Abstract
Mosquitoes (Diptera: Culicidae) are the largest group of blood-feeding insects that disturb not only humans but also other mammals and birds. This study reports the presence of native mosquito species in the regional unit of Thessaloniki and the monitoring of their population. In total, 13 mosquito species belonging to four genera were identified. The most dominant species was Culex pipiens, followed by Aedes caspius. In the present study, we report for the first time the presence of Ae. vittatus in Greece and of Anopheles plumbeus in the regional unit of Thessaloniki. Regarding the seasonal variation, species of the genus Aedes were the ones that first appeared in late March, followed by Culex species at the end of April and finally species of the genus Anopheles in July. Species of the Aedes genus were found to be the most abundant in the first quarter of the year (late March to early April). Population of Cx. pipiens remained at high levels from late April to late September. Species of the genus Anopheles were found in high densities from early August to October. The current study contributes to the knowledge of the mosquito species composition and their relative abundance in an area where West Nile virus caused severe epidemic outbreaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scourge of mosquitoes exists in Greece from early times as illustrated by various historical references (Bruce-Chwatt and Zuluetta 1980). The first attempts to record native mosquito species in Greece were initiated at the beginning of the twentieth century right after the identification of the role of Anopheles mosquitoes in malaria transmission. Cardamatis (1931) was the first researcher who recorded mosquito species throughout Greece, and his catalogue included 19 species. Pandazis’ catalogue (1935) presented 38 species belonging to 7 genera. A subsequent record was the one belonging to Sakelariou and Lane (1977) which included 38 species. In 1993, Samanidou-Voyadjoglou and Dorsie published a catalogue based mainly on previous bibliographical references which contained 7 genera, 15 subgenera, 53 species, and 2 subspecies. The most recent catalogue of the native mosquito species in Greece was published by Voyadjoglou-Samanidou (2011), including 64 species belonging to 8 genera.
The identification of mosquito species is a key factor for successful mosquito control program. Despite the wide range of taxonomic catalogues in Europe, the taxonomy of European mosquitoes still requires further investigation. Many questions arise concerning the formal status of various nominal species and subspecies, while the entire mosquito fauna is generally poorly examined. Although there are various studies on the mosquito fauna of certain countries, the vast majority of them are not up to date and lack a broader scope. Like most European countries, the catalogue of mosquito species in Greece is not investigated in depth. Studies on the mosquito fauna in different regions of Greece during the last four decades have primarily been focused on anopheline mosquitoes due to the severe malaria outbreak recorded at that time (Samanidou-Voyadjoglou and Darsie 1993) and secondarily on new distribution of mosquito species in certain area (Kaiser et al. 2001; ECDC 2014; Kioulos et al. 2014; Lytra and Emmanouel 2014; Beleri et al. 2017). The main reason why the investigation of native mosquito fauna has regained substantial scientific interest in recent years is basically attributed to the epidemic incidents of mosquito-borne viruses, such as West Nile Virus, which have sporadically resulted in human casualties, as well as to the ongoing threat of malaria reintroduction (ECDC 2010; Patsoula et al. 2016; NPHO 2019). The aim of the present study was (1) to inventory the native mosquito fauna of the regional unit (RU) of Thessaloniki, (2) to report the seasonal variation of species, and (3) to determine species imposing risk to public health.
Materials and methods
Study area
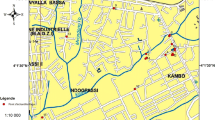
The RU of Thessaloniki is one of the biggest administrative areas of Greece, with a total area of 3,683 km2 and belongs to the Central Macedonia Region. The RU is divided into 13 municipalities and its landscape is dominated by extensive rice fields and farmlands throughout the west and northwest and mountainous areas in the north and northeast. Climate is mainly Mediterranean in the southern part with hot summers and cool to mild winters, while in the northern part, the climate is more continental with colder winters. Thessaloniki, the capital city of the RU, is the second largest city in Greece, with over one million inhabitants in its metropolitan area, located on the Thermaic Gulf, at the northwest corner of the Aegean Sea.
