Abstract
The present study provides an overview of the structures linked to fish host finding, recognition, and invasion of one of the most commonly occurring morphotypes among trematodes, furcocercariae. For this, we use free-swimming cercariae of the strigeid Cardiocephaloides longicollis (Rudolphi 1819) Dubois, 1982. Their elongated cercarial body and bifurcated tail are covered by a tegument with an irregular surface, showing numerous folds arranged in different directions and a typical syncytial organization. Both the body and the bifurcated tail are covered with short spines, rose-thorn shaped, as well as four types of sensory papillae, distinguished by the presence or absence of a cilium, its length, and their position on the cercarial body. These papillae are especially important for free-living stages that rely on external stimuli to locate and adhere to the host. A specialized anterior organ is located at the anterior part of the cercariae and is encircled by a triangle-shaped group of enlarged pre-oral spines followed by a transverse row of enlarged post-oral spines that, together with the sensory papillae, allow active finding, recognition, and penetration into fish. The ventral sucker, covered with inner-oriented spines, sensory papillae, and cilia, helps during this process. The cercariae of C. longicollis possess three types of gland cells (a head gland and two types of penetration glands), each containing different types of secretory granules that play a role in host invasion. The protonephridial excretory system consists of an excretory bladder, a system of collecting tubules, flame cells, and two excretory pores in the middle of each furcae, which serve to control osmoregulation in their marine environment, as well as to eliminate metabolic waste. Together with the four types of sensory endings, the central ganglion forms the nervous system. Our results add novel information on the ultrastructure of strigeid furcocercariae, being essential to interpret these data in relation of their functional role to better understand the transmission and penetration strategies that cercariae display to infect their fish hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trematode parasites are ubiquitous organisms in aquatic ecosystems (Poulin and Morand 2004), with digenean trematodes typically having complex life cycles involving multiple stages and hosts. Cercariae, which are infective larval stages, usually emerge from their first molluscan intermediate host into the external aquatic environment where they infect their next invertebrate or vertebrate hosts. The continuance of trematodes life cycle is highly dependent on the co-occurrence of the parasite and the host in the same space and time (Combes et al. 1994). Cercariae have therefore developed a variety of behavioral responses and host-recognition strategies to maximize encounter and transmission success and infect their next intermediate hosts (Brachs and Haas 2008; Cohen et al. 1980; Haas, 2003; Haas et al. 2008). Many of the organ systems of the adult worm are already formed, in miniature, in cercariae. However, in their relatively short life span (< 24–72 h, Combes, 2001), swimming cercariae need specific structures, as well as recognition and penetration structures, to reach, recognize, and penetrate potential hosts (Denisova and Shchenkov 2019; Haas and van de Roemer 1998; Haas 2003; Paller and Uga 2008).
Most ultrastructural studies on trematodes to date have largely focused on either species of medical or veterinary importance, such as schistosomes or liver flukes, with descriptions sometimes limited to certain areas of the organism. Some structures in trematodes have attracted more attention; for example, sensory receptors are well-documented in several families, i.e., diplostomids (Bibby and Rees 1971; Conn et al. 2008; Czubaj and Niewiadomska 1996), opecoelids (Abdul-Salam and Sreelatha 1998; Bogéa and Caira 2001a), and schistosomes (Cousin and Dorsey 1991; Dorsey et al. 2002; Göbel and Pan 1985; Morris 1971; Nittman 1971; Sakamoto and Ishii 1978; Short and Gagné 1975; Yi-Xun and Mi 1994). However, little attention has been paid to others, particularly in trematode cercariae, even though they exhibit a variety of structures related to host finding and recognition (e.g., Køie 1992; Pekkarinen 1986). While some literature exists on the ultrastructure of miracidia, metacercariae, and adult strigeids (Abdel-Aal et al. 2004; Born-Torrijos et al. 2017; Poddubnaya et al. 2010), studies on these cercariae are so far limited to the study of their tegument by SEM (Bell et al. 1996; Fernández and Hamman 2017; Gordy et al. 2017; Halton 2004). This study is thus an attempt to provide an overall view of the ultrastructure of a commonly occurring morphotype among cercariae, namely furcocercariae, which have a bifurcated tail to actively swim in the water column to encounter and infect their next intermediate host, fish. For this, we used cercariae of the strigeid Cardiocephaloides longicollis (Rudolphi 1819) Dubois, 1982, whose first general description was provided by Prévot and Bartoli (1980), lacking any SEM or TEM information. This trematode species is widely distributed along European coasts, especially in the Mediterranean, and its complex life cycle is embedded in local marine food webs (Born-Torrijos et al. 2016). While their miracidial structures have been described in a previous study (Born-Torrijos et al. 2017), we expect numerous differences compared to C. longicollis miracidium given the structural changes that digeneans undergo during their life cycle and development. Our aim is therefore to investigate the ultrastructural features of C. longicollis cercariae in relation to the transmission and penetration strategies that strigeid cercariae develop to swim, to find and infect their fish hosts, and to place this in context with closely related taxa.
Materials and methods
Specimen collection and fixation
The snail Tritia reticulata (L.), natural host for Cardiocephaloides longicollis (Born-Torrijos et al. 2016), was collected by hand during spring months at the “Beach of the Eucalyptus” (40° 37′ 35.0″ N, 0° 44′ 31.0″ E) in Els Alfacs Lagoon (Ebro Delta, Spain). After acclimatization to laboratory conditions, snails were screened for infections by incubating them individually for 24 h at 25 °C, 12:12 h light:dark cycle, and 35 psu salinity. Only freshly released active cercariae (< 6 h) were used for their morphological examination by scanning and transmission electron microscopy (SEM, TEM). Identification of the cercariae was confirmed morphologically following the descriptions by Prévot and Bartoli (1980) and molecularly as described in Born-Torrijos et al. (2016). Amplification and sequencing of ITS2 rDNA from a single cercaria revealed 99.79% and 100% similarity over 486 bp and 497 pb when compared to previously published records from C. longicollis adult and eggs, respectively (GenBank accession numbers KT454991, KT454991). The sequence was deposited as a GenBank accession number (OL362210).
