Abstract
The tegumental surface of a new strigeid metacercaria, Nematostrigea sp., which is a parasite of the freshwater fish Channa gachua (Hamilton) in central Vietnam, is described for the first time using scanning (SEM) and transmission (TEM) electron microscopy. In addition to the general tegumental surface in various parts of the body, details of the surface of the suckers, lappets and holdfast organ are presented, as are variations in the form and distribution of the body spines. As good taxonomic criteria are few in diplostomoid metacercariae at both specific and generic levels, a number of the ultrastructural features revealed may prove to represent taxonomically informative characters. These include the presence of: two rings of dome-shaped papillae localised at different levels on the rim of the oral sucker, a single ring of ciliated papillae on the inner margin of the ventral sucker and a band of dome-shaped papillae along the lateral margins of the broad body-fold in the ventral forebody; an unarmed oral sucker and anteroventral surface of the forebody, although the latter bears protuberant secretory pores; an armed ventral sucker covered by six-pointed spines, except on its rim; multi-pointed spines along the dorsal and ventral sides of the forebody, with the number of their teeth increasing posteriorly; multi-pointed spines on the forebody which gradually transform into single-pointed, more widely distributed spines on the hindbody, disappearing completely at posterior end of the body; the surface of the lappets with a particular distribution of pores leading to three types of secretory glands and three topographical modifications (areas where the surface is smooth, bears digitiform processes or bears recurved, dagger-shaped spines); and the surface of the holdfast organ which is covered with densely packed, straight or slightly curved, simple spines on its lateral surface but is smooth medially.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous ultrastructural investigations of digenean trematodes of the order Strigeida have revealed the surface features of a small number of members of the families Diplostomidae, Cyathocotylidae and Strigeidae, which together comprise the major part of the superfamily Diplostomoidea. Diplostomoid digeneans exhibit significant regional specialisation of the tegument, where a number of specialised areas have been described in both larval (cercariae and/or metacercariae) and adult stages. Examples include: diplostomids—Diplostomum phoxini (Faust, 1918) by Lee (1962), Erasmus (1969a, b; 1970a, b) and Bibby & Rees (1971); D. spathaceum (Rudolphi, 1819) by Öhman (1965); D. chromatophorum (Brown, 1931) sensu Shigin [=D. pseudospathaceum Niewiadomska, 1984] by Podvyaznaya (1999); D. tregenna Nazmi Gohar, 1932 by Ibraheem (2000); Alaria marcianae (La Rue, 1917) by Johnson et al. (1971), Bhatti & Johnson (1972) and Shoop & Corkum (1984); Cynodiplostomum azimi (Nazmi Gohar, 1933) by Ramadan & Ibraheem (2000); Ornithodiplostomum ptychocheilus (Faust, 1917) by Conn et al. (2008); cyathocotylids—Cyathocotyle bushiensis Khan, 1962 by Erasmus & Öhman (1965) and Erasmus (1967); Holostephanus luehei Szidat, 1936 by Öhman (1966b); Szidatia joyeuxi (Hughes, 1929) by Mouahid (1989); Cercaria kuwaitae II by Abdul-Salam & Sreelatha (1993); and strigeids—Apatemon gracilis minor (Yamaguti, 1933) by Öhman (1966a), Erasmus (1969b) and Bell (1995); Ichthyocotylurus erraticus (Rudolphi, 1809) and I. variegatus (Creplin, 1825) by Bell et al. (1997); Cardiocephaloides longicollis (Rudolphi, 1819) by Abdel-Aal et al. (2004).
The present study represents the first ultrastructural investigation of trematodes of the genus Nematostrigea Sandground, 1934 (family Strigeidae) and was undertaken to observe the features of the tegumental surface of a new strigeid metacercaria, which has been identified as Nematostrigea sp., a new species which is being described by A.E. Zhokhov and E. Mishina based on light microscope observations. Diplostomoid metacercariae are notoriously difficult to identify (see inter alia Shigin, 1986; Gibson, 1996). Our initial aim was to search for features which might supplement the diagnostic features for both the genus and the new species, and also to highlight any which might prove to be taxonomically informative more widely within the superfamily.
Materials and methods
Metacercariae of Nematostrigea sp. were obtained from naturally infected specimens of the freshwater fish Channa gachua (Hamilton) (Channidae) caught in the River Cai, Khanh Hoa Province, central Vietnam during March and April of 2008. Worms were recovered from the cysts on the surface of the kidneys of this intermediate host. The definitive host of these trematodes is unknown in nature, but is expected to be a piscivorous bird.
The metacercariae were excysted mechanically by means of fine needles, then the live worms were fixed entire using 5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 60 days at 5°C, rinsed 4 times for 20 min periods in the same buffer and postfixed in 1% osmium tetroxide for 1 h. The material was then dehydrated in a graded series of alcohol and acetone, and embedded in Araldite and Epon. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined using a JEM 1011 transmission electron microscope (TEM) operating at 80 kV.
For scanning electron microscopical (SEM) observations, fixed worms were dehydrated in a graded ethanol series, with a final change in absolute ethanol, and then critical-point-dried with liquid CO2. The specimens were mounted on stubs, sputter-coated with gold-palladium and examined using LEO-1420 scanning electron microscope operating at 15 kV.
Results
The long, wrinkled body (up to 30 mm) of the metacercaria of Nematostrigea sp. is divided into a short, concave forebody and a long, cylindrical hindbody [this terminology for the parts of the body is as used for diplostomoids—see Niewiadomska (2002, p. 160)]. The forebody is ventrally concave and is limited laterally by a broad body fold; this ventral depression measures c.500 × 350 μm. The oral sucker is ventrally subterminal and bordered latero-ventrally on either side by a protrusive ‘lappet’ (also known as a pseudosucker) (Fig. 1a), whereas the larger ventral sucker is present just posterior to the middle of the ventral depression and anterior to the ‘holdfast organ’ (also known as Brandes’ organ, tribocytic organ or adhesive organ), which is localised at the base of the depression (Fig. 1a).