The RU of Thessaloniki suffers from high mosquito populations every summer causing significant nuisance to inhabitants and visitors. It is one of the areas mostly affected by the recent outbreaks of West Nile Fever (WNF) in Greece (NPHO 2010; Patsoula et al. 2016; NPHO 2019). Larval collection took place from April to October through the years 2012 and 2013. In total, 11 sampling stations that covered most of the representative sites of the Thessaloniki RU were established (Metropolitan area, Kalamaria, Polichni, Thermi, Epanomi, Kalochori, Pentalofos, Chalastra, Axios, Lagadas, and Volvi) (Fig. 1). Various types of larval habitats were sampled, either permanent or temporary, e.g., rice fields, river margins, lake margins, ponds, rain water craters, marsh, and irrigation canals (Table 1).
Specimen and data collection
In the present study, larval collection was carried out using the traditional dipping method (Silver 2007) with a dipper with extendable handle (volume: 300 mL) (BioQuip Products, CA, USA). Each sample consisted of the content of 5 consecutive dipper sinks in random spots, and a total of approximately 1,500 mL water volume was placed in plastic cups (8 cm in diameter and 14 cm in height), which were afterwards carried to the Laboratory of Applied Zoology and Parasitology of the Aristotle University of Thessaloniki within 2 h. To avoid exposure to high temperatures, the plastic cups were carried in a cooler. A layer of newspaper was used to prevent direct contact of the plastic cups with ice packs at the bottom of the cooler (Andreadis et al. 2014). Samplings were executed every 15 days in each region from early April until end of October for 2 consecutive years, 2012 and 2013, reaching a total of 196 samplings. As soon as the samples were transferred to the laboratory, the larvae and pupae were counted, and microscope slides of the larvae were prepared. The third- and fourth-instar larvae were identified using the keys of Snow (1990), Darsie and Samanidou-Voyadjoglou (1997), and Becker et al. (2010). In addition, adult mosquitoes were reared from the pupae in standard laboratory conditions (25 ± 1 °C, 65 ± 5% RH, and 16:8 h L:D). Emerging adults were kept alive for 24 h, so that all the taxonomic characteristics were complete, such as color pattern or the shape of scales. The adult mosquitoes were pinned and separately classified by the sampling date to identify them using appropriate keys (Samanidou-Voyadjoglou and Harbach 2001; Becker et al. 2010).
Results
During this 2-year study, 6,227 third- and fourth-instar larvae and pupae were collected and identified to the species level based on morphological characteristics. Thirteen species within 4 genera were identified in the studied areas (Table 2). In the majority of the areas that were studied the most common species was Cx. pipiens (45.5% of total samples), followed by Ae. caspius (23.2%) (Table 2). In specific regions, Anopheles species occupied a significant percentage of the mosquito population (An. hyrcanus and An. maculipennis s.l., 8.6 and 8.5%, respectively) (Table 2). The other species were not found in large numbers and formed < 5% of the total samples (Table 2).
Regarding the seasonal variation of species, it was observed that mosquitoes of the genus Aedes appear first (late March) in 2012 and 2013 compared to the other species, followed by the genus Culex (April to May) (Fig. 2). Individuals of the genus Anopheles were the last to occur in the region in mid-July in both years. Culiseta mosquitoes were only recorded in 2013 in low populations in June. At the beginning of the period, from early April to mid-May, the most abundant species was Ae. caspius (Table 3). Culex pipiens appeared in mid-May, and its population remained high until September (Table 3). Dominant species from early August to October were species of the genus Culex and Anopheles in both years, 2012 and 2013 (Fig. 2).