Scanning and transmission electron microscopy preparations (SEM, TEM)
The cercariae used for SEM and TEM were fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS, 0.1 mol L−1, pH 7.4) and preserved at 4 °C for 24 h. For SEM, samples were later dehydrated in an ascending series of acetone and then critical point dried with liquid CO2. The specimens were mounted on stubs, sputter-coated with gold grids. Specimens used for TEM were washed three times in PBS (15 min each), post-fixed in 2% osmium tetroxide for 2 h, washed three times in phosphate buffer (PB), and dehydrated in a graded series of acetone. After this, specimens were infiltrated and embedded in Epon resin. First, semithin sections (400 nm) were cut using a glass knife, stained with toluidine blue, and observed using a light microscope. Then, ultrathin sections (60–90 nm) were cut through selected regions using a diamond knife on a Leica Ultracut UCT ultramicrotome. These were placed on copper grids, stained with uranyl acetate and lead citrate according to Reynolds (1963).
Specimen observations were carried out under the JEOL JSM 7401F scanning electronic microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 4 kV, and the JEOL JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV, equipped with a CCD digital camera Mega View III (both at the Laboratory of Electron Microscopy, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic).
Results
The general structure of the cercariae of C. longicollis, shown in Fig. 1, consists of a specialized anterior organ in the anterior part of the body, an elongated cylindrical body and a bifurcated tail (Fig. 1a). A ventral sucker is located in the mid-posterior region of the larval body. The body of cercariae contains glands of different types, a protonephridial excretory system, nerve system, and sensory organs represented by tegumental uniciliated and nonciliated sensory papillae (Figs. 1d, e, 2b, 3b, 4c, 6a, e).
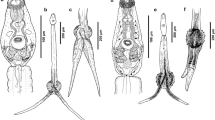
a–f Scanning electron micrographs of Cardiocephaloides longicollis cercariae. a Dorsal view of the cercaria. b Section showing the rows of spines, uniciliated and nonciliated sensory papillae on the anterior organ showing the spines and moderately long uniciliated sensory papillae. Inset: Detail through the long uniciliated sensory papillae. c On the tip of the anterior organ are sensory papillae with a single short cilium and spines. d Ventral view of cercarial body showing anterior organ and acetabulum. e Acetabulum armed with two rows of spines oriented on the inner surface and the uniciliated sensory papilla with a moderately long cilium. f The tail covered with spines and uniciliated sensory papillae with long cilium. Inset: Detail showing rose-thorn shaped spines with broad base located at the middle of the furcae. Abbreviations: A, acetabulum (ventral sucker); AO, anterior organ; EP, excretory pore; MO, mouth opening; OPG, opening of penetration gland; Sp, spines; I, uniciliated sensory papilla with a long cilium; II, uniciliated sensory papilla with a moderately long cilium; III, uniciliated sensory papilla with a short cilium; IV, nonciliated sensory papilla. Scale bars: a = 100 μm; b = 3 μm, Inset = 1 μm; c = 5 μm; d = 20 μm; e = 8 μm; f = 10 μm, Inset = 5 μm
a–f Transmission electron micrographs of Cardiocephaloides longicollis cercariae. a, b, and c General view of a cercaria showing the acetabulum and the penetration glands. Labelled are large lipid droplets, flame cells and collecting tubule cells and spines. d Detail of the outer syncytial layer of the tegument with electron-dense granules, electron-lucent vesicles and mitochondria. e Section through the cytoplasmic bridge connecting the outer layer of the tegument to the nucleated cell bodies. Note the basal lamina and the circular and longitudinal muscle layers. f A region of the tegument showing the spines that cover the tegumental surface, longitudinal and circular muscle layers and the nuclei of the cell bodies. Abbreviations: A, acetabulum; BL, basal lamina; BM, basal membrane; CB, cytoplasmic bridge; CG, central ganglion; CM, circular muscles; CT, collecting tubule; DG, dense granules; FC, flame cell; L, lipid droplets; LM, longitudinal muscles; Mt, mitochondria; N, nucleus; PoG, postacetabular gland; PrG, preacetabular gland; Sp, spines; V, vesicles. Scale bars: a, b, c = 7 μm; d = 0.7 μm; e, f = 0.2 μm
a–h Transmission electron micrographs through the tegument of the anterior organ and acetabulum of C. longicollis cercariae. a A longitudinal section through the tegument. Note the spines on the surface, the circular muscle layer and the nucleus of the subtegumental cell. b Longitudinal section through the anterior organ showing head gland, spines and uniciliated sensory papillae. c A section showing the acetabulum, muscle layers and subtegumental cells with large euchromatic nuclei. d Detail of acetabulum showing the tegumental surface and the circular and longitudinal muscle layers subjacent to the tegument. e and f TEM of the sections through the anterior organ containing ducts of head gland and postacetabular glands. g Preacetabular gland. Shown are part of the acetabulum, a large lipid droplet and osmoregulatory tubules. h A longitudinal section through the postacetabular gland and the central ganglion. Abbreviations: A, acetabulum; CG, central ganglion; CM, circular muscles; DG, dense granules; HG, head gland; dHG, head gland duct; L, lipid droplet; LM, longitudinal muscles; Mt, mitochondria; N, nucleus; NP, nerve processes; PoG, postacetabular gland duct; PrG, preacetabular gland; Sp, spine; UP, uniciliated sensory papilla. Scale bars: a, c, f, g = 0.2 μm; b = 7 μm; d, e = 2 μm; h = 5 μm
a–f Transmission electron micrographs of C. longicollis gland ducts and protonephridial excretory system. a Collecting tubules containing cilia attached to the luminal surface. b Section through the postacetabular gland. c A longitudinal section showing part of osmoregulatory system with a flame cell and collecting tubule cells. Shown are circular muscles and areas of preacetabular and postacetabular glands. d Preacetabular and postacetabular ducts reinforced by microtubules and surrounded by lipid droplets and collecting tubules. e A section through the small collecting tubules showing mitochondria and vesicles in tubule cell cytoplasma. Note septate junctions attaching the tubules to the adjacent cells. f Cross section through flame and capillary cells. Abbreviations: BB, basal body; C, cilia; CC, capillary cell; CM, circular muscles; CT, collecting tubule; PoG, postacetabular gland; dPoG, postacetabular gland duct; PrG, preacetabular gland; dPrG, preacetabular gland duct; ER, endoplasmic reticulum; FC, flame cell; L, lipid droplet; LM, longitudinal muscles; Mt, mitochondria; N, nucleus; SD, septate desmosome; V, vesicle. Scale bars: a, b, c, d, e, f = 0.2 μm
Cercarial tegument
