Abstract
Mosquitoes are important vectors of several arthropod-borne diseases, which remain a priority for epidemiological research. Mosquito vector control strategies have traditionally relied on chemical insecticides such as synthetic pyrethroids. However, the indiscriminate use of pesticides has resulted in the development of resistance in many mosquito species. In insects, resistance evolves primarily through the overexpression of one or more gene products from the cytochrome P450, carboxylesterase, and glutathione superfamilies. The current study examined the expression of cytochrome P450 CYP6M2, CYP6AA7, CYP6Z2, CYP9J34, α-Esterase, Esterase B1, and neuroactin genes in larvae and adults of a permethrin-resistant (PerRes) and susceptible (Sus) Culex quinquefasciatus strains. The results showed that the CYP6AA7 gene was overexpressed (10-fold) in larvae and adults with PerRes (p < 0.01) followed by CYPJ34 (9.0-fold) and CYP6Z2 (5.0-fold) compared to the Sus, whereas fewer changes in CYP6M gene expression were observed in PerRes adults (p < 0.05), and no expression was found in larvae. The esterase gene was overexpressed in PerRes larvae (9.0-fold) followed by adults (2.5-fold) compared to the susceptible strain. Based on data, the present study suggests that cytochrome P450, CYP6AA7, CYP6Z2, CYP9J34, α-Esterase, Esterase B1, and neuroactin genes were involved in permethrin resistance in larval and adult Cx. quinquefasciatus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases are among the leading causes of death in humans worldwide. Hence, global vector control is a top priority in reducing disease transmission pressure (Chareonviriyaphap et al. 2013; Sowndarya et al. 2017). Insecticides such as pyrethroids are the primary means of controlling mosquito vectors and related diseases (Yang et al. 2021). The continuous use of synthetic insecticides has accelerated the development of resistance to various categories of insecticide used to control mosquito species (Muthusamy and Shivakumar 2015a; Martins et al. 2019). Insecticide resistance in mosquitoes can manifest itself in four (4) ways: reduction in target site sensitivity (i.e., kdr mutations in a specific gene), reduced penetration (modification in the insect cuticle), behavioral resistance, and metabolic resistance through overproduction and expression of one or more enzymes, which are involved in the detoxification of insecticides (esterases, glutathione S-transferase, and cytochrome P450). Among the resistance mechanisms in mosquitoes and other insect species, metabolic resistance through enhanced detoxification is predominant (Hemingway and Ranson 2000; Feyereisen 2006; Zhu et al. 2010; Muthusamy and Shivakumar 2015a). Cytochrome P450s are a large superfamily in the P450 group, and the CYP6 and CYP9 families have been associated with pesticide resistance. In particular, the CYP6 gene family, which includes CYP6M2, CYP6Z2, and CYP6P3, plays an important role in insecticide resistance in Anopheles gambiae (David et al. 2005; Weedall et al. 2019). Aside from insecticide resistance, P450s could be involved in the catabolism and biosynthesis of juvenile hormone (JH) as well as the degradation of harmful xenobiotics (Zhu et al. 2013).
Esterase is another common enzyme found in most living organisms that enhances metabolic reactions and developmental regulations through the phase 1 detoxification reaction. Based on the metabolic and physiological functions, esterases are subdivided into 11 clades (Claudianos et al. 2006). Esterase enzymes in insects play an important role in the detoxification of various chemical groups such as organophosphates, carbamates, and pyrethroids through direct metabolism or sequestration (Wei et al. 2020). Increased esterase activities have been associated with enhanced detoxification in malaria vectors and other insects (Zhu et al. 2008a, b; Zhang et al. 2011; Mamatha et al. 2020). Apart from increased esterase activity, overexpression of detoxification genes can be triggered by duplication of esterase genes, cis-regulatory elements, and trans-regulatory elements in organophosphate resistance of Cx. quinquefasciatus (Wilding et al. 2012; Weetman et al. 2018; Wilding 2018). Due to the large number of gene families involved in metabolic resistance, identification of candidate genes requires examination of gene expression patterns associated with the resistance phenotype. Despite the role of Culex in the transmission of several pathogens and inducing filarial worms and West Nile virus (WNV), as well as reports of high levels of insecticide resistance, few studies have examined the relative impact of metabolic resistance in Cx. quinquefasciatus (Djouaka et al. 2008; Narayanan et al. 2020).