Discussion
According to the hitherto known facts, in Greece (mainland and islands), 64 mosquito species exist (Voyadjoglou-Samanidou 2011). In the present study, 13 of these species have been identified in the RU of Thessaloniki. The sampling method applied in this research is focused on the inspection of the larval breeding habitats. The above-mentioned sampling method apart from the fact that it provides basic information about the mosquito species composition of the study areas, it also offers specific information on the locations where larvicides could be applied and thus contributing to the quality control of mosquito control programs that are implemented over temperate areas (Michaelakis et al. 2021). One of the most significant factors of a rationally designed mosquito control program is to be mainly focused on the species which cause particular discomfort or those species which are competent vectors, posing a potential danger for transmitting disease agents (Govella et al. 2013; Majeed 2013). On the contrary, non-targeted control of mosquito and other Diptera species of no particular interest for public health could possibly disrupt the ecological balance (Majeed 2013).
The following species have been identified in the present study: Cx. pipiens, Cx. territans, Cx. theileri, Cx. mimeticus, Cx. torrentium, Ae. caspius, Ae. vexans, Ae. vittatus, An. maculipennis s.l., An. hyrcanus, An. plumbeus, Cs. annulata, and Cs. longiareolata. It is the first time that Ae. vittatus has been recorded in Greece and the first time that An. plumbeus has been found in the RU of Thessaloniki. All the other species recorded in this study have been previously reported to be native in the region (Samanidou-Voyadjoglou and Darsie 1993; Voyadjoglou-Samanidou 2011; Lytra 2015). Interestingly, although Ae. albopictus is confirmed in the RU of Thessaloniki (Badieritakis et al. 2018), it was not possible to be collected during this study due to the fact that the breeding sites that we surveyed were not suitable for its development. This species is mainly established in urban areas and therefore is very difficult to identify their breeding sites with the selected sampling method (Stefopoulou et al. 2018; Bellini et al. 2020).
Concerning the public health significance of the identified species, Ae. caspius has been reported positive to WNV, Tahyna virus, and bacterium Francisella tularensis (Milankov et al. 2009; Vázquez et al 2012). Culex pipiens mosquitoes are the main vectors of WNV causing severe epidemic outbreaks of West Nile Fever in Romania and New York in 1996 and 1999, respectively (Nicolescu 1998; Bernard et al. 2001). It is also considered responsible for the re-emergence of West Nile Fever in Greece in 2010 onwards (Gomes et al. 2013). West Nile Virus provoked serious outbreaks in the Balkan area, including Greece, Romania, Croatia, the Former Yugoslav Republic of Macedonia, Kosovo, Montenegro, and Serbia during the 2010s (Sambri et al. 2013). Climatic conditions, temperature, and humidity favor the presence and the multiplication of Culex spp. from May to October in the affected zones (ECDC 2010). Culex pipiens biotype pipiens seems to play a minor role as an arbovirus vector in Europe, whereas Cx pipiens biotype molestus presents greater medical significance (Voyadjoglou-Samanidou 2011). Culiseta annulata is known to be a potential vector of Tahina virus (Danielová 1972) and various Plasmodium sp. on birds and humans (Valkiunas 2005; Inci et al. 2012). Culex territans, Cx. theileri, Cx. mimeticus, Ae. vexans, Ae. vittatus, and Cs. longiareolata species recorded in this study are not considered of great medical importance.
When it comes to Anophelinae species, An. hyrcanus due to its exophilic nature is not considered to be an important malaria vector in the Mediterranean region (Piperaki and Daikos 2016; Tagliapietra et al. 2019). On the other hand, members of the Anopheles maculipennis complex had been reported to be responsible for the transmission of malaria to humans in Greece (Betzios 1989). Contrary to the previous scientific assumption, Papadakis (1956) claims that its sporozoic index was low, thus was considered to be less important in transmitting malaria. Nonetheless, in certain local outbreaks of the disease, An. maculipennis complex was the only Anopheles species to be found active in the region (Voyadjoglou-Samanidou 2011). Regarding An. plumbeus, even though laboratory studies have shown that it can carry Plasmodium vivax (Marchant et al. 1998), because of its feeding behavior, it is not considered epidemiologically hazardous (Becker et al. 2010).