The cercarial body is covered by a tegument with an irregular surface, presenting numerous folds arranged in different directions, i.e., dorsally, ventrally, and transversely (Fig. 1b, d), and containing numerous electron dense granules, spherical electron lucent vesicles, and mitochondria (Fig. 2d). Relatively short spines, rose-thorn shaped (broad base and single curved pointed tip), are found over the entire surface of the cercarial tegument (Figs. 2f, 3a). These spines present a different distribution depending on the area. From anterior to posterior, there is a triangle-shaped group of enlarged pre-oral spines (16 spines arranged in 4 rows) surrounded by a transverse row of enlarged post-oral spines that encircle the mouth opening (Fig. 1c). The spines are densely concentrated on the cephalic area, separating towards the body, where they transversely encircle the entire body surface. Thereafter, the spine distribution on the body trunk can be divided into two sections: (i) while the anterior spines are sparsely distributed, and arranged in nine transversely encircling single rows, being denser on the flanks (Figs. 1b, 2b, c), (ii) the spines posterior to the ventral sucker are numerous and small, and transversely encircle the surface, being less numerous on the ventral side and absent on the dorsal side (Fig. 1d). The tegument consists of a syncytial, anucleated cytoplasm connected by cytoplasmic bridges crossing the muscle layers to nucleated cell bodies lying in the medullary parenchyma. Its outer layer is delimited from the underlying longitudinal and circular muscle layers by a thin basal lamina. A basal membrane lines the surface of the outer layer of the tegument and separates it from the basal lamina. Below the basal lamina are arranged the circular and longitudinal muscle layers, with numerous mitochondria scattered below them (Fig. 2e). Large nuclei of the transversely arranged subtegumental cells were observed (Figs. 2b, c, f, 3a). The ducts of the penetration glands run laterally in the anterior middle of the body (Fig. 2f). The anterior part of the body is a specialized head region containing the head gland and the ducts of the penetration glands (Fig. 3b). It is encircled by transverse rows of spines and sensory papillae, each with a single cilium (Fig. 1b, Inset, c). The ventral sucker (acetabulum) is located on the ventral surface of the mid-posterior half of cercarial body (Figs. 1d, 2a). It is a well-developed round structure covered with tegument, similar to the tegument covering the rest of the body (Fig. 3c). Below the tegument of the sucker, the muscles are arranged at various angles (Fig. 3c, d). Bundles of circular muscle are attached to the connective tissue that joins the ventral sucker to the body (Fig. 3d). Also, nerve fibers from the body enter the ventral sucker (Fig. 3d). The acetabulum, which is slightly pedunculated, is armed with two rows of spike-like spines oriented to the inner surface (Fig. 1e). Its tegument is connected to the subtegumental cells, which possess large euchromatic nuclei and numerous granules (Fig. 3c, d, g). The tail stem is straight, covered with two parallel rows of rose-thorn spines with a broad base along the dorsal side of the tail (Fig. 1f, inset). It bifurcates into two broad furca, the edges/margins of which are covered with tegumental spines. All spines on the cercarial body, tail stem, and furcae are directed posteriorly. Two types of uniciliated sensory papillae were observed on the surface of the cercarial body, the tail stem, and the furcae (Fig. 1f).
Cercarial glands
The glandular system of C. longicollis cercaria consists of three different types of unicellular glands and their corresponding ducts. These are a head gland located in the center of the muscular anterior organ and two types of penetration glands, located in the vicinity of the acetabulum and designated as preacetabular gland around the acetabulum (Figs. 2a, b, 3g) and postacetabular lateral penetration gland (Figs. 2b, c, 3h). All three types of gland cells are packed with distinctive types of secretory granules that differ in size, shape, and electron density (Figs. 3b, 4a, b). The head gland is densely packed with large, irregularly oval granules filled with lightly dense homogeneous material (Fig. 3f). The ducts open into the tegument at the anterior end of the head region (Fig. 3b), with openings visible around the pre-oral spines (Fig. 1c). The content of preacetabular gland is composed of abundant, densely packed elongated electron dense granules (Figs. 2b, 3g, 4a). Another type of gland, which runs in the middle of the body beneath the acetabulum, is the postacetabular gland (Figs. 2a, c, 3h). The secretion of this type of gland cells consists of dense granules that are smaller than those of the preacetabular glands and are embedded in a more electron-lucent matrix (Fig. 4a, b). The secretion granules are concentrated in ducts that run as lateral bundles in the middle of the body (Fig. 4c). They open to the exterior at the surface of the tegument (Fig. 2f). Large droplets, which could be composed of lipid, are observed around the acetabulum and between the penetration glands (Fig. 4a).
Protonephridial excretory system
The excretory system of C. longicollis cercariae consists of an excretory bladder, collecting tubules, flame cells, and excretory pores. The small excretory bladder, located at the posterior end of the body (Fig. 5a, b), is divided into collecting tubules. The collecting tubules are composed of large cells wrapped around the lumen and exhibit numerous cilia adjacent to the luminal surface, thus increasing its surface area (Figs. 4f, 5c). The anterior and posterior longitudinal collecting tubules, situated on both sides of the cercarial body, are connected to small tubules that possess a well-defined large nucleus with condensed chromatin, a thin layer of homogeneous cytoplasm containing mitochondria and vesicles, and are connected to the adjacent cells by septate desmosomes (Fig. 4e). The terminal cells of the protonephridial excretory system are located in the body periphery near the external surface. They consist of flame cells and capillary cells, which are continuous with the small collecting tubule cells (Figs. 2b, c, 4f). The flame cells are irregularly shaped cells bearing a tuft of hexagonally arranged cilia. Inside the ciliary tuft, there is a bundle of long cilia with the typical 9 + 2 arrangement of microtubules that terminate in the capillary cells. The cilia of each flame cell are anchored to its cytoplasm by basal bodies (Fig. 4f). The capillaries of the flame cells are connected to the small collecting tubule cells (Fig. 4c). The cytoplasm of the capillaries contains mitochondria, a rough endoplasmic reticulum, and free ribosomes (Fig. 4f). Two excretory pores are visible in the middle of the two furcae (Fig. 1a). The excretory pore appears to be circular and attached by a septate desmosome to the tegument (Figs. 1f, 5d, e, f).