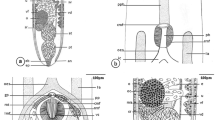
SEM observations of the antero-ventral surface of the forebody of the metacercaria of Nematostrigea sp.: a. forebody; b. anterior extremity with unspined, ridged area and spined area covered with toothed spines; note the numerous protuberances of the secretory pores; c. unspined oral sucker with double row of dome-shaped papillae; d. unspined, ridges region of the anterior extremity merges into the spined region; note the secretory protuberances and sensory papillae; e. spines protruding from surface depressions; f. multi-pointed spines with 10–13 teeth. Abbreviations: BF broad body fold, DR double row of dome-shaped papillae, Fb forebody, HO holdfast organ, Lp lappet, OS oral sucker, PS protuberance of secretory pore, RA ridged area, S spine, SA spined area, SP sensory papilla, VS ventral sucker. Scale-bars: a, 100 μm; b, 10 μm; c, 20 μm; d, e, 2 μm; f, 1 μm
The antero-ventral surface of the forebody
The surface of the oval oral sucker (c. 36 × 48 μm in size), the visible region of the lappets, and the areas between the oral sucker and the lappets and between the lappets are all devoid of spines but irregularly ridged (Fig. 1a–d). On the rim of the oral sucker is a double row of dome-shaped papillae bearing sensory endings (Figs. 1c, 2a). Such sensory papillae are scattered over the entire antero-ventral surface and are especially numerous between the lappets interspersed among dome-shaped protuberances of secretory pores (Figs. 1b, d, 2a, d).
TEM observations of the surface coverings of the metacercaria of Nematostrigea sp.: a. sensory papilla surrounding a sensory ending; b. multi-pointed spine of the ventral forebody; c. dagger-shaped spine of the lappet region; d. secretory duct from a unicellular gland in the forebody discharging to the exterior with the formation of surface protuberances; e. distal region of a multi-pointed spine showing the separate teeth; f. fragment of a spine attached to the basement membrane in the posterior spineless area of the body; g. three-pointed spine from the antero-ventral surface; h. straight, simple spine on a lateral lobe of the holdfast organ. Abbreviations: BM basement membrane, BR basal region of the spine, DB dense bodies in the tegument, Mt microtubules, PS protuberance of secretory pore, S spine, SE sensory ending, SG secretory granule, SJ septate junction, SM surface membrane of the tegument, SP sensory papilla, ST syncytial layer of the tegument, T tooth. Scale-bars: a, b, d, f, g, 2 μm; c, e, h, 1 μm
The antero-ventral surface of the forebody is devoid of spines but gradually merges into a spined area further posteriorly (Fig. 1b, d). Under the SEM, emerging spines are multi-pointed, bearing 3–5 teeth (Figs. 1d, 2g). Further posteriorly, the spines are densely distributed and uniformly arranged, protruding from narrow, horizontal surface depressions (furrows) and with increased numbers of teeth to 8 (SEM spine view c.0.4 μm in length and 0.6 μm in width) (Figs. 1e, 2b). The mid-ventral surface between the suckers is covered with the same kind of spines, but their exposed regions are longer (c.1.8 μm) and wider (c.2 μm), and the number of teeth is increased to 10–11 (Figs. 1f, 2e). The spines are arranged in alternating rows (quincuncially) and directed posteriorly (Fig. 1b), with the distance between the spines in each row being c.0.5 μm and between the rows c.1.7 μm.
TEM observations show (Fig. 2b, c, e, g, h) that the spines arise from, and are anchored to, the basal plasma membrane of the distal cytoplasm, exhibit an electron-dense structure and are covered distally by a surface plasma membrane. However, the basal region of the spines appears more electron-dense and represents a co-called ‘basal plate’ (Fig. 2b, c, f–h). The points of the multi-pointed spines are parallel in orientation (Fig. 2b, e, g), separated from each other and measure up to c.0.6 μm in length and c.0.2 μm in width. The full length of the multi-pointed spines of the mid-ventral surface, from the base to the exposed distal extremity, may reach up to 6 μm.
Surface of the lappets
The present SEM and TEM observations show that in encysted metacercariae of Nematostrigea sp. each lappet is retracted into a pit and covered by a convex, lobe-like projection (Figs. 1a, 3a, b). The surface of this projection is devoid of spines but bears small, dome-shaped sensory papillae (Fig. 3a). The specialised surface of the lappets lies within the pit (Fig. 3b), the base of which is covered with digitiform processes (Fig. 3c, f, i) and the tegumental cytoplasm of which is perforated by glandular ducts filled with secretory granules (Fig. 3c, d, f, i). These ducts are supported by peripheral microtubules (Fig. 3g) and are attached to the epithelial cytoplasm by a circular septate junction (Fig. 3d); they represent the terminal end of unicellular gland-cells and their perikarya are localised deep within the parenchymal region of the forebody.