Seasonal abundance of mosquitoes is affected greatly by climate variables such as temperature and rainfall that alter the quantity and quality of mosquito breeding habitats (Bashar and Tuno 2014; Roiz et al. 2014). Drakou et al. (2020) reported that monthly relative humidity showed positive correlation with the numbers of the species that were sampled, i.e., Cx. pipiens, Ae. detritus, and Ae. caspius. Moreover, mosquito abundance of Cx. pipiens and Ae. detritus was strongly correlated to seasonal precipitation (Drakou et al. 2020). The relationship between environmental factors and mosquito populations provides crucial information on parasite activity levels; thus, it contributes towards prediction of mosquito-borne disease outbreaks (Bashar and Tuno 2014; Roiz et al. 2014; Meuti and Short 2019; Drakou et al. 2020). Other factors influencing mosquito populations include the presence of potential predators or competitors in larval habitats and human activities such as cultural practices in rice fields (Lytra and Emmanouel 2014). In the present study, the seasonal distributions of the most abundant species reveal population fluctuations in different months. Culex pipiens was the dominant species present almost throughout the year with peaks in June and August and smaller peaks in September. In contrast, Anopheles species were found during July to September only, with a peak observed in August to September. Aedes species were the first to appear in the season with a peak of their population in April to May. Our findings are in accordance with a previous study conducted in various geographical areas of Central and Southern Greece during the years 2009–2011 (Lytra 2015).
Thorough notes of the composition and seasonal abundance of mosquitoes in a specific region is fundamental for the development of efficient mosquito control programs (Alten et al. 2000). Moreover, detailed studies of bionomics, surveillance, and disease transmission dynamics of mosquitoes are required for predicting disease outbreaks and vector control in a region (Bashar and Tuno 2014). Taking this into consideration, recording the mosquito fauna in the RU of Thessaloniki as well as their seasonal abundance is essential for designing an integrated control program especially against species with medical importance. Such catalogues should be created in all prefectures of Greece, and mosquito populations should be monitored in a regular basis which will eventually contribute to the most effective control of the mosquitoes.
References
Alten B, Bellini R, Caglar SS, Simsek FM, Kaynas S (2000) Species composition and seasonal dynamics of mosquitoes in the Belek region of Turkey. J Vector Ecol 25:146–154
Andreadis SS, Dimotsiou OC, Savopoulou-Soultani M (2014) Variation in adult longevity of Culex pipiens form pipiens, vector of the West Nile Virus. Parasitol Res 113:4315–4319
Badieritakis Ε, Papachristos D, Latinopoulos D, Stefopoulou A, Kolimenakis A, Bithas K, Patsoula E, Beleri S, Maselou D, Balatsos G, Michaelakis A (2018) Aedes albopictus (Skuse, 1895) (Diptera: Culicidae) in Greece: 13 years of living with the Asian tiger mosquito. Parasitol Res 117:453–460
Bashar K, Tuno N (2014) Seasonal abundance of An. mosquitoes and their association with meteorological factors and malaria incidence in Bangladesh. Parasit Vectors 7:442
Becker N, Petrić D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A (2010) Mosquitoes and their control, 2nd edn. Springer, Heidelberg
Beleri S, Chatzinikolaou S, Nearchou A, Patsoula E (2017) Entomological study of the mosquito fauna in the Regional Unit of Drama, Region of East Macedonia-Thrace, Greece (2015 to 2016). Vector Borne Zoonotic Dis 17:665–671