a-f Sections through the excretory system of C. longicollis. a A section showing the fusion of main collecting tubules and the circular muscles underlying the tegument. b Distal parts of the main collecting tubules which merge and form the thin-walled excretory bladder. c A cross section through the collecting tubule cells surrounded by a large nucleus of perikarya. d, e and f Sections through the excretory pores. Abbreviations: CM, circular muscles; CT, collecting tubule; DG, dense granules; EP, excretory pore; LM, longitudinal muscles; Mt, mitochondria; N, nucleus; SD, septate desmosome. Scale bars: a= 2 μm; b= 1 μm; c, e = 0.2 μm; d = 0.6 μm; f = 0.1 μm
The nervous system
The nervous system of C. longicollis cercariae consists of a central ganglion (a neuropile), from which nerve processes run longitudinally through the body, and tegumental sensory papillae (Figs. 6a, 3b). The neuropile is centrally located in the anterior region of the body and is surrounded by irregularly shaped cell bodies. The cell bodies have large, prominent heterochromatic nuclei and a small amount of cytoplasm filled with mitochondria and vesicles (Fig. 6a, b). The neuropile is composed of a dense network of unsheathed nerve fibers that possess numerous mitochondria and vesicles. Most of the nerve fibers contain spheroidal electron-dense vesicles approximately 150 nm in diameter (Fig. 6c). In the neuropile, there are numerous synapses between the nerve processes.
a-f Sections through the tegumental surface and the central ganglion of C. longicollis. a and b Longitudinal sections showing the heterochromatic nuclei of perikarya surrounding the neuropile. The cytoplasm around the nucleus contains mitochondria, and vesicles and flame cells. c Cross section through the central ganglion showing nerve processes with electron dense neurosecretory vesicles. Synaptic junction and mitochondria are shown. d Longitudinal section of a uniciliated sensory papilla containing electron-lucent neurosecretory vesicles. Shown is the excretory pore. e Detail through the uniciliated sensory papilla attached to the tegument with septate desmosomes. Shown are electron-dense rings. f A section showing the cilia lying above the surface tegument. Inset: Section through the nonciliated sensory papillae containing electron-lucent vesicles. Abbreviations: BB, basal body; C, cilia; CG, central ganglion; CM, circular muscles; DG, dense granules; DR, electron-dense rings; EP, excretory pore; FC, flame cell; LM, longitudinal muscles; LV, lucent vesicles; Mt, mitochondria; N, nucleus; NP, nerve processes; S, synapses; Sp, spine; SD, septate desmosome; V, vesicles. Scale bars: a, = 2 μm; b = 0.1 μm; c, f = 0.2 μm, Inset = 0.6 μm; d = 1 μm; e = 0.4 μm
Based on the morphology observed with SEM, four types of sensory papillae were distinguished on the cercarial tegument. These types are distinguished by the presence or absence of the cilium, its length and its position on the cercarial body. The first type of sensory receptor, with a long cilium (Fig. 1b, Inset, d) and a high tegumental collar (Fig. 1b, Inset), is found mainly on the anterior part of the cercarial body, while the type surrounded by a low tegumental collar (Fig. 1f,) is situated on the tail stem and the furca, especially in the posterior half. The second type, with a moderately long or intermediate cilium, occurs on the anterior organ of the body, including the ventral sucker, outside the rows of spines (Fig. 1b, e). The third type, each with a short cilium, is concentrated on the anterior organ (Fig. 1c), and the fourth nonciliated type is present dorsally on the anterior, unarmed body (Fig. 1b). Each uniciliated sensory structure consists of a bulbous nerve ending attached to the tegument by septate desmosomes and electron-dense rings. A septate desmosome forms almost a complete ring around the cilium at the level of the basal body (Fig. 6e). Another type of inclusion, observed mainly in the cytoplasm of the nerve bulb of the ciliated sensory papillae, are electron-lucent vesicles, ca. 100 nm in diameter (Fig. 6d). The terminal cilium projects from the bulb beyond the tegument and is not covered by a sheath of tegumental material (Figs. 1b, Inset, 3b). The cilium contains both central and peripheral microtubules in a typical 9 + 2 pattern (Fig. 6f). Nonciliate sensory endings, situated on the anterior end of the body, contain a sensory bulb without a cilium, a basal body and electron-dense rings. Their cytoplasm is filled with electron-lucent vesicles of various sizes (Fig. 6 Inset).
Discussion
The present study provides detailed information on the ultrastructural features of C. longicollis cercariae, and describes the diversity of structures required by swimming strigeids to actively locate, recognize, and infect their fish intermediate host. Our results revealed that C. longicollis cercariae possess a tegument with an irregular surface, with numerous dorsally and ventrally located folds and a syncytial organization. A mouth opening was found in C. longicollis cercariae, probably non-functional at this stage as cercariae do not actively feed, but rely on their limited glycogen energy reserves, which are concentrated mainly in the tail and are depleted if a suitable next intermediate host is not found (Anderson and Whitfield 1975; Lawson and Wilson, 1980). Nevertheless, the tegumental ridges observed in regions of close contact with the host, such as the strigeoid adhesive organs (Erasmus 1970), likely evidence a tegumental absorptive function (Lumsden 1975). According to Goater et al. (2005) and Conn et al. (2008), the tegumental folds described in other strigeoids could also be preliminary structures that transform into microvilli involved in nutrient acquisition in metacercariae. Moreover, during larval development, the tegument fills with numerous secretory inclusions that are synthesized in the cytons and exported to the cytoplasmic surface layer. This indicates an intense metabolic activity, including protection from external damage, osmoregulation and excretion, and evasion of the host’s immune system (Lumsden 1975; Halton 2004; Smyth and Halton 1983). The absence of an alimentary tract in the cercariae of C. longicollis contrasts with what has been described in other digeneans, although descriptions of intestinal differentiation/digestive tracts in cercariae are scarce (e.g., Cheng and Bier 1972; Halton and McCrae 1985; Podvyaznaya 2006, 2011). The existence of few individuals could also explain that this structure was not localized in our material. In addition, the non-functionality of a rudimentary canal, as described by Dorsey et al. (2002) in schistosome cercariae, probably argues for the non-existence of such structures in non-feeding larval stages.