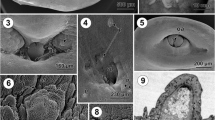
The ultrastructure of the lappets of the metacercaria of Nematostrigea sp.: a. SEM view of a retracted lappet showing the lobe-like projection; b. TEM view of a retracted lappet; c. three types of secretory glands in the glandular region of a lappet; d. Type I secretory glands grouped in the central area of the glandular region; note the smooth lappet surface of the tegument; e. modifications of the surface coverings of the lappets; f. sensory ending between the glandular region covered by digitiform projections and the region with a smooth surface; g. granules of Type III secretory glands; h. granules of Type I secretory glands; i. tegument covered with digitiform projections and penetrated by Types II and III secretory ducts. Abbreviations: B base of the lappet pit, D dense region at one pole of a Type I secretory granule, DP digitiform surface projection, GR glandular region of the lappet surface, LP lobe-like projection situated on the outer region of a retracted lappet, Mt microtubules, OS oral sucker, S1, S2, S3 secretions of Types I, II, III glands, SE sensory ending, SG secretory granule, SJ septate junction, SP sensory papilla, SR spined surface region, SS smooth surface, ST syncytial layer of the tegument. Scale-bars: a, e, 20 μm; b, 100 μm; c, f, 5 μm; d, i, 2 μm; g, h, 1 μm
Three types of secretory ducts from 3 types of unicellular glands with 3 distinctive kinds of granules are associated with the lappet surface (Fig. 3c, d, f–i). Type I ducts are grouped in the central region of the base of the lappet pit, where the surface is smooth (Fig. 3c, d). Granules of Type I are tightly packed, moderately electron-dense, irregularly rounded and c.0.5 μm in diameter. One pole of the granule is marked by a more electron-dense region (Fig. 3d, h). Type II and III ducts are randomly intermixed with each other and situated beside Type I ducts (Fig. 3c). The least common type (Type II) can be recognised by the presence of small, homogenous, spheroidal, electron-dense granules of c.0.3 μm in diameter (Figs. 3c, f, i). Type III secretion is characterised by loosely packed, spheroidal granules of c.0.6 μm in diameter, with a particulate appearance and which are intermediate in density between Types I and II granules (Fig. 3c, g, i).
Modifications of the tegument occur over the surface of the lappets (Fig. 3e). The glandular region of each lappet (in the basal region of the pit), most of which is covered with digitiform surface structures, is always demarcated by receptors (Fig. 3f). This region adjoins a smooth area covering the internal and external surfaces of the lobe-like projection (Fig. 3b) and persists for a short distance on the inner wall of the withdrawn lappet (Fig. 3b, e) until it reaches a spined area (Fig. 3b, e). The spines of the latter area are recurved, dagger-shaped and 4.5 μm in length (Fig. 2c).
Surface of the ventral sucker
The round ventral sucker (c.65 μm in diameter) is raised c.25 μm above the general surface of the ventral depression and most of its circumference is encircled by rows of spines (Fig. 4a). These spines are 6-pointed, and SEM measurements indicate that their exposed region is c.1.5 μm in length and c.1 μm in width. They are uniformly distributed at intervals of c.1.5 μm between the spines in each row and c.2 μm between the rows. The margin (rim) of this sucker is c.8 μm wide, devoid of spines, has a smooth tegumental surface (Fig. 4a) and bears a single row of ciliated papillae on its inner edge (Fig. 4a, c).
Surface of the ventral sucker, holdfast organ and broad body fold of the metacercaria of Nematostrigea sp.: a. ventral sucker; note the spined and smooth areas and ring of sensory papillae; b. six-pointed spines on the ventral sucker; c. ciliated papillae on the ventral sucker; d. holdfast organ; e. part of a lobe of the holdfast organ showing the spined lateral and smooth medial surfaces; f. TEM view of the two sides of a holdfast lobe showing spined and unspined surfaces; g. densely packed straight spines on the lateral surface of the holdfast organ; h. region of the broad body fold showing a band of sensory papillae; i. surface of the broad body fold showing spines protruding from surface depressions and sensory papillae. Abbreviations: BF broad body fold, LS lateral surface of the holdfast organ, MS medial surface of the holdfast organ, S spine, SP sensory papilla, SS smooth surface. Scale-bars: a, d, e, h, 10 μm; b, c, i, 2 μm; f, 20 μm; g, 1 μm
Surface of the holdfast organ
The holdfast organ of this metacercaria comprises two globular, horseshoe-shape lobes situated side-by-side at the base of the ventral depression. The surface of each lobe is differentiated into two distinct surface regions (Fig. 4d–f). Laterally, each lobe is covered with spines, but medially their surface is smooth (Fig. 4d–g). The spines on the outer side of the lobes are densely packed (Fig. 4f, g), straight or slightly curved, stout and simple (Figs. 2h, 4f, g). SEM measurements indicate that the exposed regions of the spines are c.3.6 μm in length, but TEM measurements of the entire spine show that they may reach 4.2 μm. The medial surface does not bear any superficial tegumentary structures (Fig. 4f).
Surface of the lateral margins of the forebody
As indicated above, the ventral concavity in the forebody is limited laterally and posteriorly by a broad U-shaped body fold (Fig. 1a). The surface of this fold is covered with spines (Fig. 4i), which are similar to those of the antero-ventral region of the forebody in appearance, i.e. they are multi-pointed and arise from elongate depressions (Fig. 4i). A band of irregularly distributed, dome-shaped papillae is also visible along the lateral margins of this fold (Fig. 4h, i).
Dorsal surface of the forebody and hindbody
The dorsal surface of the forebody at the level of the ventral concavity is convex, spined (Fig. 5a) and divided into two regions, according to the shape of the posteriorly directed and uniformly distributed spines. Antero-dorsally, the surface is covered with 6-pointed spines that are embedded in the tegumental cytoplasm with only their teeth protruding (Fig. 5b); the distance between these spines is c.0.8 μm in each row and c.2 μm between the rows. The remainder of the dorsal surface of the forebody is covered with 8-pointed spines, which extend c.1.2 μm above the general tegumental surface; spines in each row are c.3.0 μm apart and the rows are separated by c.1.6 μm (Fig. 5c). Most of the dorsal surface of hindbody bears spines, the closely packed teeth of which differ in size, with the central tooth being larger than the lateral ones (Fig. 5d); these spines are distributed at intervals of c.4.5 μm in each row and c.4.0 μm between the rows. However, the spines of the postero-dorsal region of the hindbody are more deeply embedded (Fig. 5i) and appear to disappear entirely posteriorly (Fig. 5e). Throughout the entire dorsal surface of the metacercarial body, both between the furrows from which the spines arise and in the region devoid of spines, the tegument lacks any sort of small surface fold and is smooth (Fig. 5b–d, i).