Previous research found that laboratory selection of permethrin resistance (PerRes) in Cx. quinquefasciatus was associated with increased cytochrome P450 and esterase enzyme activity (Ramkumar and Shivakumar 2015). However, there is no information on the expression profiles of P450 and esterase genes involved in permethrin resistance in the PerRes strain. Hence, the current study investigated the expression profiles of selected candidate genes P450 and esterase, which are involved in metabolic resistance mechanisms. For this study, the gene-specific primers of six cytochrome P450 (CYP6M2, CYP6AA7, CYP6Z2, CYP9J34, CYP6BTQ6B7, and CYP6AE14) and five esterase genes (α-esterase, esterase B1, neuroactin, KM234968, and KM234962) were selected based on the Cx. quinquefasciatus genome platform and previously reported permethrin resistance studies (Arensburger et al. 2010; Yang et al. 2021).
Materials and methods
Mosquito strains and rearing
Two Cx. quinquefasciatus mosquito strains were studied: a laboratory susceptible strain (Sus) originally obtained from the National Centre for Disease Control (NCDC), Mettupalayam, Tamil Nadu, India, and not exposed to any insecticide and a permethrin-resistant (PerRes) field strain collected from Salem District in Tamil Nadu, India, and subjected to permethrin selection for 10 consecutive generations with LC50 value previously determined (Ramkumar and Shivakumar 2015). All the samples were maintained at 27 ± 2 °C under a photoperiod of 14: 10 h (L:D) and fed blood samples on a strained chick in the hatchery cages (60 × 60 cm).
Larval and adult bioassays
Bioassays and P450 gene induction assays were performed on both Sus and PerRes larval and adult Cx. quinquefasciatus. The larval bioassay was performed based on the WHO standard protocol for susceptibility or resistance testing (WHO 1981). Twenty-five (25) early fourth (4th) instar larvae were introduced in a 250 ml test solution of permethrin and an ethanol mixture in a 300-ml paper cup for 24 h. Concentrations were obtained by diluting commercial permethrin stock solution (25% a.i. w/v) with absolute ethanol. For the control, 1 ml of absolute ethanol was added to 249 ml of distilled water. Different concentrations of permethrin were used in this bioassay based on mortality caused by them ranging from 10–90%. The tests were replicated three times per concentration. Larval mortality was observed after 24 h.
CDC bottle bioassays were performed on adult mosquitoes (Brogdon and Chan 2010; Brogdon and McAllister 1998). A previously diagnosed dose of permethrin insecticide 20 μg/bottle (data not shown) was diluted in acetone and used to coat the inside of 250-ml glass bottles, and a control bottle was coated with acetone alone. The acetone was allowed to evaporate over the course of several hours. After the acetone had completely evaporated, 20–25 mosquitoes were introduced into each bottle by aspiration. The number of live and dead mosquitoes was counted every 15 min for 3 h. After 3 h, the mosquitoes were kept in separate paper cups with 10% sucrose solution under laboratory conditions. At least three replicates were performed for 20 µg of insecticide. After 24 h, mortality was assessed, and mortality in the control greater than 5% but lower than 20% was corrected using Abbott’s formula (Abbotts 1925).
