative trait locus analysis reveals multiple diabetes susceptibility loci mapped to intervals <800 kb in the species-conserved Niddm1i of the GK rat. Genetics 174:1565–1572
Grear KE, Ling IF, Simpson JF, Furman JL, Simmons CR, Peterson SL, Schmitt FA, Markesbery WR, Liu Q, Crook JE, Younkin SG, Bu G, Estus S (2009) Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain. Mol Neurodegener 4:46
Guo LH, Westerteicher C, Wang XH, Kratzer M, Tsolakidou A, Jiang M, Grimmer T, Laws SM, Alexopoulos P, Bujo H, Kurz A, Perneczky R (2012) SORL1 genetic variants and cerebrospinal fluid biomarkers of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 262:529–534
Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN (2010) The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci 123:3703–3717
Harrison MS, Hung CS, Liu TT, Christiano R, Walther TC, Burd CG (2014) A mechanism for retromer endosomal coat complex assembly with cargo. Proc Natl Acad Sci USA 111:267–272
Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, van Weering JR, van Heesbeen RG, Middelkoop TC, Basler K, Cullen PJ, Korswagen HC (2011) A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 13:914–923
He Y, Fang Z, Yu G (2012) Sortilin-related VPS10 domain containing receptor 1 and Alzheimer’s disease-associated allelic variations preferentially exist in female or type 2 diabetes mellitus patients in southern Han Chinese. Psychogeriatrics 12:215–225
Hermey G, Riedel IB, Rezgaoui M, Westergaard UB, Schaller C, Hermans-Borgmeyer I (2001) SorCS1, a member of the novel sorting receptor family, is localized in somata and dendrites of neurons throughout the murine brain. Neurosci Lett 313:83–87
Hermey G, Plath N, Hubner CA, Kuhl D, Schaller HC, Hermans-Borgmeyer I (2004) The three sorCS genes are differentially expressed and regulated by synaptic activity. J Neurochem 88:1470–1476
Hermey G, Mahlke C, Gutzmann JJ, Schreiber J, Bluthgen N, Kuhl D (2013) Genome-wide profiling of the activity-dependent hippocampal transcriptome. PLoS One 8:e76903
Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW (2004) A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem 279:54291–54303
Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH (2007) Functional architecture of the retromer cargo-recognition complex. Nature 449:1063–1067
Hong Z, Yang Y, Zhang C, Niu Y, Li K, Zhao X, Liu JJ (2009) The retromer component SNX6 interacts with dynactin p150(Glued) and mediates endosome-to-TGN transport. Cell Res 19:1334–1349
Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD (1997) A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell 8:1529–1541
Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM (2010) Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68:654–667
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482:216–220
Izzo G, Forlenza OV, Santos B, Bertolucci PH, Ojopi EB, Gattaz WF, Kerr DS (2013) Single-nucleotide polymorphisms of GSK3B, GAB2 and SORL1 in late-onset Alzheimer’s disease: interactions with the APOE genotype. Clinics (Sao Paulo) 68:277–280
Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM (2001) Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem 276:22788–22796
Jedrychowski MP, Gartner CA, Gygi SP, Zhou L, Herz J, Kandror KV, Pilch PF (2010) Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J Biol Chem 285:104–114
Jia D, Gomez TS, Metlagel Z, Umetani J, Otwinowski Z, Rosen MK, Billadeau DD (2010) WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci USA 107:10442–10447