Swimming furcocercariae require specific structures that enable them to actively find and infect their next suitable host, with the bifurcated tail being the main propulsion organ. The well-developed musculature not only allows the cercariae to swim, but can also help during the vigorous movements to crawl on the host surface, when cercariae also use their bodies and suckers to adhere to the host body (Dorsey et al. 2002; Haas 2003; Haas and van de Roemer 1998). The spines distributed over the entire body surface aid in this process, facilitating the burrowing into the host (Conn et al. 2008; Whitfield et al. 2003). Spine distribution and morphology have been described in various cercariae, including schistosome cercariae (Abdul-Salam and Sreelatha 1998; Choi et al. 2006; Conn et al. 2008; Paller and Uga 2008; Sakamoto and Ishii 1978; Whitfield et al. 2003), but to our knowledge, there are few detailed descriptions in strigeids (Bakry et al. 2018; Fernández and Hamman 2017; Gordy et al. 2017). In contrast to Prévot and Bartoli (1980), our results show only nine rows of spines in the anterior body (instead of 10), but the distribution of spines on the tail and furcae is consistent with their description. While most caudate cercariae shed their tails during host penetration (Erasmus 1972; McKerrow and Salter 2002), Whitfield et al. (2003) suggested that human schistosome cercariae retain their tails during skin invasion, thanks to numerous backward-pointing spines that prevent retrograde movement. Even if this is not the case for C. longicollis, the function of the spines along the tail margins of C. longicollis, which are also posteriorly directed, remains unclear, apart from possibly serving as anchors while crawling on the fish’s body surface once cercariae are there (Fernández and Hamman 2017; Gordy et al. 2017). Thus, this tail, which the cercariae use as a temporary locomotion organ, loses its function after penetrating the host, as it detaches during this process (Erasmus 1972; Haas and van de Roemer 1998). Once the host is localized, the cercariae need to actively penetrate into it, by first attaching to the body surface with help of a stylet or adherent substances and then burrowing into the skin (Erasmus 1972; McKerrow and Salter 2002). In furcocercariae, the penetration apparatus, in which the anterior organ is transformed into an oral sucker in adults, has a strong musculature that allows this area to be everted/elongated or retracted (Czubaj and Niewiadomska 1997; Ginetsinskaya 1988). The powerful rose-thorn spines enable the cercariae to break open the fish skin and push themselves into the host, using the hooks/spines as anchors, as described in Diplostomum spathaceum (Höglund 1991). Bartoli and Prévot (1980) mentioned the presence of penetration spines, including three rows each staggered with 4 or 5, 6 or 7, and 4 or 5 spines. This partially agrees with our observations, as we observe an additional frontal row of two spines, forming a triangle-shaped group of pre-oral spines similar to that described by Niewiadomska and Našincová (1990) for a diplostomid. The penetration structures of furcocercariae bear a group of 8–12 anteriorly directed penetration spines arranged in front of the oral aperture (Ginetsinskaya, 1988), confirming this as a common feature for this cercarial type.
The pre- and postacetabular glands of C. longicollis cercariae provide secretions with proteolytic activity and mucus-like substances involved in host skin penetration and adhesion, similar to other species (Dorsey 1976; Dorsey and Stirewalt 1971; Ligasová et al. 2011; McKerrow and Doenhoff 1988; Stirewalt and Austin 1973; Stirewalt and Walters 1973). Penetration glands have openings on both sides of the oral sucker, and the acetabulum probably also aids in host’ surface exploration and penetration by helping with attachment thanks to secreted mucous substances (Ligasová et al. 2011; Stirewalt and Dorsey 1974).
Sensory papillae are particularly important for digenean free-living stages (miracidia and cercariae) that rely on external stimuli. Their function is likely related to host localization and penetration site selection and involves photo-, chemo-, tango-, thermo-, and mechanoreception (e.g. Brachs and Haas 2008; Cohen et al. 1980; Dorsey et al. 2002; Dunn et al. 1987; Niewiadomska and Czubaj 1996). Since no eyespots were observed in cercariae of C. longicollis, their behavior of swimming and finding and penetrating the host could rely on their sensory papillae, which are in direct contact with the environment. In trematode species, numerous types of sensory endings have been described based on ultrastructural studies distinguishing various general features mentioned previously (e.g. Bell et al. 1996; Bogéa and Cairo 2001a; Czubaj and Niewiadomska 1996; Žďárská 1992). The present study has revealed the presence of four types of putative sensory structures scattered over the body and tail surfaces of the cercariae of C. longicollis. From our results, they are distinguished by the presence or absence of a cilium, its length, and their position on the cercarial body, the latter likely reflecting the motility and function of this area. These four types of sensory endings in C. longicollis cercariae resemble some of those described for other furcocercariae (e.g., Czubaj and Niewiadomska 1996) and follow a pattern similar to that in other strigeids (Bell et al. 1996). No multiciliated receptors were observed in C. longicollis cercariae, even if present in miracidia of the same species (Born-Torrijos et al. 2017) or in other cercariae (Abdul-Salam and Sreelatha 2004). According to Czubaj and Niewedomska (1996), the ultrastructural diversity of sensory endings demonstrated in different systematic groups as well as in different generations of a species may be the result of localization on the cercarial body and motility of the respective body part or tail. In addition, Bell et al. (1996) suggested that the lateral tail stem sensilla may have taxonomic value at a subgenus/genus level, possessing C. longicollis papillae distributed along the entire length of the tail stem and the posterior half of the furcae. The uniciliated receptors located in the anterior region of the cercarial body and the tail stem and furcae (type I) have long cilia, which correspond to receptors with low mechanical stimuli such as water currents (Bogéa and Caira 2001a, 2001b). This is probably indicative of the locomotor function of the tail during swimming periods of C. longicollis cercariae, which could use these receptors to orient themselves in the water column by alternating between swimming and resting periods (Prévot and Bartoli 1980). Receptors with a long cilium could also serve as drag anchors during resting periods, as suggested for other furcocercariae (Czubaj and Niewiadomska 1996). In addition, the presence of vesicles in the space around the cilium could indicate an additional secretory function of these cells (Czubaj and Niewedomska 1988). Those with moderately long cilia (type II), located around the anterior organ and body, including the ventral sucker, are likely intended to locate and recognize suitable sites for host penetration. Furthermore, sensory papillae with hairs/moderately long cilia and concentrated at the end of the anterior body and also on the acetabulum (type II) help also to settle on the host to find the most suitable site for penetration, usually soft areas such as those around the eyes or oral cavity or with easy access to the target organ (e.g., Erasmus 1959; Paller and Uga 2008; van Beest et al. 2019). Sensory papillae with shorter cilium, found on the anterior organ in C. longicollis (type III), may be more sensitive to different pressures, suggesting a tango-/mechanoreceptive function to perceive of the host once the cercariae touch the host (Denisova and Shchenkov 2019). The nonciliate sensory endings (type IV) are suggested to serve as mechanoreceptors. Their subsurface localization, and the electron-dense collar at the tip, may be an adaptation that enables responses to stretch and contraction in different directions (Czubaj and Niewedomska 1996). The nonciliate receptors observed in the tegument of C. longicollis cercariae resemble the receptors previously described in cercaria of Echinostoma revolutum (Žďárská 1992). Together with the tegumental sensory papillae, the central ganglion forms the nervous system of cercariae, being its largest component. The central ganglion of C. longicollis is located in the anterior area of the body, similar to other cercariae (Denisova and Shchenkov 2019; Niewiadomska 1994).