RNA preparation and cDNA synthesis
Total RNA was extracted from live pooled samples of fifteen early 4th instar larvae and 3–5-day-old adults (without blood feeding) PerRes and Sus strains using the RNeasy Miniprep kit from QIAGEN. One microgram of RNA from both resistant and susceptible samples was reverse transcribed into 20µl of the ABI reverse transcriptase kit. Quantitative analysis of the cDNA sample was performed using a UV-Vis double-beam spectrophotometer (Systronics, India). After quantification, reverse transcriptase polymerase chain reaction was performed in a Pepseq thermocycler at 94 °C for 5 min, followed by 35 cycles at 94 °C denaturation for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 15 s, and a final extension at 72 °C for 10 min. After amplification, the PCR amplicons were confirmed by 1.5% agarose gel electrophoresis. Each experiment was repeated 3 times with independent RNA preparation (Liu et al. 2004).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
The total RNA sample (0.5 µg/sample) from larval and adult mosquitoes (PerRes and Sus) was reverse transcribed using the ABI 7500 Real-Time PCR system (Applied Biosystems) supplied with the SYBR Green Master Mix Kit in a total volume of 25 µl. Each qRT-PCR reaction contains SYBR Green Master Mix (1x), 4 µl of cDNA from each amplicon, and a P450 and esterase gene-specific forward and reverse primer designed according to each of the P450 and esterase gene sequences (http: //quinquefasciatus. vector base. org/) at a final concentration of 3–5 µM. The primer pairs are listed in Table 1. All samples, including the negative control gene (non-template control), were performed in triplicates. The reaction was carried out for 40 cycles with initial melting at 50 °C for 2 min followed by 95 °C for 10 min, 95 °C for 15 s, and 60 °C for 60 s in a Light Cycler 480 II (Roche Applied Science, Switzerland). The relative expression levels for P450 and esterase genes were calculated using the 2−∆∆CT method of MIQE (minimum information necessary for evaluating qPCR experiment) guidelines. This method used in this study was due to the non-paring and different amplification efficiency of some of the selected gene primers (amplification efficiency for each gene was given in Table 1). The elongation factor 1 (EF-1α) gene was used as an endogenous control to normalize the target gene manifestation level.
Statistical analysis
Mortality in larva and adult PerRes and Sus Cx. quinquefasciatus mosquitos and their relative gene expressions were presented as standard error of the mean replicates (n = 3). The significances in the bioassay and P450 and esterase gene expression levels were subjected to parametric population analysis at *p < 0.05 and **p < 0.01 (Student’s t-test column statistics) using GraphPad PRISM software (version 9.0).
Results
Bioassay
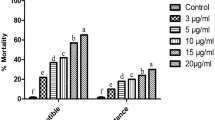
The percentage mortality in the permethrin-resistant (PerRes) and susceptible (Sus) Culex quinquefasciatus larvae and adults is represented in Fig. 1. After exposure to different concentrations of permethrin, the permethrin-resistant Cx. quinquefasciatus larvae and adults showed lower mortality (10–15%) than the Sus strain (92–83%) p < 0.05.
P450 and esterase gene expression
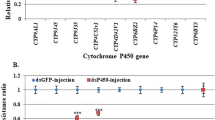
The current work investigated the expression of six P450 and five esterase genes. Out of these genes, four P450 and three esterase genes were amplified in both permethrin-resistant and susceptible strains along with the internal reference gene (EF-1α). Followed by amplification, CYP6AA7, CYP9J34, and CYP6Z2 genes were overexpressed in PerRes larvae (10, 9.0, and 5.0-fold) and adults (4.5 to 4.0-fold) compared to the Sus strain (p < 0.01) with a standard error value of 0.1–0.2 (Fig. 2a–c), whereas the CYP6M2 gene (Fig. 2d) was not differentially expressed in larval and adult strains (S.E. ± 0.4). Next, the esterase B1 and α-esterase genes were 9.0-fold expressed in the larval stage and 2.5-fold in the adult stage Fig. 3a, b, p < 0.01, whereas 4.0-fold expression was increased in the neuroactin gene in the PerRes larval stage p < 0.01 and 1.2-fold in adult (S.E. ± 0.2) p < 0.05, Fig. 4.