Jin C, Zhang L, Xian Y, Liu X, Wu Y, Zhang F, Zhu J, Zhang G, Chen C, Gong R, Yuan J, Tian L, Wang G, Cheng Z (2014) The SORL1 polymorphism rs985421 may confer the risk for amnestic mild cognitive impairment and Alzheimer’s disease in the Han Chinese population. Neurosci Lett 563:80–84
Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J (2004) New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci 117:5367–5379
K Tc, Lunetta KL, Baldwin CT, McKee AC, Guo J, Cupples LA, Green RC, St George-Hyslop PH, Chui H, DeCarli C, Farrer LA (2008) Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol 65:1640–1648
Kerr MC, Bennetts JS, Simpson F, Thomas EC, Flegg C, Gleeson PA, Wicking C, Teasdale RD (2005) A novel mammalian retromer component, Vps26B. Traffic 6:991–1001
Kimura R, Yamamoto M, Morihara T, Akatsu H, Kudo T, Kamino K, Takeda M (2009) SORL1 is genetically associated with Alzheimer disease in a Japanese population. Neurosci Lett 461:177–180
Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, Toga AW, Jack CR Jr, Weiner MW, de Zubicaray GI, McMahon KL, Hansell NK, Martin NG, Wright MJ, Thompson PM (2012) Discovery and replication of gene influences on brain structure using LASSO regression. Front Neurosci 6:115
Kolsch H, Jessen F, Wiltfang J, Lewczuk P, Dichgans M, Kornhuber J, Frolich L, Heuser I, Peters O, Schulz JB, Schwab SG, Maier W (2008) Influence of SORL1 gene variants: association with CSF amyloid-beta products in probable Alzheimer’s disease. Neurosci Lett 440:68–71
Kolsch H, Jessen F, Wiltfang J, Lewczuk P, Dichgans M, Teipel SJ, Kornhuber J, Frolich L, Heuser I, Peters O, Wiese B, Kaduszkiewicz H, van den Bussche H, Hull M, Kurz A, Ruther E, Henn FA, Maier W (2009) Association of SORL1 gene variants with Alzheimer’s disease. Brain Res 1264:1–6
Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, Been LF, Chia KS, Dimas AS, Hassanali N, Jafar T, Jowett JB, Li X, Radha V, Rees SD, Takeuchi F, Young R, Aung T, Basit A, Chidambaram M, Das D, Grundberg E, Hedman AK, Hydrie ZI, Islam M, Khor CC, Kowlessur S, Kristensen MM, Liju S, Lim WY, Matthews DR, Liu J, Morris AP, Nica AC, Pinidiyapathirage JM, Prokopenko I, Rasheed A, Samuel M, Shah N, Shera AS, Small KS, Suo C, Wickremasinghe AR, Wong TY, Yang M, Zhang F, Abecasis GR, Barnett AH, Caulfield M, Deloukas P, Frayling TM, Froguel P, Kato N, Katulanda P, Kelly MA, Liang J, Mohan V, Sanghera DK, Scott J, Seielstad M, Zimmet PZ, Elliott P, Teo YY, McCarthy MI, Danesh J, Tai ES, Chambers JC (2011) Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 43:984–989
Koumandou VL, Klute MJ, Herman EK, Nunez-Miguel R, Dacks JB, Field MC (2011) Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J Cell Sci 124:1496–1509
Kumar KR, Weissbach A, Heldmann M, Kasten M, Tunc S, Sue CM, Svetel M, Kostic VS, Segura-Aguilar J, Ramirez A, Simon DK, Vieregge P, Munte TF, Hagenah J, Klein C, Lohmann K (2012) Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch Neurol 69:1360–1364
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Granier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45:1452–1458
Lane RF, Raines SM, Steele JW, Ehrlich ME, Lah JA, Small SA, Tanzi RE, Attie AD, Gandy S (2010) Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: evidence for involvement of SorL1 and the retromer complex. J Neurosci 30:13110–13115
Lane RF, St George-Hyslop P, Hempstead BL, Small SA, Strittmatter SM, Gandy S (2012) Vps10 family proteins and the retromer complex in aging-related neurodegeneration and diabetes. J Neurosci 32:14080–14086
Lao A, Schmidt V, Schmitz Y, Willnow TE, Wolkenhauer O (2012) Multi-compartmental modeling of SORLA’s influence on amyloidogenic processing in Alzheimer’s disease. BMC Syst Biol 6:74
Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M (2010) SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190:565–574
Laumet G, Chouraki V, Grenier-Boley B, Legry V, Heath S, Zelenika D, Fievet N, Hannequin D, Delepine M, Pasquier F, Hanon O, Brice A, Epelbaum J, Berr C, Dartigues JF, Tzourio C, Campion D, Lathrop M, Bertram L, Amouyel P, Lambert JC (2010) Systematic analysis of candidate genes for Alzheimer’s disease in a French, genome-wide association study. J Alzheimers Dis 20:1181–1188
Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, Rogaeva E, Wakutani Y, Farrer L, St George-Hyslop P, Mayeux R (2007) The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol 64:501–506
Lee JH, Shibata N, Cheng R, Mayeux R (2008) Possible association between SORL and Alzheimer disease? Reanalysing the data of Shibata. Dement Geriatr Cogn Disord 26:482
Lesage S, Condroyer C, Klebe S, Honore A, Tison F, Brefel-Courbon C, Durr A, Brice A (2012) Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology 78:1449–1450
Li Y, Rowland C, Catanese J, Morris J, Lovestone S, O’Donovan MC, Goate A, Owen M, Williams J, Grupe A (2008) SORL1 variants and risk of late-onset Alzheimer’s disease. Neurobiol Dis 29:293–296
Liang X, Slifer M, Martin ER, Schnetz-Boutaud N, Bartlett J, Anderson B, Zuchner S, Gwirtsman H, Gilbert JR, Pericak-Vance MA, Haines JL (2009) Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat 30:463–471
Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME (2008) Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455:1198–1204
Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, Gazzellone M, Carson AR, Howe JL, Wang Z, Wei J, Stewart AF, Roberts R, McPherson R, Fiebig A, Franke A, Schreiber S, Zwaigenbaum L, Fernandez BA, Roberts W, Arnold PD, Szatmari P, Marshall CR, Schachar R, Scherer SW (2011) Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med 3:95ra75
Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG (2012) Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Mol Biol Cell 23:2505–2515
Loebrich S, Nedivi E (2009) The function of activity-regulated genes in the nervous system. Physiol Rev 89:1079–1103
Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154:635–641
Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR 3rd, Slesinger PA (2007) A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci 10:1249–1259
MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 77:425–439
McAllister AK, Lo DC, Katz LC (1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15:791–803
McMillan CT, Toledo JB, Avants BB, Cook PA, Wood EM, Suh E, Irwin DJ, Powers J, Olm C, Elman L, McCluskey L, Schellenberg GD, Lee VM, Trojanowski JQ, Van Deerlin VM, Grossman M (2014) Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol Aging 35:1473–1482
Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA (2014) Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol 10:443–449
Meng Y, Lee JH, Cheng R, St George-Hyslop P, Mayeux R, Farrer LA (2007) Association between SORL1 and Alzheimer’s disease in a genome-wide study. Neuro Report 18:1761–1764
Miyashita A, Koike A, Jun G, Wang LS, Takahashi S, Matsubara E, Kawarabayashi T, Shoji M, Tomita N, Arai H, Asada T, Harigaya Y, Ikeda M, Amari M, Hanyu H, Higuchi S, Ikeuchi T, Nishizawa M, Suga M, Kawase Y, Akatsu H, Kosaka K, Yamamoto T, Imagawa M, Hamaguchi T, Yamada M, Moriaha T, Takeda M, Takao T, Nakata K, Fujisawa Y, Sasaki K, Watanabe K, Nakashima K, Urakami K, Ooya T, Takahashi M, Yuzuriha T, Serikawa K, Yoshimoto S, Nakagawa R, Kim JW, Ki CS, Won HH, Na DL, Seo SW, Mook-Jung I, St George-Hyslop P, Mayeux R, Haines JL, Pericak-Vance MA, Yoshida M, Nishida N, Tokunaga K, Yamamoto K, Tsuji S, Kanazawa I, Ihara Y, Schellenberg GD, Farrer LA, Kuwano R (2013) SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese Koreans and Caucasians. PLoS One 8:e58618