The protonephridial system of C. longicollis cercariae is composed of multiple functional units, i.e., flame cells bearing a tuft of cilia, a system of collecting tubules and an excretory vesicle that opens into the external environment through the two excretory pores in each furcae. These cercariae spend their short lives in a marine environment searching for their next suitable host, using their protonephridial system to control their osmoregulation. This function complements the role of protonephridia in the elimination of waste products from the organism, thus being this system involved in both osmoregulation and removal of metabolic waste (Dorsey et al. 2002; Smyth and Halton 1983; Wilson and Webster 1974). Cercarial stages of C. longicollis do not possess germinal material at this stage, unlike the earlier larval miracidial stages (Born-Torrijos et al. 2017).
Cercariae are critical for the continuance of trematodes’ life cycle, and given their wide range of transmission strategies involving different morphotypes, swimming behaviors, attachment, and penetration structures, detailed studies on these cercarial traits are fundamental. Our results add novel data on the morphological structures of furcocercariae, a commonly occurring morphotype among trematodes. The combined analysis of the ultrastructural data and the functional role of these structures provides a better understanding of the behaviors exhibited by cercariae after their emergence from molluscan hosts, reflecting the transmission and penetration strategies displayed by strigeid cercariae to infect fish hosts.
References
Abdel-Aal AA, Soliman MFM, Shalaby IM (2004) Surface ultrastructure of Cardiocephalus longicollis (Digenea: Strigeidae) from herring gull, Larus argentatus, and its associated pathological lesions. Helminthologia 41:175–178
Abdul-Salam J, Sreelatha BS (1998) Studies on cercariae from Kuwait Bay. VIII. Description and surface topography of Cercaria kuwaitae VIII sp. n. (Digenea: Opecoelidae). Parasitol Int 47:87–94. https://doi.org/10.1016/S1383-5769(98)00002-6
Abdul-Salam J, Sreelatha BS (2004) Description and surface topography of the cercaria of Austrobilharzia sp. (Digenea: Schistosomatidae). Parasitol Int 53:11–21. https://doi.org/10.1016/j.parint.2003.10.001
Anderson RM, Whitfield PJ (1975) Survival characteristics of the free-living cercarial population of the ectoparasitic digenean Transversotrema patialensis (Soparker, 1924). Parasitology 70:295–310. https://doi.org/10.1017/S0031182000052082
Bakry FA, Atwa MT, Attia MM (2018) Three Strigeid cercariae from Littorina littorea snail, Qarun Lake, Fayoum. Egypt. Vet Worl 11:310–315. https://doi.org/10.14202/vetworld.2018.310-315
Bell AS, Gibson DI, Sommerville C (1996) Chaetotaxy and armature of Ichthyocotylurus erraticus (Rudolphi, 1809) and I. variegatus (Creplin, 1825) cercariae (Digenea, Strigeidae). Parasitol Res 83:70–76. https://doi.org/10.1007/s004360050211
Bibby MC, Rees G (1971) The ultrastructure of the epidermis and associated structures in the metacercaria, cercaria and sporocyst of Diplostomum phoxini (Faust, 1918). Z Parasitenk 37:169–186. https://doi.org/10.1007/BF00259497
Bogéa T, Caira JN (2001a) Ultrastructure and chaetotaxy of sensory receptors in the cercaria of a species of Allopodocotyle Pritchard, 1966 (Digenea: Opecoelidae). Mem I Oswaldo Cruz 96:205–214. https://doi.org/10.1590/S0074-02762001000200012
Bogéa T, Caira JN (2001b) Ultrastructure and chaetotaxy of sensory receptors in the cercariae of a species of Crepidostomum Braun, 1900 and Bunodera Railliet, 1896 (Digenea:Allocreadiidae). J Parasitol 87:273–286. https://doi.org/10.1645/0022-3395(2001)087[0273:UACOSR]2.0.CO;2
Born-Torrijos A, Poulin R, Pérez-del-Olmo A, Culurgioni J, Raga JA, Holzer AS (2016) An optimised multi-host trematode life cycle: fishery discards enhance trophic parasite transmission to scavenging birds. Int J Parasitol 46:745–753. https://doi.org/10.1016/j.ijpara.2016.06.005
Born-Torrijos A, Holzer AS, Raga JA, van Beest GS, Yoneva A (2017) Description of embryonic development and ultrastructure in miracidia of Cardiocephaloides longicollis (Digenea, Strigeidae) in relation to active host finding strategy in a marine environment. J Morphol 278:1137–1148. https://doi.org/10.1002/jmor.20700
Brachs S, Haas W (2008) Swimming behaviour of Schistosoma mansoni cercariae: responses to irradiance changes and skin attractants. Parasitol Res 102:685–690. https://doi.org/10.1007/s00436-007-0812-4
Cheng TC, Bier JW (1972) Studies on molluscan schistosomiasis: an analysis of the development of the cercaria of Schistosoma mansoni. Parasitology 64:129–141. https://doi.org/10.1017/S003118200004470X
Choi MH, Kim SH, Chung JH, Jang HJ, Eom JH, Chung BS, Sohn WM, Chai JY, Hong ST (2006) Morphological observations of Echinochasmus japonicus cercariae and the in vitro maintenance of its life cycle from cercariae to adults. J Parasitol 92:236–241. https://doi.org/10.1645/GE-354R1.1
Cohen LM, Neimark H, Eveland LK (1980) Schistosoma mansoni: response of cercariae to a thermal gradient. J Parasitol 66:362–364. https://doi.org/10.2307/3280843
Combes C, Fournier A, Moné H, Théron A (1994) Behaviours in trematode cercariae that enhance parasite transmission: patterns and processes. Parasitology 109:S3–S13. https://doi.org/10.1017/S0031182000085048
Combes C (2001) Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press
Conn DB, Goater CP, Bray D (2008) Developmental and functional ultrastructure of Ornithodiplostomum ptychocheilus diplostomula (Trematoda: Strigeoidea) during invasion of the brain of the fish intermediate host, Pimephales promelas. J Parasitol 94:635–642. https://doi.org/10.1645/GE-1421.1