SEM observations of the surface of the dorsal forebody and dorsal and ventral hindbody of the metacercaria of Nematostrigea sp.: a. dorsal forebody; b. protruded teeth of six-pointed spines on the antero-dorsal forebody; c. eight-pointed spines on dorsal side of the mid-forebody; d. spines on the dorsal hindbody; e. posterior region of the hindbody; f. spines on the ventral hindbody; g. knobbly ventral surface close to the posterior extremity; h. single-pointed spines on the ventral surface of the posterior hindbody; i. deeply embedded spines of the postero-dorsal hindbody. Abbreviations: AD antero-dorsal end of the forebody, S spine, KP knobbly surface of the posterior end of the ventral hindbody, T tooth. Scale-bars: a, 50 μm; b, 1 μm; c–e, g–j, 2 μm; f, 100 μm
Surface of the ventral hindbody
Variation in the shape and distribution of the spines on the ventral side of the hindbody is similar to that on the dorsal side, but, unlike the latter, the surface is folded (Fig. 5f, h). The number of ventral surface folds gradually decreases posteriorly. The multi-pointed spines of the ventral forebody are gradually modified to form more widely distributed, single-pointed spines (Fig. 5f, h). SEM views of the terminal end of the ventral hindbody show only an irregular, knobbly surface (Fig. 5g) and no sign of spines. Nevertheless, TEM sections of the tegumental cytoplasm of the spineless area indicate the presence of portions of the spines attached to the basement membrane of the tegument (Fig. 2f).
Discussion
As indicated above, the identification of diplostomoid metacercariae is notoriously difficult, as useful features of the reproductive system are not developed. Many of these larvae occur in freshwater fish and are known to be pathogenic when present in certain regions of the body; for example, Diplostomum spp. in the eye have long been recognised as causing blindness (e.g. Rushton, 1937). In terms of prophylaxis, identification and knowledge of the life-history are important.
Distribution of tegumental papillae
The numbers and distribution patterns of sensory papillae (often referred to as chaetotaxy) have been considered as diagnostic features for larval digeneans, i.e. usually at the cercarial (e.g. Combes, 1980; Bell et al., 1997) stage but also as metacercariae (e.g. Komalamisra et al., 2005). In their review of ultrastructural studies related the taxonomy and biology of trematodes, Jamjoom & Shalaby, (2006) cite numerous examples where such features have been used at the specific and generic levels to distinguish taxa. The arrangement of the papillae around the oral and ventral suckers of newly excysted metacercariae has been used as taxonomic markers for the identification of Paragonimus Baun, 1899 and its species by Higo & Ishii (1984, 1987) and Komalamisra et al. (2005) and for adults of the genera Echinostoma Rudolphi, 1809 by Fried et al. (1990) and Diplostomum von Nordmann, 1832 by McKeown & Irwin (1995). In addition to the present investigation on Nematostrigea, previous ultrastructural studies on diplostomoids have shown: the presence of a double row of sensory papillae around the rim of the oral and ventral suckers in adult Diplostomum phoxini (Diplostomidae) (Erasmus, 1970b); several circles of papillae on the oral and ventral suckers in the mesocercaria of Alaria marcianae (Diplostomidae) (Shoop & Corkum, 1984); two concentric cycles of dome-shaped papillae on the ventral sucker and numerous papillae around the oral sucker and along the lateral margins of the forebody in adult Szidatia joyeuxi (Cyathocotylidae) (Mouahid, 1989); ciliated sensory papillae on the oral sucker and two rings of sensilla at different levels on the ventral sucker of Ichthyocotylurus erraticus and I. variegatus metacercariae (Strigeidae) (Bell, 1995); a bilateral arrangement of sensilla on the oral sucker in the metacercariae of Apatemon gracilis (Rudolphi, 1819) and A. annuligerum (von Nordmann, 1832) (Strigeidae) (Bell, 1995); and both large and small sensory papillae on the oral sucker, numerous large papillae on the ventral sucker and 16 papillae on the lateral margins of the ventral forebody of adult D. tregenna (Diplostomidae) (Ibraheem, 2000). To summarise, in terms of the distribution of sensory papillae in those diplostomoids examined at the ultrastructural level, it seems that the number of rings of sensory papillae on the surfaces of the oral and ventral suckers, the presence of ciliated (with long or nipple-shaped cilia) or unciliated papillae, and the presence or absence of the papillae along lateral margins of the ventral forebody may be useful as diagnostic traits for this group of trematodes.
Gland-cells in the forebody
Numerous pores of unicellular glands are present in the antero-ventral surface of the forebody of Nematostrigea sp. These gland-cells are associated with neither the oral sucker nor the lappets, but open externally within the base of the anterior region of the concavity of the forebody and are especially numerous between the two lappets. The gland-cell bodies lie beneath the tegument and ducts pass distally, penetrating the distal cytoplasm of the tegument. Erasmus (1969b) mentioned a mass of gland-cells at the base of the forebody ‘cup’ of the strigeid Apatemon gracilis minor. However, he did not consider their secretion as adhesive, although the same mass of glands has been referred to as ‘adhesive organ gland-cells’ in different trematode species (see review by Whittington & Cribb, 2001). In support of this hypothesis, observations of other strigeid trematodes have suggested attachment is effected by the cup-shaped forebody, which functions as a sucker (Öhman, 1965; Johnson et al., 1971; Bhatti & Johnson, 1972; Shoop & Corkum, 1984; Mouahid, 1989).