a–d Relative expression of cytochrome P450 genes from larvae and adults of permethrin-resistant (PerRes) and susceptible (Sus) strains of Cx. quinquefasciatus. The results are shown as the mean ± S.E. Asterisks indicate significant difference in cytochrome P450 genes expression level in resistant strain compared with susceptible strain (Student’s t-test **p < 0.01)
a, b Relative expression of esterase B1 and α-esterase genes from larvae and adults of permethrin-resistant (PerRes) and susceptible (Sus) Cx. quinquefasciatus. The results are shown as the mean ± S.E. Asterisks indicate significant difference in gene expression level in resistant strain compared with susceptible strain *p < 0.05 and **p < 0.01 (Student’s t-test)
Relative expression of neuroactin from larvae and adults of permethrin-resistant (PerRes) and susceptible (Sus) strains of Cx. quinquefasciatus. The results are shown as the mean ± S.E. Asterisks indicate significant difference in neuroactin expression level in resistant strain compared with susceptible strain *p < 0.05 and **p < 0.01 (Student’s t-test)
Discussion
Insecticide resistance is common in many mosquito vectors and has been linked to both target site and detoxification mechanisms (Muthusamy et al. 2014; Muthusamy and Shivakumar 2015b; Ramkumar et al. 2022). In our previous study, Cx. quinquefasciatus showed metabolic resistance (P450 and esterase enzyme) to permethrin selection under laboratory conditions (Ramkumar and Shivakumar 2015). Therefore, the present study further investigated the susceptibility and expression pattern of cytochrome P450 and esterase genes involved in permethrin resistance in Cx. quinquefasciatus larvae and adults. As a result of bioassay, the PerRes Culex strain showed high permethrin resistance compared to the Sus strain through reduced mortality and overexpression of one or more P450 and esterase genes in both larvae and adults. In a similar study with significant overexpression of P450 genes CYP6AA7 and CYP6Z10 and esterase A and esterase B genes, they were reported to be involved in insecticide resistance in Cx. quinquefasciatus compared to the S-lab strain (Talipouo et al. 2021). Guntay et al. (2018) investigated the susceptibility status of Culex pipiens in the northern Izmir Province of Turkey and found that the species had high resistance to all tested pyrethroids compared to the susceptible population. Cytochrome P450 belongs to a superfamily of metabolic enzymes found in all living organisms (Liu 2015). Overexpression of P450 genes as a result of increased P450 protein levels has been linked to resistance to mosquito insecticide resistances (Hemingway and Ranson 2000; Donnelly et al. 2009; Muthusamy and Shivakumar 2015b). The present study showed overexpression of CYP6AA7, CYP9J34, and CYP6Z2 in both larva and adult permethrin-resistant strains, whereas no significant difference was found in the expression of CYP6M2 at the adult and larval stage among susceptible and PerRes strain. These results suggest that CYP6M2 plays no role in the development of resistance in the PerRes strain. These results further suggest that different mechanisms and/or P450 genes may be involved in the response to insecticide pressure for different developmental stages of mosquitoes (Liu et al. 2011). Several P450 genes from the CYP6, CYP4, and CYP9 gene families were found to be up-regulated and constitutively overexpressed in the permethrin-resistant Musca domestica ALHF strain (Zhu et al. 2008a, b). Similar studies on overexpression of CYP6Z1, CYP6P3, CYP9J32, CYP4H34, CYP6F1, CYP9M10, and CYP6AA7 were reported in An. gambiae, Ae. aegypti, and Cx. quinquefasciatus in different parts of the world (Mueller et al. 2008; Komagata et al. 2010; Gong et al. 2017; Omotayo et al. 2022).
