Abstract
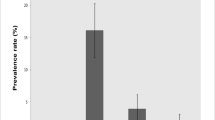
The clinical effect of Trypanosoma congolense infection on Dutch belted (does) rabbits was investigated. Sixteen Dutch belted rabbits weighing between 1.6 and 1.8 kg were grouped into two groups of eight each. Animals were accessed for packed cell volume (PCV), total leucocyte count (TLC), rectal temperature (RT), heart rate (HR), and body weight (BW) before infection as well as 18, 25, and 58 days post inoculation (PI). The level of parasitaemia was estimated on a weekly basis and was graded by number of parasites/field. There was a significant difference (P < 0.05) in the mean PCV between treatment and control groups of the rabbits on all days PI. The other parameters were not significantly different between uninfected controls and treatment group although the rectal temperature fluctuated. The mean PCV of infected rabbits was 36.0 ± 0.53%, 35.3 ± 0.19%, and 28.0 ± 0.89% at days 18, 25, and 58 PI, while for uninfected, the mean PCV was 40.8 ± 0.11%, 41.8 ± 0.19%, and 41.3 ± 0.08% across the same time periods. Parasitaemia was detected at 6th day PI and remained high to the end of the study. The study suggests that the use of haematinics and anti-pyrexia treatments as part of disease management for rabbits would be useful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
African trypanosomes are protozoan parasites transmitted by various species of flies of the genus Glossina (cyclically), and Stomoxys or Tabunus (mechanically). They cause devastating medical, veterinary, and socioeconomic disorders that have a negative impact on Sub-Saharan Africa’s economic development (Aksoy et al. 2017). Each parasite species is adapted to certain climatic and ecological parameters that correspond to the habitat of the tsetse fly vector (Glossina spp.), often known as the “belt” of tsetse flies (Shaw et al. 2014). In humans, the disease is known as “sleeping sickness” or human African trypanosomiasis (HAT) and “nagana” or animal African trypanosomiasis (AAT) in domestic animals (Courtin et al. 2008). HAT is caused by two distinct Trypanosoma brucei subspecies: Trypanosoma brucei gambiense (chronic form) found in western and central Africa, and Trypanosoma brucei rhodesiense (acute form) prevalent in eastern and southern Africa (Ruiz et al. 2015). T. congolense, T. brucei, and T. vivax are the most common trypanosomes that cause disease in domestic animals (Morrison et al. 2016). The bovidae and suidae families of game animals are major reservoirs of infections with minor clinical indications, but the disease in domestic animals is severe often with high fatality (Yaro et al. 2016). The severity of infection with various species of trypanosomes is dependent on the parasite’s highly sophisticated immune escape mechanisms, which allow them to manipulate the entire host immune response to evade elimination via antigenic variation of the variant surface glycoproteins (VSG) (Stijlemans et al. 2016). The most common pathogen in livestock is Trypanosoma congolense (Henry et al. 2004). Studies have demonstrated phagocytosis of T. brucei or T. congolense by macrophages in the presence of immunological sera in vitro (Henry et al. 2004). Although several studies have used T. vivax or T. brucei and have been conducted in rats, cattle, sheep, and goats, there is very little research on rabbits. In Nigeria, rabbits are non-traditional meat source in many households and their husbandry is becoming increasingly important for a variety of reasons, including low initial investment and high fecundity. Trypanotolerant rabbits would result in increased rabbit production and more protein available to the population. Therefore, this study aimed to investigate clinical effect of experimental infection with T. congolense in Dutch belted rabbits.
Materials and methods
Trypanosomes, animals, and experimental infection
The T. congolense Kaura strain used for the study was isolated from cattle in Kaduna State and was passaged in mice. Sixteen Dutch belted rabbits (does) between 7 and 12 months of age were used for the experiment. The base line parameters for mean TLC (109/L), RT (0C), HR (/min), and BW (kg) were recorded. The rabbits were placed in two groups (infected = T) and (control = C) of eight rabbits each. The infected group was inoculated 1 ml T. congolense suspension equivalent to 4 × 109 trypanosomes in diluted mouse blood used as a donor via intra-peritoneal injection (Eisler et al. 2001). Parasitaemia was confirmed by wet mount microscopy of blood collected from the rabbits’ ear veins on alternate days. On days 18, 25, and 58 PI, blood was taken from the rabbits’ ear veins into ethylene diamine tetra acetic acid (EDTA) tubes to determine PCV and TLC. A suspended scale and sac were used to weigh all of the rabbits on days 18, 25, and 58 PI. Rectal temperature and heart rate were measured using a digital thermometer and a stethoscope respectively, every other day including days 18, 25, and 58 PI. Level of parasitaemia was estimated on a weekly basis and was graded by number of parasites/field.
Statistical analysis
Data collected was analyzed using the T-test (independent samples test) in SPSS version 23 and the value was regarded significant at P < 0.05. Data was presented as mean ± standard error of mean (SE).