Mufson EJ, Wuu J, Counts SE, Nykjaer A (2010) Preservation of cortical sortilin protein levels in MCI and Alzheimer’s disease. Neurosci Lett 471:129–133
Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA (2008) Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci USA 105:7327–7332
Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466:714–719
Naslavsky N, Caplan S (2011) EHD proteins: key conductors of endocytic transport. Trends Cell Biol 21:122–131
Nicholson AM, Finch NA, Thomas CS, Wojtas A, Rutherford NJ, Mielke MM, Roberts RO, Boeve BF, Knopman DS, Petersen RC, Rademakers R (2014a) Progranulin protein levels are differently regulated in plasma and CSF. Neurology 82:1871–1878
Nicholson AM, Finch NA, Thomas CS, Wojtas A, Rutherford NJ, Mielke MM, Roberts RO, Boeve BF, Knopman DS, Petersen RC, Rademakers R (2014b) Progranulin protein levels are differently regulated in plasma and CSF. Neurology
Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM (2001) The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 20:2180–2190
Ning M, Yang Y, Zhang Z, Chen Z, Zhao T, Zhang D, Zhou D, Xu J, Liu Z, Wang Y, Liu Y, Zhao X, Li W, Li S, He L (2010) Amyloid-beta-related genes SORL1 and ACE are genetically associated with risk for late-onset Alzheimer disease in the Chinese population. Alzheimer Dis Assoc Disord 24:390–396
Nuytemans K, Bademci G, Inchausti V, Dressen A, Kinnamon DD, Mehta A, Wang L, Zuchner S, Beecham GW, Martin ER, Scott WK, Vance JM (2013) Whole exome sequencing of rare variants in EIF4G1 and VPS35 in Parkinson disease. Neurology 80:982–989
Nykjaer A, Willnow TE (2012) Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci 35:261–270
Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ (2006) The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci 26:1596–1603
Olgiati P, Politis A, Albani D, Rodilossi S, Polito L, Ateri E, Zisaki A, Piperi C, Liappas I, Stamouli E, Mailis A, Atti AR, Ferrari B, Morini V, Moretti F, Biella G, Forloni G, Papadimitriou GN, Ronchi DD, Kalofoutis A, Serretti A (2012) Association of SORL1 alleles with late-onset Alzheimer’s disease findings from the GIGAS_LOAD study and mega-analysis. Curr Alzheimer Res 9:491–499
Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM (1996) Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 39:1392–1397
Parks WT, Frank DB, Huff C, Renfrew Haft C, Martin J, Meng X, de Caestecker MP, McNally JG, Reddi A, Taylor SI, Roberts AB, Wang T, Lechleider RJ (2001) Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-beta family of receptor serine-threonine kinases. J Biol Chem 276:19332–19339
Paterson AD, Waggott D, Boright AP, Hosseini SM, Shen E, Sylvestre MP, Wong I, Bharaj B, Cleary PA, Lachin JM, Below JE, Nicolae D, Cox NJ, Canty AJ, Sun L, Bull SB (2010) A genome-wide association study identifies a novel major locus for glycemic control in type 1 diabetes, as measured by both A1C and glucose. Diabetes 59:539–549
Pottier C, Hannequin D, Coutant S, Rovelet-Lecrux A, Wallon D, Rousseau S, Legallic S, Paquet C, Bombois S, Pariente J, Thomas-Anterion C, Michon A, Croisile B, Etcharry-Bouyx F, Berr C, Dartigues JF, Amouyel P, Dauchel H, Boutoleau-Bretonniere C, Thauvin C, Frebourg T, Lambert JC, Campion D (2012) High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry 17:875–879
Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A (2011) Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell 146:359–371
Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, Bettens K, Sleegers K, Tan EK, Kimura R, Shibata N, Arai H, Kamboh MI, Prince JA, Maier W, Riemenschneider M, Owen M, Harold D, Hollingworth P, Cellini E, Sorbi S, Nacmias B, Takeda M, Pericak-Vance MA, Haines JL, Younkin S, Williams J, van Broeckhoven C, Farrer LA, St George-Hyslop PH, Mayeux R (2011a) Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol 68:99–106
Reitz C, Lee JH, Rogers RS, Mayeux R (2011b) Impact of genetic variation in SORCS1 on memory retention. PLoS One 6:e24588
Reitz C, Tokuhiro S, Clark LN, Conrad C, Vonsattel JP, Hazrati LN, Palotas A, Lantigua R, Medrano M, ZJ-V I, Vardarajan B, Simkin I, Haines JL, Pericak-Vance MA, Farrer LA, Lee JH, Rogaeva E, George-Hyslop PS, Mayeux R (2011c) SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer’s disease risk. Ann Neurol 69:47–64
Reitz C, Tosto G, Vardarajan B, Rogaeva E, Ghani M, Rogers RS, Conrad C, Haines JL, Pericak-Vance MA, Fallin MD, Foroud T, Farrer LA, Schellenberg GD, George-Hyslop PS, Mayeux R (2013) Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP). Transl Psychiatry 3:e256
Reynolds CA, Hong MG, Eriksson UK, Blennow K, Johansson B, Malmberg B, Berg S, Gatz M, Pedersen NL, Bennet AM, Prince JA (2010) Sequence variation in SORL1 and dementia risk in Swedes. Neurogenetics 11:139–142
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P (2007) The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39:168–177
Sager KL, Wuu J, Leurgans SE, Rees HD, Gearing M, Mufson EJ, Levey AI, Lah JJ (2007) Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol 62:640–647
Saratzis A, Bown MJ (2014) The genetic basis for aortic aneurysmal disease. Heart 100:916–922
Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, Willnow TE (2007) SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem 282:32956–32964
Schmidt V, Baum K, Lao A, Rateitschak K, Schmitz Y, Teichmann A, Wiesner B, Petersen CM, Nykjaer A, Wolf J, Wolkenhauer O, Willnow TE (2012) Quantitative modelling of amyloidogenic processing and its influence by SORLA in Alzheimer’s disease. EMBO J 31:187–200
Seaman MN (2007) Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci 120:2378–2389
Seaman MN (2012) The retromer complex—endosomal protein recycling and beyond. J Cell Sci 125:4693–4702
Seaman MN, Marcusson EG, Cereghino JL, Emr SD (1997) Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 137:79–92
Seaman MN, McCaffery JM, Emr SD (1998) A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 142:665–681
Seaman MN, Harbour ME, Tattersall D, Read E, Bright N (2009) Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci 122:2371–2382
Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D’Agostino RB Sr, Decarli C, Atwood LD, Wolf PA (2007) Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet 8(Suppl 1):S15
Shannon B, Soto-Ortolaza A, Rayaprolu S, Cannon HD, Labbe C, Benitez BA, Choi J, Lynch T, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Van Gerpen JA, Uitti RJ, Springer W, Cruchaga C, Wszolek ZK, Ross OA (2014) Genetic variation of the retromer subunits VPS26A/B-VPS29 in Parkinson’s disease. Neurobiol Aging 35(1958):e1951–e1952