Esterase is another important metabolic resistance gene from the carboxylesterase family because it can hydrolyze the ester bonds in the chemical structure of most organophosphate and pyrethroid insecticides (Wei et al. 2020; Ramkumar et al. 2021; Shyam-Sundar et al. 2022). Resistance to synthetic pyrethroids and carbamates, associated with esterase hydrolysis and carboxylesterase overexpression, has been shown to be involved in metabolic resistance in insects (Wheelock et al. 2005; Prasannakumar et al. 2023). The expression of esterase B1, α-esterase, and neuroactin genes was found to be higher in larval and adult Cx. quinquefasciatus PerRes strains. These results suggest the possibility of esterase metabolism resistance in the PerRes strain. Similar studies on esterase overexpression caused by organophosphate resistance in An. stephensi larvae have been published (Vivekanandhan et al. 2021; Prasannakumar et al. 2021). Wang et al. (2018) reported that overexpression of the carboxyl esterase gene (RpCarE) was associated with isoprocarb and cyhalothrin resistance in R. padi. Studies by Marcombe et al. (2019) showed that overexpression of the CCEAe3a gene was reported to be involved in permethrin resistance in Ae. aegypti mosquito. Based on the results of this present study, we conclude that the selection of permethrin resistance in Cx. quinquefasciatus mosquitoes can develop metabolic resistance through overexpression of one or more cytochrome P450 and esterase genes (CYP6AA7, CYP9J34, CYP6Z2, and esterase B1, α-esterase, and neuroactin) in larvae and adults. Therefore, careful application of recommended pesticide is essential to reduce the development of resistance in Culex mosquito.
Data availability
Data will be made available on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW (2010) Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330(6000):86–8
Brogdon WG, Chan A (2010) Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. Centers for Disease Control and Prevention, Atlanta, pp 1–28
Brogdon WG, McAllister JC (1998) Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Contr Assoc 14:159–164
Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, Ngoen-Klan R (2013) Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors 6(1):1–28
Claudianos C, Ranson H, Johnson R, Biswas S, Schuler M, Berenbaum M, Feyereisen R, Oakeshott JG (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15(5):615–636
David JP, Strode C, Vontas J, Nikou D, Vaughan A, Pignatelli PM, Louis C, Hemingway J, Ranson H (2005) The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proc Natl Acad Sci 102(11):4080–4084
Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, Strode C (2008) Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae ss. from Southern Benin and Nigeria. BMC Genomics 9(1):1–10
Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC IV (2009) Does kdr genotype predicts insecticide-resistance phenotype in mosquitoes. Trends Parasitol 25(5):213–219
Feyereisen R (2006) Evolution of insect P450. Biochem Soc Trans 34:1252–1255
Gong Y, Li T, Feng Y, Liu N (2017) The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci Rep 7:587
Guntay O, Yikilmaz MS, Ozaydin H, Izzetoglu S, Suner A (2018) Evaluation of pyrethroid susceptibility in Culex pipiens of Northern Izmir Province, Turkey. J Arthropod Borne Dis 12(4):370–377
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annual Rev Entomol 45(1):371–391
Komagata O, Kasai S, Tomita T (2010) Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus. Insect Biochem Mol Biol 40(2):146–152
Liu N (2015) Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60:537–559
Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M (2004) An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci 101(26):9740–9744
Liu N, Li T, Reid WR, Yang T, Zhang L (2011) Multiple cytochrome P450 genes: their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS One 6:e23403