Results and discussion
The mean PCV, TLC, RT, HR, and BW of the rabbits before the experiment and the mean values of infected and uninfected animals at days 18, 25, and 58 PI, as well as level of parasitaemia in the infected group are shown in Table 1. The values before inoculation showed that they were within the normal range, an indication that the rabbits used in the study were in good health. The values in the treated group showed some variations from the control group; however, no significant differences (P < 0.05) were recorded between them. The values for mean PCV decreased significantly (P < 0.05) in the treated group compared to the untreated control group on day 18, 25, and 58 PI until the end of the trial. Parasitaemia was observed on the 6th day PI and the level was represented based on the number of trypanosomes found in the microscopic field (Table 1).
In this study, anemia was observed in infected rabbits, as revealed by decreased PCV PI. This finding agrees with Toma et al. (2008) that anemia is a common pathological feature associated with trypanosomiasis. Egbe – Nwiyi et al. (2005) recorded significantly lower PCV in infected rats (P < 0.05) compared to uninfected rats. Similarly, Toma et al. (2008) reported anemia in rabbits infected with T. congolense. Studies have shown that RBCs from infected mice exhibited increased osmotic fragility and a different fatty acid membrane composition than RBCs from uninfected mice (Cnops et al. 2015). The change in RBC fragility was not due to interferon (IFN), but rather by host-derived factors such as tumor necrotic factors (TNF) (Balogun et al. 2014; Szempruch et al. 2016). In addition, parasite-derived substances such as sialidases in T. congolense infections and extracellular vehicles (EVs) in T. brucei infections may contribute to RBC alteration (Szempruch et al. 2016). The mechanisms by which the parasite causes anemia are complex and poorly understood, but most likely include differences in erythropoietic potential and hemodilution factors involved in erythrolysis and eryhtrophagocytosis (Habila et al. 2012). The regulation of erythropoietin homeostasis, the host’s potential to raise neutralizing antibodies against secreted trypanosome virulent factors, and the general mechanism involved in inflammation control such as the regulation of the interferon-gamma and interleukin (IFNγ/IL-10) balance, as well as other cytokines are also thought to play a role (Stijlemans et al. 2018). The hallmarks of anemia are arguably the most well-known and well-described features of AT (African trypanosomiasis) to date. The rectal temperature of infected rabbits in the present study showed fluctuating pyrexia, as trypanosomiasis has been linked to variable pyrexia in animals (Ogwu and Njoku 1986). Low body weight, anemia, and stress associated with immune complex reactions could cause anoestrus in females resulting to poor reproductive performance. The presence of trypanosomes in the blood samples of infected animals was first observed six days post inoculation, and the report was consistent with previous reports that T. congolense prefers the capillary bed (Losos and Ikede 1972). Reports showed that phagocytosis of trypanosomes by kupfer cells, mediated by actively produced antibodies, was first noticed on day 5 post infection in mice infected with T. congolense, and greatly increased on day 6 post infection (Henry et al. 2004). Given the 6 days incubation period observed in the present study and that of Toma et al. (2008), who reported an 8 days incubation period in rabbits, a 7-day incubation period for T. congolense may be considered the average incubation period. However, the length of the incubation time may not be determined by the animal species involved but the species of the trypanosomes. As the parasites multiplied, the PCV level decreased and remained low, resulting in pale mucous membranes and chronic anemia. The present study reaffirmed that T. congolense induced severe anemia in infected rabbits and suggests that in addition to trypanocides and antipyretics, haematinic therapy be given priority in the treatment of rabbits infected with T. congolense.
Data availability
The data that were generated or analyzed in the course of this study were included in the article.
References
Aksoy S, Buscher P, Lehane M, Solano P, Van Den AJ (2017) Human African trypanosomiasis control: achievements and challenges. PLoS Negl Trop Dis 11(4):e0005454. https://doi.org/10.1371/journal.pntd.0005454
Balogun EO, Balogun JB, Yusuf S, Inuwa HM, Ndams IS, Sheridan P et al (2014) Anemia amelioration by lactose infusion during trypanosomosis could be associated with erythrocytes membrane de-galactosylation. Vet Parasitol 199:259–263. https://doi.org/10.1016/j.vetpar.2013.10.013
Cnops J, De Trez C, Stijlemans B, Keirsse J, Kauffmann F, Barkhuizen M, Keeton R, Boon L, Brombacher F, Magez S (2015) NK-, NKT- and CD8-derived IFNγ drives myeloid cell activation and erythrophagocytosis, resulting in trypanosomosis-associated acute anemia. PLoS Pathog 11(6):e1004964. https://doi.org/10.1371/journal.ppat.1004964
Courtin D, Berthier D, Thevenon S, Dayo GK, Garcia A, Bucheton B (2008) Host genetics in African trypanosomiasis. Infect Genet Evol 8:229–238. https://doi.org/10.1016/j.meegid.2008.02.007
Egbe – Nwiyi TN, Igbokwe IO, Onyeyili PA, (2005) Pathogenicity of Trypanosome congolense infection following oral calcium chloride administration in rats. Afr J Biomed Res 8(3):197–201. https://doi.org/10.4314/ajbr.v8i3.35751
Eisler MC, Brandt J, Bauer B, Clausen PH, Delespaux V, Holmes PH, Ilemobade A, Machila N, Mambo H, McDermott J, Mehlitz D, Murilla G, Ndung’u JM, Peregrine AS, Sidibe I, Sinyangwe L, Geerts S, (2001) Standardized tests in mice and cattle for the detection of drug resistance in tsetse-transmitted s of African domestic cattle. Vet Parasitol 97:171–182
Habila N, Inuwa MH, Aimola IA, Udeh MU, Haruna E (2012) Pathogenic mechanisms of Trypanosoma evansi infections. Res Vet Sci 93:13–17. https://doi.org/10.1016/j.rvsc.2011.08.011
Henry T, Wanling P, Guiana W, Meiqing S (2004) Trypanosoma congolense infections: antibody – mediated phagocytosis by Kupfer cells. J Leukoc Biol 76:399 – 405. https://doi.org/10.1189/jlb.1003500
Losos GJ, Ikede BO (1972) Review of pathology of diseases of domestic and laboratory animals caused by Trypanosoma congolense, T. vivax, T. brucei, T. rhodesiense and T. gambiense. Vet Path 9 [suppl] 1-79.