Surface structures and types of gland-cells in the lappets
In diplostomoids, specialisation occurs on the surface of the lappets in the form of musculo-glandular areas with surface features referred to as digitiform, finger-like or microvilliform processes respectively (Present study; Erasmus, 1969a, 1970a; Bibby & Rees, 1971) and unicellular secretory glands: one type in adult Apatemon gracilias minor (see Erasmus, 1969b), two types in adult Diplostomum phoxini (see Erasmus, 1969a, 1970a) or three types in metacercariae of D. phoxini and Nematostrigea sp. (see Bibby & Rees, 1971; Present study). With respect to the function of the lappets, Bibby & Rees (1971) considered them functioning in attachment for adults and fully-formed metacercariae of D. phoxini, with the secretion of the gland-cells being adhesive. Histochemical tests of the metacercariae of the latter species demonstrated non-specific esterase activity in the lappet gland-cells (Lee, 1962) and PAS positive carbohydrate-containing material (Arvy, 1954), and in adult A. gracilis minor, with a single type of lappet gland-cell, an esterase secretion has been demonstrated (Öhman, 1966a; Erasmus, 1969b).
Our results from Nematostrigea sp. show the presence of three types of secretory gland-cells, with a special distribution of their pores along the surface of the lappet, and the presence of three modifications of this surface (smooth, with digitiform processes and with recurved, dagger-shaped spines). Such a specific arrangement of the pores for a particular type of secretory gland-cell with a specific surface topography has not been reported for the lappets of other diplostomoids (cf. Erasmus, 1969a, 1970a; Bibby & Rees, 1971).
Surface of the holdfast organ
The present study of Nematostrigea sp. shows the presence of two different surface coverings for the holdfast organ, i.e. a heavily spined lateral surface with simple, recurved spines which taper to a fine point and an unarmed medial surface. This confirms the results of previous studies on this organ in diplostomoids in terms of surface specialisation; for example, in Cyathocotyle bushiensis (see Erasmus,1967; Erasmus & Öhman, 1965), Diplostomum spathaceum and D. phoxini (see Lee, 1962; Öhman, 1965; Erasmus, 1970a; Bibby & Rees, 1971), Apatemon gracilis minor (see Öhman, 1966a, Erasmus, 1969b), Holostephanus luehei (see Öhman, 1966b); Alaria marcianae (see Johnson et al., 1971; Shoop & Corkum, 1984) and Szidatia joyeuxi (see Mouahid, 1989). A suggested function of the holdfast organ is extracorporeal digestion (Lee, 1962; Öhman, 1966a; Johnson et al., 1971; Bhatti & Johnson, 1972). The presence of alkaline phosphatase activity in this organ, at the metacercarial stage of D. phoxini, has been indicated by Arvy (1954) and Lee (1962), and a nonspecific esterase has been demonstrated in adult D. phoxini and D. spathaceum by Lee (1962) and in adult A. gracilis minor by Öhman (1966a). Erasmus (1970b, p. 39), with reference to the spines on its lateral surface, has stated that they “are extremely suitable for anchorage to the tissue and could also produce considerable abrasion of the host tissue”.
The present study of Nematostrigea sp. shows that it has a well-formed holdfast organ at the metacercarial stage, as has previously been noted for the metacercariae of Alaria marcianae (see Shoop & Corkum, 1984) and Szidatia joyeuxi (see Mouahid, 1989), for which there is also an indication of spined lateral and smooth medial areas. Nevertheless, as has been shown for D. phoxini by Bibby & Rees (1971), this organ is not fully functional at the metacercarial stage, although alkaline phosphatase is present in its epithelium. The modifications of the covering of the medial surface of the lobes of the holdfast organ between the metacercarial and adult stages result in an expansion of the surface area due to the appearance of microvilli-like processes and gland-cells (Erasmus, 1969b, 1970a), which indicate a change in functionality.
Shape and distribution of the tegumental spines
The armature of the tegumental surface of trematodes provides insights into aspects of their biology and provides a wealth of taxonomic criteria in some groups. It has been shown that the shape, size and distribution of tegumental spines differ between species (see Jamjoom & Shalaby, 2006, for examples) and even at higher taxonomic levels (see chapters in Jones et al., 2005). In the present study, it is apparent that the surface of the metacercaria of Nematostrigea sp. has multi-pointed spines with numerous teeth, except for the most antero-ventral area, lappets and holdfast organ, but posteriorly the spines become progressively less numerous, two-pointed, single-pointed and disappear completely near the posterior extremity of the hindbody. It has been shown that, in certain cases during digenean development, simple spines became modified and acquired a more complex, serrate shape (Køie, 1992). Furthermore, Bennett (1975) noted that the divisions at the tips of the tegumental spines in juvenile Fasciola hepatica L., 1758 occurred with the worm growth, and this author associated this division with the abrasion of the host tissue and locomotion within the host. Moreover, Køie (1977) has suggested that simple spines occur in highly migratory forms, and that serrate spines are characteristic of non-migratory stages.