Bellini R, Michaelakis A, Petrić D, Schaffner F, Alten B, Angelini P, Aranda C, Becker N, Carrieri M, Di Luca M, Fălcuţă E, Flacio E, Klobučar A, Lagneau C, Merdić E, Mikov O, Pajovic I, Papachristos D, Sousa CA, Stroo A, Toma L, Vasquez MI, Velo E, Venturelli C, Zgomba M (2020) Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med Infect Dis 35:101691
Bernard KA, Maffei JG, Jones SA, Kauffman EB et al (2001) West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis 7:679
Betzios BC (1989) Arthropods of medical importance: morphology, biology, ecology, medical importance, control [in Greek]. Stamoulis Publications, Athens
Bruce-Chwatt LJ, De Zulueta J (1980) The rise and fall of malaria in Europe: a historico-epidemiological study. Oxford University Press (on behalf of the Regional Office for Europe of the World Health Organization), Oxford
Cardamatis JP (1931) Les espèces de moustiques en Grèce et tout particulièrement d’Athènes. Bull Soc Pathol Exot 24:122–131
European Centre for Disease Prevention and Control, ECDC (2010) West Nile virus infection outbreak in humans in Central Macedonia, Greece: July–August 2010. ECDC, Stockholm
European Centre for Disease Prevention and Control, ECDC (2014) Guidelines for the surveillance of native mosquitoes in Europe. ECDC, Stockholm
Danielová V (1972) The vector efficiency of Culiseta annulata mosquito in relation to Tahyna virus. Folia Parasitol 19:259–262
Darsie RF Jr, Samanidou-Voyadjoglou A (1997) Keys for the identification of the mosquitoes of Greece. J Am Mosq Control Assoc 13:247–254
Drakou K, Nikolaou T, Vasquez M, Petric D, Michaelakis A, Kapranas A, Papatheodoulou A, Koliou M (2020) The effect of weather variables on mosquito activity: a snapshot of the main point of entry of Cyprus. Int J Environ Res Public Health 17:1403
Gomes B, Kioulos E, Papa A, Almeida AP, Vontas J, Pinto J (2013) Distribution and hybridization of Culex pipiens forms in Greece during the West Nile virus outbreak of 2010. Infect Genet Evol 16:218–225
Govella NJ, Chaki PP, Killeen GF (2013) Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J 12:124
Inci A, Yildirim A, Njabo KY, Duzlu O, Biski Z, Ciloglu A (2012) Detection and molecular characterization of avian Plasmodium from mosquitoes in central Turkey. Vet Parasitol 188:179–184
Kaiser A, Jerrentrup H, Samanidou-Voyadjoglou A, Becker N (2001) Contribution to the distribution of European mosquitoes (Diptera: Culicidae): four new country records from northern Greece. Eur Mosq Bull 10:9–12
Kioulos I, Michaelakis A, Kioulos N, Samanidou-Voyadjoglou A, Koliopoulos G (2014) Mosquito (Diptera: Culicidae) fauna in natural breeding sites of Attica basin, Greece. Hell Plant Prot J 7:31–34
Lytra I, Emmanouel N (2014) Study of Culex tritaeniorhynchus and species composition of mosquitoes in a rice field in Greece. Acta Trop 134:66–71
Lytra I (2015) Study of the presence of Culicidae in Greece, of their molecular classification and of the seasonal variation and of the present mosquitoes control system in rice fields: evaluation of biocides about their effectiveness against Aedes albopictus (Diptera: Culicidae). Dissertation, Agricultural University of Athens
Majeed S (2013) Odour-mediated host preference in mosquitoes: the role of the maxillary palps in host recognition. Dissertation, Swedish University of Agricultural Sciences
Marchant P, Eling W, van Gemert G-J, Leake CJ, Curtis CF (1998) Could British mosquitoes transmit Falciparum malaria? Parasitol Today 14:344–345
Meuti ME, Short SM (2019) Physiological and environmental factors affecting the composition of the ejaculate in mosquitoes and other insects. Insects 10:74