Morrison LJ, Vezza L, Rowan T, Hope JC (2016) Animal African trypanosomiasis: time to increase focus on clinically relevant parasite and host species. Trends Parasitol 32:599–607. https://doi.org/10.1016/j.pt.2016.04.012
Ogwu D, Njoku N (1986) Effects of the reproductive status in Zebu Heifers on the immunoglobin M and G levels in bovine Trypanosoma vivax infection. Anim Reprod Sci 12:179–187. https://doi.org/10.1016/0378-4320(86)90038
Ruiz JP, Nyingilili HS, Mbata GH et al (2015) The role of domestic animals in the epidemiology of human african trypanosomiasis in Ngorongoro conservation area. Tanzania Parasites Vectors 8:510. https://doi.org/10.1186/s13071-015-1125-6
Shaw AP, Cecchi G, Wint GR, Mattioli RC, Robinson TP (2014) Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev Vet Med 113:197–210. https://doi.org/10.1016/j.prevetmed.2013.10.024
Stijlemans B, Caljon G, Van Den AJ, Van Ginderachter JA, Magez S, De Trez C (2016) Immune evasion strategies of Trypanosoma brucei within the mammalian host: progression to pathogenicity. Front Immunol 7:233. https://doi.org/10.3389/fimmu.2016.00233
Stijlemans B, De Baetselier P, Magez S, Van Ginderachter JA, De Trez C (2018) African trypanosomiasis-associated anemia: the contribution of the interplay between parasites and the mononuclear phagocyte system. Front Immunol 9:218. https://doi.org/10.3389/fimmu.2018.00218
Szempruch AJ et al (2016) Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164:246–257. https://doi.org/10.1016/j.cell.2015.11.051
Toma I, Shinggu DV, Ezekiel W, Barminas JT (2008) Effect of intraperitoneal administration of vitamine C (ascorbic acid) on anaemia in experimental Trypanosoma congolense infected rabbits. Afr J Pure Appl Chem 2(4):037–040
Yaro M, Munyard KA, Stear MJ, Groth DM (2016) Combatting African animal trypanosomiasis (AAT) in livestock: the potential role of trypanotolerance. Vet Parasitol 225:43–52. https://doi.org/10.1016/j.vetpar.2016.05.003
Acknowledgements
The authors express their appreciation to the Technologists of Parasitology and Entomology, and that of Theriogenology Laboratories, Faculty of Veterinary Medicine, University of Abuja for their support and technical assistance.
Author information
Authors and Affiliations
Contributions
Sylvester Sunday Obeta - Conceptualization, Simon Azubuike Ubah – Supervision, Charles Ejike Ejiofor - Edited manuscript, Adikpe Oluwa Agbonu – Investigation, Columbus Philemon Kwinjoh - Resources, Kenneth Owoicho Abah – Resources, Alapa Baba Ikpe – Resources, Samuel Bankole Abayomi – Resources, Prisca Adaoma Ezinwo – Resources, Joy Iyojo Itodo – Resources, Isaac Oluwatobi Akefe - Drafted Original manuscript, Charles Amaechi Ubah – Review the manuscript, Samuel Mailafia – Review the manuscript, All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the University of Abuja Ethical Committee on Animal Use (UAECAU) (UAECAU/2022/008). The procedures used in this study adhered to the tenets of the Declaration of UAECAU.
Consent to participate
Not applicable.
Consent for publication
All authors have agreed to this final version of the manuscript and have given their consent for its publication in the Parasitology Research journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obeta, S.S., Ubah, S.A., Ejiofor, C.E. et al. The clinical effect of experimental infection with Trypanosoma congolense on Dutch belted rabbits. Parasitol Res 122, 113–116 (2023). https://doi.org/10.1007/s00436-022-07702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07702-5