Mamatha V, Muthusamy R, Murugan JM, Kweka EJ (2020) Effect of cypermethrin on worker and soldier termites of subterranean termites Odontotermes brunneus (Hagen)(Termitidae: Isoptera). Proc Zool Soc 73:40–45
Marcombe S, Fustec B, Cattel J, Chonephetsarath S, Thammavong P, Phommavanh N, David JP, Corbel V, Sutherland IW, Hertz JC, Brey PT (2019) Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl Trop Dis 13(12):e0007852
Martins WFS, Wilding CS, Isaacs AT, Rippon EJ, Megy K, Donnelly MJ (2019) Transcriptomic analysis of insecticide resistance in the lymphatic filariasis vector Culex quinquefasciatus. Sci Rep 9(1):1–13
Mueller P, Chouaibou M, Pignatelli P, Etang J, Walker ED, Donnelly MJ, Simard F, Ranson H (2008) Pyrethroid tolerance is associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in Northern Cameroon. Mol Ecol 17(4):1145–1155
Muthusamy R, Shivakumar MS (2015a) Involvement of metabolic resistance and F1534C kdr mutation in the pyrethroid resistance mechanisms of Aedes aegypti in India. Acta Tropica 148:137–141
Muthusamy R, Shivakumar MS (2015b) Resistance selection and molecular mechanisms of cypermethrin resistance in red hairy caterpillar (Amsacta albistriga Walker). Pestic Biochem Physiol 117:54–61
Muthusamy R, Vishnupriya M, Shivakumar MS (2014) Biochemical mechanism of chlorantraniliprole resistance in Spodoptera litura (Fab) (Lepidoptera: Noctuidae). J Asia-Pac Entomol 17(4):865–869
Narayanan M, Muthusamy R, Shivakumar MS, Suresh K, Sabariswaran K (2020) Toxicity of cypermethrin and enzyme inhibitor synergists in red hairy caterpillar Amsacta albistriga (Lepidoptera: Arctiidae). J Basic Appl Zool 81(1):1–8
Omotayo AI, Dogara MM, Sufi D, Shuaibu T, Balogun J, Dawaki S, Muktar B, Adeniyi K, Garba N, Namadi I, Adam HA, Adamu S, Abdullahi H, Sulaiman A, Oduola AO (2022) High pyrethroid-resistance intensity in Culex quinquefasciatus (Say) (Diptera: Culicidae) populations from Jigawa, North-West, Nigeria. PLoS Negl Trop Dis 16(6):e0010525
Prasannakumar NR, Jyothi N, Ramkumar G, Asokan R, Sridhar V (2021) Assessment of cross-resistance in South American tomato moth, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pest Manag Hortic Ecosyst 27(2):265–269
Prasannakumar NR, Rao VK, Jyothi N, Saroja S, Lokesha AN, Ramkumar G (2023) Evaluation of insecticidal properties of botanicals for sustainable management of sucking pests of horticultural crops. J Appl Entomol 147:105–115
Ramkumar G, Shivakumar MS (2015) Laboratory development of permethrin resistance and cross-resistance pattern of Culex quinquefasciatus to other insecticides. Parasitol Res 114(7):2553–2560
Ramkumar G, Asokan R, Prasannakumar NR, Kariyanna B, Karthi S, Alwahibi MS, Elshikh MS, Abdel-Megeed A, Ghaith A, Senthil-Nathan S, Kalaivani K (2021) RNA interference suppression of V-ATPase B and juvenile hormone binding protein genes through topically applied DsRNA on tomato leaves: developing biopesticides to control the South American pinworm, Tuta Absoluta (Lepidoptera: Gelechiidae). Front Physiol 12:742871
Ramkumar G, Muthusamy R, Narayanan M, Dhanapal R, Karthik C, Shivakumar MS, Malathi G, Kariyanna B (2022) Pretreatment of mosquito larvae with ultraviolet-B and nitropolycyclic aromatic hydrocarbons induces increased sensitivity to permethrin toxicity. Heliyon 8(10):e11094
Shyam-Sundar N, Ramasubramanian R, Karthi S, Senthil-Nathan S, Chanthini KMP, Sivanesh H, Stanley-Raja V, Ramkumar G, Narayanan KR, Mahboob S, Al-Ghanim KA (2022) Effects of phytocompound Precocene 1 on the expression and functionality of the P450 gene in λ-cyhalothrin-resistant Spodoptera litura (Fab.). Front Physiol 13:900570
Sowndarya P, Ramkumar G, Shivakumar MS (2017) Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif Cells Nanomed Biotechnol 45(8):1490–1495
Talipouo A, Mavridis K, Nchoutpouen E, Djiappi-Tchamen B, Fotakis EA, Kopya E, Bamou R, Kekeunou S, Awono-Ambene P, Balabanidou V, Balaska S, Wondji CS, Vontas J, Antonio-Nkondjio C (2021) High insecticide resistance mediated by different mechanisms in Culex quinquefasciatus populations from the city of Yaoundé, Cameroon. Sci Rep 11(1):7322