With regard to the diplostomoids, metacercariae of Diplostomum phoxini bear simple tegumentary spines on the ventral surface (Bibby & Rees, 1971), whereas the distribution of serrate spines on adults of the same species has been described by Erasmus (1970b). As another example, in the mesocercariae of the diplostomid Alaria marcianae, simple spines are located along the margins of the ventral surface to the posterior end, but during transformation to the adult stage the spines give rise to a three-pointed dentation (Shoop & Corkum, 1984). Furthermore, the occurrence of multi-pointed spines has been reported in adult D. tregenna (see Ibraheem, 2000), and single-pointed spines become double-pointed and then multi-pointed during the growth of adults of the cyathocotylid Szidatia joyeuxi in the definitive host (Mouahid, 1989). Multi-pointed spines have also been observed on the surface of adult Cynodiplostomum azimi (see Ramadan & Ibraheem, 2000). A change from the pointed cercarial spines to scale-like, serrate spines in infective metacercariae and adults has been observed in other groups of trematodes, e.g. the families Heterophyidae, Lepocreadiidae, Gymnophallidae and Microphallidae (Køie, 1977, 1985, 1992; Fujino et al., 1979; Pekkarinen, 1986; Scholz et al., 1991; Lim et al., 2008).
Due to the absence of any other ultrastructural studies on specimens of Nematostrigea with regard to detailed information on tegumental transformations that may take place during the migration of the Nematostrigea sp. in both intermediate and definitive hosts, we can only suggest, in view of the aforementioned changes in spine shape during development, that the presence of multi-pointed spines on the body of the metacercaria is a final phase of spine modification and that multi-pointed spines are present in the unknown adult. Nevertheless, diplostomoids are normally considered as unarmed (at least at the light microscope level). Gibson (1996), in a superfamily diagnosis for the Diplostomoidea in relation to metacercariae, stated: ‘Tegument unarmed’ and listed it as a key feature of the group, and Niewiadomska (2002) made no mention of body spination for adult diplostomoids or in the diagnoses of families and genera in related chapters. However, in ultrastructural observations on the metacercarial stage of another group of trematodes, Fujino et al. (1979) found that in the opisthorchiid Clonorchis sinensis (Cobbold, 1875) the spines of the metacercariae gradually disappear and are completely absent from the fully-developed adults; their explanation being that “these spines may be absorbed into the tegument during the late stage of development” (p. 585). Kobayashi (1915) also mentioned that the disappearance of body spines in this species may be due to abrasion during growth within the host. However, according to Conn et al. (2008, p. 641), “various structures associated with the tegument of several larval trematodes appear to play a role in migration and site selection… perhaps it is not surprising that these structures regress following location within particular sites”. It should be noted, however, that the spines observed on diplostomoids using EM observations are very small and not usually reported in LM observations, even at the metacercarial stage. Body spination observed with the LM in adult digeneans occurs in all three orders of the Digenea recognised by Gibson & Bray (1994), although it is very restricted in the Strigeida compared to the other two orders.
It should be noted that the TEM study of the spineless area at the posterior end of the hindbody of Nematostrigea sp. indicates that the fragments of spines are attached to the basement membrane of the tegument, despite the apparent absence of these structures on the surface under SEM observation. This suggests either the presence of spines along the entire trematode body at an earlier stage of its evolution (in this case the fragments are the vestiges of spines), or the appearance of spines in adults (i.e. they are primordial spines).
The spination pattern on the suckers of diplostomoids has been examined by EM in only a few species: unspined surfaces of the oral and ventral suckers have been illustrated in adult Diplostomum phoxini by Erasmus (1970b); 14–15 rows of simple spines on the oral sucker and 3–4 rows on the ventral sucker have been observed in mesocercarial Alaria marcianae, a species whose spines on the suckers are apparently lost during the ‘diplostomulum’ stage (Shoop & Corkum, 1984); an unspined oral sucker and a spined ventral sucker have been demonstrated in adults of Cynodiplostomum azimi (see Ramadan & Ibraheem, 2000); spined oral and ventral suckers are present in adult Szidatia joyeuxi (see Mouahid, 1989); and the metacercaria of Nematostrigea sp. has an unspined oral sucker and, except for the rim, the circumference of the ventral sucker is covered with uniformly distributed, six-pointed spines (Present study).
Final comments
In the present work, the topographical features of the tegumental surface of the metacercaria of Nematostrigea sp. are described for the first time. Among the ultrastructural features found are a number which might prove useful as identification criteria for the species Nematostrigea sp., and even for the genus Nematostrigea. These are as follows:
-
1.
the ventral forebody papillae are distributed in three permanent regions (as two rings of dome-shaped papillae localised at different levels on the rim of the oral sucker, as a single ring of ciliated papillae on the inner margin of the ventral sucker, and as a band of dome-shaped papillae along the lateral margins of the broad body fold of the ventral forebody); there are also irregularly dome-shaped papillae which occur all over the body but are more numerous on the ventral forebody, including the lappets and holdfast organ;
-
2.
the oral sucker and anteroventral surface of the forebody are unspined, but the latter bears protuberant secretory pores;
-
3.
the armed ventral sucker, which, except for the smooth surface of the rim, is covered by six-pointed spines;
-
4.
the presence of multi-pointed spines along the ventral and dorsal sides of the forebody, with the number of teeth increasing posteriorly;
-
5.
the multi-pointed spines of the forebody gradually transform into single-pointed, more widely distributed spines in the hindbody, disappearing completely at the posterior end of the body;
-
6.
the surface of the pseudosuckers has a particular distribution of pores that lead to three types of secretory glands, plus three topographical modifications (areas where the surface is smooth, bears digitiform processes or bears recurved, dagger-shaped spines);
-
7.
the surface of the holdfast organ bears densely packed, straight or slightly curved, simple spines on its lateral surface but is smooth medially.
The present study extends our knowledge of diplostomoids by taking into account details of another genus, Nematostrigea. The basic topographical and ultrastructural features of the tegument of this genus resemble those described for other members of the group. Particular specialisations observed in Nematostrigea sp. appear to represent taxonomically informative characters, some of which may be useful at the generic level—such data may prove to be important for a genus which is essentially based on body-shape (cf. Niewiadomska, 2002).