Cousin CE, Dorsey CH (1991) Nervous system of Schistosoma mansoni cercaria: organization and fine structure. Parasitol Res 77:132–141. https://doi.org/10.1007/BF00935427
Czubaj A, Niewiadomska K (1996) Ultrastructure of sensory endings in Diplostomum pseudospathaceum Niewiadomska, 1984 cercariae (Digenea, Diplostomidae). Int J Parasitol 26:1217–1225. https://doi.org/10.1016/S0020-7519(96)00085-9
Czubaj A, Niewiadomska K (1997) The muscular system of the cercaria of Diplostomum pseudospathaceum Niewiadomska, 1984 (Digenea): a phalloidin-rhodamine fluorescence and TEM study. Acta Parasitol 42:199–121
Denisova S, Shchenkov S (2019) New data on the nervous system of Cercaria parvicaudata Stunkard & Shaw, 1931 (Trematoda: Renicolidae): revisiting old hypotheses. J Helminthol 94:e52. https://doi.org/10.1017/S0022149X1900035X
Dorsey CH (1976) Schistosoma mansoni: description of the head gland of cercariae and schistosomules at the ultrastructural level. Exp Parasitol 39:444–459. 10.1016/0014-4894(76)90049-7
Dorsey CH, Cousin CE, Lewis FA, Stirewalt MA (2002) Ultrastructure of the Schistosoma mansoni cercaria. Micron 33:279–323. https://doi.org/10.1016/S0968-4328(01)00019-1
Dorsey CH, Stirewalt MA (1971) Schistosoma mansoni: fine structure of cercarial acetabular glands. Exp Parasitol 30:199–214. 10.1016/0014-4894(71)90084-1
Dunn TS, Hanna REB, Nizami WA (1987) Ultrastructural and histochemical observations on the epidermis, presumptive tegument and glands of the miracidium of Gygantocotyle explanatum (Trematoda: Paramphistomidae). Int J Parasitol 17:885–895. https://doi.org/10.1016/0020-7519(87)90004-x
Erasmus DA (1959) The migration of Cercaria X Baylis (Strigeida) within the fish intermediate host. Parasitology 49:73–190. https://doi.org/10.1017/S0031182000026810
Erasmus D (1970) The host-parasite interface of strigeoid trematodes. VII. Ultrastructural observations on the adhesive organ of Diplostomum phoxini Faust, 1918. Z Parasitenk 33:211–224. https://doi.org/10.1017/S0031182000040828
Erasmus D (1972) The biology of trematodes. Edward Arnold, London
Fernández MV, Hamann MI (2017) Cercariae (Digenea: Strigeidae, Diplostomidae) in Biomphalaria straminea (Planorbidae) from a rice field in Northeastern Argentina. Rev. Biol. Trop 65:551–563. https://doi.org/10.15517/rbt.v65i2.24713
Ginetsinskaya TA (1988) Trematodes; their life-cycles, biology and evolution. New Delhi, India
Goater CP, Bray D, Conn DB (2005) Cellular aspects of early development of Ornithodiplostomum ptychocheilus metacercariae in the brain of fathead minnows, Pimephales promelas. J Parasitol 91:814–821. https://doi.org/10.1645/GE-3485.1
Göbel E, Pan JP (1985) Ultrastructure of the daughter sporocyst and developing cercaria of Schistosoma japonicum in experimentally infected snails, Oncomelania hupensis hypensis. Z Parasitenkd 71:227–240. https://doi.org/10.1007/BF00926273
Gordy MA, Locke SA, Rawlings TA, Lapierre AR, Hanington PC (2017) Molecular and morphological evidence for nine species in North American Australapatemon (Sudarikov, 1959): a phylogeny expansion with description of the zygocercous Australapatemon mclaughlini n. sp. Parasitol Res 116:2181–2198. https://doi.org/10.1007/s00436-017-5523-x
Haas W (2003) Parasitic worms: strategies of host finding, recognition and invasion. Zoology 106:349–364. https://doi.org/10.1078/0944-2006-00125
Haas W, Beran B, Loy C (2008) Selection of the host's habitat by cercariae: from laboratory experiments to the field. J Parasitol 94:1233–1238. https://doi.org/10.1645/GE-1192.1
Haas W, van de Roemer A (1998) Invasion of the vertebrate skin by cercariae of Trichobilharzia ocellata: penetration processes and stimulating host signals. Parasitol Res 84:787–795. https://doi.org/10.1007/s004360050489
Halton DW (2004) Microscopy and the helminth parasite. Micron 35:361–390. https://doi.org/10.1016/j.micron.2003.12.001
Halton DW, McCrae JM (1985) Development of the tegument and alimentary tract in a digenetic trematode, Fellodistomum fellis. Parasitology 90:193–204. https://doi.org/10.1017/S0031182000049131
Höglund J (1991) Ultrastructural observations and radiometrie assay on cerearial penetration and migration of the digenean Diplostomum spathaceum in the rainbow trout Oncorhynchus mykiss. Parasitol Res 77:283–289. https://doi.org/10.1007/BF00930902
Køie M (1992) Scanning electron microscopy of cercariae, metacercariae and adults of Pygidiopsis ardeae Køie, 1990 (Digenea, Heterophyidae). Parasitol Res 78:469–474. https://doi.org/10.1007/BF00931565
Lawson JR, Wilson RA (1980) The survival of the cercariae of Schistosoma mansoni in relation to water temperature and glycogen utilization. Parasitology 81:337–348. https://doi.org/10.1017/s0031182000056079
Ligasová A, Bulantová J, Šebesta O, Kašný M, Koberna K, Mikeš L (2011) Secretory glands in cercaria of the neuropathogenic schistosome Trichobilharzia regenti-ultrastructural characterization, 3-D modelling, volume and pH estimations. Parasites Vectors 4:1–12. https://doi.org/10.1186/1756-3305-4-162
Lumsden RD (1975) Surface ultrastructure and cytochemistry of parasitic helminths. Exp Parasitol 37:367–339. https://doi.org/10.1016/0014-4894(75)90078-8
McKerrow JH, Doenhoff M (1988) Schistosome proteases. Parasitol Today 4:334–340. https://doi.org/10.1016/0169-4758(88)90002-6
McKerrow JH, Salter J (2002) Invasion of skin by Schistosoma cercariae. Trends Parasitol 18:193–195. https://doi.org/10.1016/S1471-4922(02)02309-7