Vivekanandhan P, Ayyavu T, Kweka EJ, Mahande AM (2021) Resistance to temephos in Anopheles stephensi larvae is associated with increased cytochrome P450 and α-esterase genes overexpression. Int J Trop Insect Sci 41(4):2543–2548
Wang K, Huang Y, Li X, Chen M (2018) Functional analysis of a carboxylesterase gene associated with isoprocarb and cyhalothrin resistance in Rhopalosiphum padi (L.). Front Physiol 9:992
Weedall GD, Mugenzi LM, Menze BD, Tchouakui M, Ibrahim SS, Amvongo-Adjia N, Irving H, Wondji MJ, Tchoupo M, Djouaka R (2019) A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci Trans Med 11:484
Weetman D, Djogbenou LS, Lucas E (2018) Copy number variation (CNV) and insecticide resistance in mosquitoes: evolving knowledge or an evolving problem. Curr Opin Insect Sci 27:82–88
Wei DD, He W, Miao ZQ, Tu YQ, Wang L, Dou W, Wang JJ (2020) Characterization of esterase genes involving malathion detoxification and establishment of an RNA interference method in Liposcelis bostrychophila. Front Physiol 11:274
Wheelock CE, Shan G, Ottea J (2005) Overview of carboxylesterases and their role in the metabolism of insecticides. J Pestic Sci 30(2):75–83
WHO (1981) Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides, WHO/VBC/81.805. World Health Organization, Geneva
Wilding CS (2018) Regulating resistance: CncC: Maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Curr Opin Insect Sci 27:89–96
Wilding CS, Smith I, Lynd A, Yawson AE, Weetman D, Paine MJ, Donnelly MJ (2012) A cis-regulatory sequence driving metabolic insecticide resistance in mosquitoes: functional characterization and signatures of selection. Insect Biochem Mol Biol 42(9):699–707
Yang T, Li T, Feng X, Li M, Liu S, Liu N (2021) Multiple cytochrome P450 genes: conferring high levels of permethrin resistance in mosquitoes, Culex quinquefasciatus. Sci Rep 11:9041
Zhang Y, Zhang B, Yan D, Dong W, Yang W, Li Q, Zeng L, Wang J, Wang L, Hicks LM (2011) Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellins deactivation. Plant J 67(2):342–353
Zhu F, Feng JN, Zhang L, Liu N (2008) Characterization of two novel cytochrome P450 genes in insecticide-resistant house-flies. Insect Mol Biol 17(1):27–37
Zhu F, Li T, Zhang L, Liu N (2008) Co-up-regulation of three P450 genes in response to permethrin exposure in permethrin resistant house flies. Musca domestica. BMC Physiol 8:18
Zhu F, Parthasarathy R, Bai H, Woithe K, Kaussmann M, Nauen R, Harrison DA, Palli SR (2010) A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc Natl Acad Sci 107(19):8557–8562
Zhu F, Moural TW, Shah K, Palli SR (2013) Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genomics 14(1):1–12
Acknowledgements
The authors thank the Department of Biotechnology, Periyar University, Salem, Tamil Nadu, India, and PG and Research Center for Biotechnology, MGR College, Hosur, India, for providing the infrastructural facility. The authors would also like to thank the reviewer of this draft for valuable suggestions and changes.
Author information
Authors and Affiliations
Contributions
Govindaraju Ramkumar: Planned and conducted research, analyzed the data and wrote the original manuscript; Mathiyazhagan Narayanan: Help with RT-PCR analysis; Ranganathan Muthusamy: planning the research and editing the manuscript; Muthugoundar Subramanian Shivakumar: planning and supervising the research work; Eliningaya J. Kweka: Edited and reviewed the language and draft manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Julia Walochnik
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramkumar, G., Muthusamy, R., Narayanan, M. et al. Overexpression of cytochrome P450 and esterase genes involved in permethrin resistance in larvae and adults of Culex quinquefasciatus. Parasitol Res 122, 3205–3212 (2023). https://doi.org/10.1007/s00436-023-08010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-08010-2