Sharma M, Ioannidis JP, Aasly JO, Annesi G, Brice A, Bertram L, Bozi M, Barcikowska M, Crosiers D, Clarke CE, Facheris MF, Farrer M, Garraux G, Gispert S, Auburger G, Vilarino-Guell C, Hadjigeorgiou GM, Hicks AA, Hattori N, Jeon BS, Jamrozik Z, Krygowska-Wajs A, Lesage S, Lill CM, Lin JJ, Lynch T, Lichtner P, Lang AE, Libioulle C, Murata M, Mok V, Jasinska-Myga B, Mellick GD, Morrison KE, Meitnger T, Zimprich A, Opala G, Pramstaller PP, Pichler I, Park SS, Quattrone A, Rogaeva E, Ross OA, Stefanis L, Stockton JD, Satake W, Silburn PA, Strom TM, Theuns J, Tan EK, Toda T, Tomiyama H, Uitti RJ, Van Broeckhoven C, Wirdefeldt K, Wszolek Z, Xiromerisiou G, Yomono HS, Yueh KC, Zhao Y, Gasser T, Maraganore D, Kruger R (2012) A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J Med Genet 49:721–726
Sheerin UM, Charlesworth G, Bras J, Guerreiro R, Bhatia K, Foltynie T, Limousin P, Silveira-Moriyama L, Lees A, Wood N (2012) Screening for VPS35 mutations in Parkinson’s disease. Neurobiol Aging 33(838):e831–e835
Shi H, Rojas R, Bonifacino JS, Hurley JH (2006) The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol 13:540–548
Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel JP, Kim TW (2005) Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol 58:909–919
Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627–638
Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavare JM, Cullen PJ (2013) A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 15:461–471
Strochlic TI, Setty TG, Sitaram A, Burd CG (2007) Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol 177:115–125
Strochlic TI, Schmiedekamp BC, Lee J, Katzmann DJ, Burd CG (2008) Opposing activities of the Snx3-retromer complex and ESCRT proteins mediate regulated cargo sorting at a common endosome. Mol Biol Cell 19:4694–4706
Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, Wells JM, Morin PJ (2011) Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis 43:338–345
Tan EK, Lee J, Chen CP, Teo YY, Zhao Y, Lee WL (2009) SORL1 haplotypes modulate risk of Alzheimer’s disease in Chinese. Neurobiol Aging 30:1048–1051
Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M (2011) SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13:715–721
Teng KK, Felice S, Kim T, Hempstead BL (2010) Understanding proneurotrophin actions: recent advances and challenges. Dev Neurobiol 70:350–359
Tsika E, Glauser L, Moser R, Fiser A, Daniel G, Sheerin UM, Lees A, Troncoso JC, Lewis PA, Bandopadhyay R, Schneider BL, Moore DJ (2014) Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum Mol Genet
Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, Lewin GR, Willnow TE, Chao MV, Nykjaer A (2011) Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 14:54–61
van Weering JR, Verkade P, Cullen PJ (2012) SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13:94–107
Vardarajan BN, Bruesegem SY, Harbour ME, Inzelberg R, Friedland R, St George-Hyslop P, Seaman MN, Farrer LA (2012) Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol Aging 33: 2231 e2215-2231 e2230
Vassilopoulos S, Esk C, Hoshino S, Funke BH, Chen CY, Plocik AM, Wright WE, Kucherlapati R, Brodsky FM (2009) A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science 324:1192–1196
Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, Small SA, Gandy S, da Cruz ESEF, da Cruz ESOA (2010) Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener 5:40
Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ (2011) VPS35 mutations in Parkinson disease. Am J Hum Genet 89:162–167
Vilarino-Guell C, Rajput A, Milnerwood AJ, Shah B, Szu-Tu C, Trinh J, Yu I, Encarnacion M, Munsie LN, Tapia L, Gustavsson EK, Chou P, Tatarnikov I, Evans DM, Pishotta FT, Volta M, Beccano-Kelly D, Thompson C, Lin MK, Sherman HE, Han HJ, Guenther BL, Wasserman WW, Bernard V, Ross CJ, Appel-Cresswell S, Stoessl AJ, Robinson CA, Dickson DW, Ross OA, Wszolek ZK, Aasly JO, Wu RM, Hentati F, Gibson RA, McPherson PS, Girard M, Rajput M, Rajput AH, Farrer MJ (2014) DNAJC13 mutations in Parkinson disease. Hum Mol Genet 23:1794–1801