References
Abdel-Aal, A. A., Soliman, M. F. M., & Shalaby, I. M. (2004). Surface ultrastructure of Cardiocephalus longicollis (Digenea: Strigeidae) from herring gull, Larus argentatus, and its associated pathological lesions. Helminthologia, 41, 175–178.
Abdul-Salam, J., & Sreelatha, B. N. S. (1993). Studies on cercariae from Kuwait Bay. II. Description and surface topography of Cercaria kuwaitae II sp.n. (Digenea, Cyathocotylidae). Acta Parasitologica, 38, 1–7.
Arvy, I. (1954). Distomatose cêrébro-rachidienne due à Diplostomum phoxini (Faust), Hughes 1920, chez Phoxinus laevis Ag. Annales de Parasitologie Humaine et Comparée, 29, 510–520.
Bell, A. S. (1995). Studies on the biosystematics and biology of strigeids (Digenea) parasitic in freshwater fish. PhD Thesis, Institute of Aquaculture, University of Stirling, 402 pp.
Bell, A. S., Gibson, D. I., & Sommerville, C. (1997). Chaetotaxy and armature of Ichthyocotylurus erraticus (Rudolphi, 1809) and I. variegatus (Creplin, 1825) cercariae (Digenea, Strigeidae). Parasitology Research, 83, 70–76.
Bennett, C. E. (1975). Scanning electron microscopy of Fasciola hepatica L. during growth and maturation in the mouse. Journal of Parasitology, 61, 892–898.
Bhatti, I., & Johnson, A. D. (1972). Enzyme histochemistry of the holdfast and forebody gland cells of Alaria marcianae (La Rue, 1917) (Trematoda: Diplostomatidae). Proceedings of the Helminthological Society of Washington, 39, 78–87.
Bibby, M. C., & Rees, G. (1971). The ultrastructure of the epidermis and associated structures in the metacercaria, cercaria and sporocyst of Diplostomum phoxini (Faust, 1918). Zeitschrift für Parasitenkunde, 37, 169–186.
Combes, C. (1980). Atlas, mondial des cercairae. Mémoires du Muséum National d’Histoire Naturelle, Ser. A Zoologie, 115, 1–235.
Conn, D. B., Goater, C. P., & Bray, D. (2008). Developmental and functional ultrastructure of Ornithodiplostomum ptychocheilus diplostomula (Trematoda: Strigeoidea) during invasion of the brain of the fish intermediate host, Pimephales promelas. Journal of Parasitology, 94, 635–642.
Erasmus, D. A. (1967). The host-parasite interface of Cyathocotyle bushiensis Khan, 1962 (Trematoda: Strigeoidea). II. Electron microscope studies of the tegument. Journal of Parasitology, 53, 703–714.
Erasmus, D. A. (1969a). Studies on the host-parasite interface of strigeoid trematodes. VI. Ultrastructural observations on the lappets of Diplostomum phoxini Faust, 1918. Zeitschrift für Parasitenkunde, 32, 48–58.
Erasmus, D. A. (1969b). Studies on the host-parasite interface of strigeoid trematodes. V. Regional differentiation of the adhesive organ of Apatemon gracilis minor Yamaguti, 1933. Parasitology, 59, 245–256.
Erasmus, D. A. (1970a). The host-parasite interface of strigeoid trematodes. VII. Ultrastructural observation on the adhesive organ of Diplostomum phoxini Faust, 1918. Zeitschrift für Parasitenkunde, 33, 211–224.
Erasmus, D. A. (1970b). The host-parasite interface of strigeoid trematodes. IX. A probe and transmission electron microscope study of the tegument of Diplostomum phoxini Faust, 1918. Parasitology, 61, 35–41.
Erasmus, D. A., & Öhman, C. (1965). Electron microscope studies of the gland cells and host-parasite interface of the adhesive organ of Cyathocotyle bushiensis Khan, 1962. Journal of Parasitology, 51, 761–769.
Fried, B., Irwin, S. W. B., & Lowry, S. F. (1990). Scanning electron microscopy of Echinostoma trivolvis and E. caproni (Trematoda) adults from experimental infections in the golden hamster. Journal of Natural History, 24, 433–440.
Fujino, T., Ishii, Y., & Choi, D. W. (1979). Surface ultrastructure of the tegument of Clonorchis sinensis newly excysted juveniles and adult worms. Journal of Parasitology, 65, 579–590.
Gibson, D. I. (1996). Trematoda. In: L. Margolis & Z. Kabata (Eds.), Guide to the parasites of fishes of Canada. Part IV. Canadian Special Publication of Fisheries and Aquatic Sciences No. 124. Ottawa: NRC Press, 373 pp.
Gibson, D. I., & Bray, R. A. (1994). The evolutionary expansion and host-parasite relationships of the Digenea. International Journal for Parasitology, 24, 1213–1226.
Higo, H., & Ishii, Y. (1984). Scanning electron microscopy of the newly excysted juveniles Paragonimus westermani (Kerbert, 1878) Braun, 1899 (parthenogenetic type) and P. miyazakii Kamo, Nishida, Hatsushika and Tominura, 1961. Japanese Journal of Parasitology, 33, 421–427.
Higo, H., & Ishii, Y. (1987). Comparative studies on the surface ultrastructure of newly excysted metacercariae of Japanese lung flukes. Parasitology Research, 73, 541–549.
Ibraheem, M. H. (2000). A light and electron microscope study on Diplostomum tregenna Nazmi Gohar, 1932 (Digenea: Diplostomatidae). Helminthologia, 37, 137–142.