Morris JP (1971) The fine structure of the tegument and associated structures of the cercaria of Schistosoma mansoni. Z Parasitenk 36:15–31. https://doi.org/10.1007/BF00328972
Niewiadomska K (1994) Adjusting of the pattern of chaetotaxy in cercariae of Diplostomum Nordmann, 1832 (Digenea) to the structure of their nervous system. Acta Parasitol 39:187–191
Niewiadomska K, Czubaj A (1996) Sensory endings in Diplostomum pseudospathaceum Niewiadomska, 1984 cercariae (Digenea, Diplostomidae). Acta Parasitol 41:20–25
Niewiadomska K, Našincová V (1990) SEM verification of armature and chaetotaxy of Diplostomum paracaudum (Iles, 1959) cercaria (Digenea, Diplostomidae). Ann Parasit Hum Comp 65:69–73. https://doi.org/10.1051/parasite/1990652069
Nittman CJ (1971) The fine structure of ciliated nerve endings in the cercaria of Schistosoma mansoni. J Parasitol 57:855–859. https://doi.org/10.2307/3277814
Paller VGV, Uga S (2008) Attachment and penetration of Centrocestus armatus (Digenea: Heterophyidae) cercariae to gills of secondary intermediate fish hosts. J Parasitol 94:578–583. https://doi.org/10.1645/GE-1402.1
Pekkarinen M (1986) Development of the cercaria of Lacunovermis macomae (Trematoda: Gymnophallidae) to the metacercaria in brackish-water Macoma balthica. Ann Zool Fenn 23:237–250 http://www.jstor.org/stable/23736133
Poddubnaya LG, Mishina E, Zhokhov AE, Gibson DI (2010) Ultrastructural features of the tegumental surface of a new metacercaria, Nematostrigea sp. (Trematoda: Strigeidae), with a search for potential taxonomically informative characters. Syst Parasitol 75:59–73. https://doi.org/10.1007/s11230-009-9207-5
Prévot G, Bartoli P (1980) Démonstration de l’existence d’un cycle marin chez les Strigeides: Cardiocephalus longicollis Szidat, 1928 (Trematoda: Strigeidae). Ann Parasit Hum Comp 55:407–425. https://doi.org/10.1051/parasite/1980554407
Podvyaznaya IM (2006) An ultrastructural study of alimentary tract development in the cercariae of Diplostomum pseudospathaceum (Digenea: Diplostomidae). Parasitol Res 99:362–367. https://doi.org/10.1007/s00436-006-0167-2
Podvyaznaya IM (2011) An ultrastructural study of alimentary tract development in the cercariae of Prosorhynchoides borealis (Digenea, Bucephalidae). Acta Zool-Stockholm 92:170–178. https://doi.org/10.1111/j.1463-6395.2010.00486.x
Poulin R, Morand S (2004) Parasite biodiversity. Smithsonian Institution Books
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. https://doi.org/10.1083/jcb.17.1.208
Short RB, Gagné HT (1975) Fine structure of a possible photoreceptor in cercariae of Schistosoma mansoni. J Parasitol 61:69–74. https://doi.org/10.2307/3279110
Stirewalt MA, Austin BE (1973) Collection of secreted protease from the preacetabular glands of cercariae of Schistosoma mansoni. J Parasitol 59:741–743. https://doi.org/10.2307/3278879
Stirewalt MA, Dorsey CH (1974) Schistosoma mansoni: cercarial penetration of the host epidermis at the ultrastructural level. Exp Parasitol 35:1–15. 10.1016/0014-4894(74)90002-2
Stirewalt MA, Walters M (1973) Schistosoma mansoni: histochemical analysis of the postacetabular gland secretion of cercariae. Exp Parasitol 33:56–72. 10.1016/0014-4894(73)90009-x
Smyth JD, Halton DW (1983) The physiology of trematodes, 2nd edn. Cambridge University Press
Sakamoto K, Ishii Y (1978) Scanning electron microscope observations on miracidium, cercaria, and cercarial papillar patterns of Schistosoma japonicum. J Parasitol 64(1):59–68. https://doi.org/10.2307/3279610
van Beest GS, Villar-Torres M, Raga JA, Montero FE, Born-Torrijos A (2019) In vivo fluorescent cercariae reveal the entry portals of Cardiocephaloides longicollis (Rudolphi, 1819) Dubois, 1982 (Strigeidae) into the gilthead seabream Sparus aurata L. Parasit Vectors 12:92. 10.1186/s13071-019-3351-9
Whitfield PJ, Bartlett A, Khammo N, Brain APR, Brown B, Marriott MC, Clothier R (2003) Delayed tail loss during the invasion of human skin by schistosome cercariae. Parasitology 126:135–140. https://doi.org/10.1017/S0031182002002676
Wilson RA, Webster LA (1974) Protonephridia. Biol Rev 49:127–160. https://doi.org/10.1111/j.1469-185X.1974.tb01572.x
Yi-Xun H, Mi X (1994) Ultrastructural observations on cercaria of Schistosoma japonicum. Se Asian J Trop Med 25:501–508
Žďárská Z (1992) Transmission electron microscopy of sensory receptors of Echinostoma revolutum (Froelich 1802) cercaria (Digenea: Echinostomatidae). Parasitol Res 78:598–606. 10.1007/BF00936459
Acknowledgements
We acknowledge the core facility Laboratory of Electron Microscopy (LEM), Biology Centre of ASCR institution, supported by the MEYS CR (LM2018129 Czech-BioImaging). We are especially grateful to Martina Tesařová for her invaluable help in the preparation of samples. Sincere thanks to two anonymous reviewers for constructive inputs that improved the paper.
Availability of data and material
Not applicable
Code availability
Not applicable
Funding
Financial support was provided by Czech Science Foundation (no. 20-14903Y).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
All authors approved the final version of this manuscript for its submission and publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Shokoofeh Shamsi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoneva, A., van Beest, G.S. & Born-Torrijos, A. Search, find, and penetrate: ultrastructural data of furcocercariae of Cardiocephaloides longicollis (Digenea, Strigeidae) explain their transmission and infection strategy into fish hosts. Parasitol Res 121, 877–889 (2022). https://doi.org/10.1007/s00436-022-07448-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07448-0