Wang HF, Yu JT, Zhang W, Wang W, Liu QY, Ma XY, Ding HM, Tan L (2012) SORCS1 and APOE polymorphisms interact to confer risk for late-onset Alzheimer’s disease in a Northern Han Chinese population. Brain Res 1448:111–116
Wang X, Zhao Y, Zhang X, Badie H, Zhou Y, Mu Y, Loo LS, Cai L, Thompson RC, Yang B, Chen Y, Johnson PF, Wu C, Bu G, Mobley WC, Zhang D, Gage FH, Ranscht B, Zhang YW, Lipton SA, Hong W, Xu H (2013) Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat Med 19:473–480
Wang S, Tan KL, Agosto MA, Xiong B, Yamamoto S, Sandoval H, Jaiswal M, Bayat V, Zhang K, Charng WL, David G, Duraine L, Venkatachalam K, Wensel TG, Bellen HJ (2014) The retromer complex is required for rhodopsin recycling and its loss leads to photoreceptor degeneration. PLoS Biol 12:e1001847
Wassmer T, Attar N, Harterink M, van Weering JR, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, Cullen PJ (2009) The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell 17:110–122
Webster JA, Myers AJ, Pearson JV, Craig DW, Hu-Lince D, Coon KD, Zismann VL, Beach T, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Huentelman MJ, Joshipura K, Walker D, Heward CB, Ravid R, Rogers J, Papassotiropoulos A, Hardy J, Reiman EM, Stephan DA (2008) Sorl1 as an Alzheimer’s disease predisposition gene? Neurodegener Dis 5:60–64
Wei WH, Hemani G, Gyenesei A, Vitart V, Navarro P, Hayward C, Cabrera CP, Huffman JE, Knott SA, Hicks AA, Rudan I, Pramstaller PP, Wild SH, Wilson JF, Campbell H, Hastie ND, Wright AF, Haley CS (2012) Genome-wide analysis of epistasis in body mass index using multiple human populations. Eur J Hum Genet 20:857–862
Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, Zhang QG, Brann D, Kim TW, Yan R, Mei L, Xiong WC (2011) VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J Cell Biol 195:765–779
Wen Y, Miyashita A, Kitamura N, Tsukie T, Saito Y, Hatsuta H, Murayama S, Kakita A, Takahashi H, Akatsu H, Yamamoto T, Kosaka K, Yamaguchi H, Akazawa K, Ihara Y, Kuwano R (2013) SORL1 is genetically associated with neuropathologically characterized late-onset Alzheimer’s disease. J Alzheimers Dis 35:387–394
Westergaard UB, Kirkegaard K, Sorensen ES, Jacobsen C, Nielsen MS, Petersen CM, Madsen P (2005) SorCS3 does not require propeptide cleavage to bind nerve growth factor. FEBS Lett 579:1172–1176
Xu W, Xu J, Wang Y, Tang H, Deng Y, Ren R, Wang G, Niu W, Ma J, Wu Y, Zheng J, Chen S, Ding J (2013) The genetic variation of SORCS1 is associated with late-onset Alzheimer’s disease in Chinese Han population. PLoS One 8:e63621
Xue X, Zhang M, Lin Y, Xu E, Jia J (2014) Association between the SORL1 rs2070045 polymorphism and late-onset Alzheimer’s disease: interaction with the ApoE genotype in the Chinese Han population. Neurosci Lett 559:94–98
Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC (2014) Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun 5:3828
Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM (2011) A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 89:168–175
Acknowledgments
Dr. Reitz was supported by a Paul B. Beeson Career Development Award (K23AG034550).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Rights and permissions
About this article
Cite this article
Reitz, C. The role of the retromer complex in aging-related neurodegeneration: a molecular and genomic review. Mol Genet Genomics 290, 413–427 (2015). https://doi.org/10.1007/s00438-014-0939-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-014-0939-9