Jamjoom, M. B., & Shalaby, I. (2006). The contribution of electron microscopic studies to the taxonomy and biology of parasitic trematodes. World Journal of Zoology, 1, 64–81.
Johnson, A. D., Bhatti, I., & Kanemoto, N. (1971). Structure and function of the holdfast organ and lappets of Alaria marcianae (La Rue, 1917) (Trematoda: Diplostomatidae). Journal of Parasitology, 57, 235–243.
Jones, A., Bray, R. A., & Gibson, D. I. (Eds.). (2005). Keys to the Trematoda. Vol. 2. Wallingford: CAB International, 745 pp.
Kobayashi, H. (1915). On the life-history and morphology of Clonorchi sinensis. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten (und Hygiene), 75, 299–313.
Køie, M. (1977). Stereoscan studies of cercariae, metacercariae, and adults of Cryptocotyle lingua (Creplin, 1925) Fischoeder, 1903 (Trematoda: Heterophyidae). Journal of Parasitology, 63, 835–839.
Køie, M. (1985). On the morphology and life-history of Lepidapedon elongatum (Lebour, 1908) Nicoll, 1910 (Trematoda, Lepocreadiidae). Ophelia, 24, 135–153.
Køie, M. (1992). Scanning electron microscopy of cercariae, metacercariae and adults of Pygidiopsis ardeae Køie, 1990 (Digenea, Heterophyidae). Parasitology Research, 78, 469–474.
Komalamisra, C., Bunchuen, S., Waikagul, J., & Pongponratn, E. (2005). Chaetotaxy of newly excysted metacercariae among five species of Thai Paragonimus. Journal of Tropical Medical Parasitology, 28, 1–7.
Lee, D. L. (1962). Studies on the function of the pseudosuckers and holdfast organ of Diplostomum phoxini Faust (Strigeida, Trematoda). Parasitology, 52, 103–112.
Lim, D., Choi, K., Guk, S., Chai, J., Park, I., Park, Y., et al. (2008). Tegumental ultrastructure of adult Gynaecotyla squatarolae (Digenea: Microphallidae). Korean Journal of Parasitology, 46, 87–90.
McKeown, C. A., & Irwin, S. W. B. (1995). The life cycle stages of three Diplostomum species maintained in the laboratory. International Journal for Parasitology, 25, 897–906.
Mouahid, A. (1989). Szidatia joyeuxi (Trematoda, Cyathocotylidae): morphological and tegumental changes during growth in the definitive host. Systematic Parasitology, 13, 125–134.
Niewiadomska, K. (2002). Superfamily Diplostomoidea Poirier, 1886. In: D. I. Gibson, A. Jones, & R. A. Bray (Eds.), Keys to the Trematoda. Vol. 1. Wallingford: CAB International, pp. 159–166.
Öhman, C. (1965). The structure and function of the adhesive organ in strigeid trematodes. Part II. Diplostomum spathaceum Braun, 1893. Parasitology, 55, 481–502.
Öhman, C. (1966a). The structure and function of the adhesive organ in strigeid trematodes. Part III. Apatemon gracilis minor Yamaguti, 1933. Parasitology, 56, 209–226.
Öhman, C. (1966b). The structure and function of the adhesive organ in strigeid trematodes. IV. Holostephanus lühei Szidat, 1936. Parasitology, 56, 633–637.
Pekkarinen, M. (1986). Development of the cercaria of Lacunovermis macomae (Trematoda: Gymnophallidae) to the metacercaria in brackish-water Macoma balthica. Annales Zoologici Fennici, 23, 237–250.
Podvyaznaya, I. M. (1999). The fine structure of the tegument of cercariae and developing metacercariae of Diplostomum chromatophoprum (Trematoda: Diplostomidae). Parazitologiya, 33, 507–519. (In Russian).
Ramadan, M. A., & Ibraheem, M. H. (2000). A contribution to the surface ultrastructure of adult Cynodiplostomum azimi (Gohar, 1933) Dubois, 1936 (Digenea: Cynodiplostomatidae): with special reference to its internal anatomy. Journal of the Union Arab Biologists, Cairo, Zoology, 13A, 531–544.
Rushton, W. (1937). Blindness in freshwater fish. Nature, 140, 1014.
Scholz, T., Ditrich, O., Tuma, M., & Giboda, M. (1991). Study of the body surface of Haplorchis yokogawai (Katsuta, 1932) and H. taichui (Nishigori, 1924) (Trematoda: Heterophyidae). Southeast Asian Journal of Medicine and Public Health, 22, 443–448.
Shigin, A. A. (1986). Trematodes of the fauna of the USSR. Genus Diplostomum metacercariae. Moscow: Academii Nauk SSSR, 253 pp. (In Russian).
Shoop, W., & Corkum, K. C. (1984). Tegumental changes of Alaria marcianae (Trematoda) during migration in the domestic cat. Journal of Parasitology, 70, 244–252.
Whittington, I. D., & Cribb, B. W. (2001). Adhesive secretions in the Platyhelminthes. Advances in Parasitology, 48, 101–223.
Acknowledgements
The authors would like to thank the workers of the Coastal Department of the Russian-Vietnamese Tropical Centre in Vietnam for their continuous support and the staff of the Centre of Electron Microscopy, Institute of Biology of Inland Waters, Russia. The present study was supported by the Russian Foundation for Fundamental Research (project no. 09-04-00342a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poddubnaya, L.G., Mishina, E., Zhokhov, A.E. et al. Ultrastructural features of the tegumental surface of a new metacercaria, Nematostrigea sp. (Trematoda: Strigeidae), with a search for potential taxonomically informative characters. Syst Parasitol 75, 59–73 (2010). https://doi.org/10.1007/s11230-009-9207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-009-9207-5