Michaelakis A, Balestrino F, Becker N, Bellini R, Caputo B, della Torre A, Figuerola J, L’Ambert G, Petric D, Robert V, Roiz D, Saratsis A, Sousa CA, Wint WGR, Papadopoulos NT (2021) A case for systematic quality management in mosquito control programmes in Europe. Int J Environ Res Public Health 18:3478
Milankov V, Petric D, Vujic A, Vapa L (2009) Taxonomy, biology, genetic variability and medical importance of Ochlerotatus caspius (Pallas, 1771) and O. dorsalis (Meigen, 1830) (Diptera: Culicidae). Acta Entomol Serbica 14:195–207
National Public Health Organization, NPHO (2010) Epidemiological data for West Nile Virus infection outbreak, Greece, 2010 [in Greek]. https://eody.gov.gr/wp-content/uploads/2019/01/etisia_ekthesi_idn_2010_ellada_revised_2013.pdf. Accessed 29 Dec 2020
National Public Health Organization, NPHO (2019) Annual epidemiological report for West Nile virus human infection, Greece, 2019. https://eody.gov.gr/wp-content/uploads/2019/01/WNV_annual_report_2019_ENG.pdf. Accessed 29 Dec 2020
Nicolescu G (1998) A general characterization of the mosquito’s fauna (Diptera: Culicidae) in the endemic area of West Nile Virus in the South of Romania. Europ Mosq Bull 2:13–19
Pandazis G (1935) La faune des Culcides de Grèce. Acta Inst Mus Zool Univ Athen 1:1–27
Papadakis AM (1956) Parasitology: protozoa, helminths, arthropods and parasitic diseases [in Greek]. Private edition, Athens
Patsoula E, Vakali A, Balatsos G, Pervanidou D, Beleri S, Tegos N, Baka A, Spanakos G, Georgakopoulou T, Tserkezou P, Van Bortel W, Zeller H, Menounos P, Kremastinou J, Hadjichristodoulou C (2016) West Nile virus circulation in mosquitoes in Greece (2010–2013). Biomed Res Int 2450683:1–13
Piperaki ET, Daikos GL (2016) Malaria in Europe: emerging threat or minnow nuisance? Clin Microbiol Infect 22:487–493
Roiz D, Ruiz S, Soriguer R, Figuerola J (2014) Climatic effects on mosquito abundance in Mediterranean wetlands. Parasit Vectors 7:1–13
Sakelariou M, Lane J (1977) Notes on the Culicidae recorded in the Copais District of Greece, 1974–1976. Ann Benaki Phytopathol Inst 11:321–328
Samanidou-Voyadjoglou A, Darsie RF Jr (1993) An annotated checklist and bibliography of the mosquitoes of Greece (Diptera: Culicidae). Mosq Syst 25:177–185
Samanidou-Voyadjoglou A, Harbach RE (2001) Keys to the adult female mosquitoes (Culicidae) of Greece. Europ Mosq Bull 10:13–20
Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, Niedrig M, Papa A, Pierro A, Rossini G, Varani S, Vocale C, Landini MP (2013) West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect 19:699–704
Silver JB (2007) Mosquito ecology: field sampling methods, 3rd edn. Springer, Dordrecht
Snow KR (1990) Mosquitoes, naturalists’ handbooks 14. Richmond Publishing Ltd., Slough
Stefopoulou Α, Balatsos G, Petraki A, LaDeau SL, Papachristos D, Michaelakis A (2018) Reducing Aedes albopictus breeding sites through education: a study in urban area. PLOS ONE 13(11):e0202451
Tagliapietra V, Arnoldi D, Di Luca M, Toma L, Rizzoli A (2019) Investigation on potential malaria vectors (Anopheles spp.) in the Province of Trento, Italy. Malar J 18:151
Valkiunas G (2005) Avian malaria parasites and other Haemosporidia. CRC Press, Boca Raton
Vázquez A, Sánchez-Seco M-P, Palacios G, Molero F, Reyes N, Ruiz S, Aranda C, Marqués E, Escosa R, Moreno J, Figuerola J, Tenorio A (2012) Novel flaviviruses detected in different species of mosquitoes in Spain. Vector Borne Zoonotic Dis 12:223–229
Voyadjoglou-Samanidou A (2011) The mosquitoes of Greece: morphology, biology, public health, identification keys, control [in Greek]. Agrotypos Publishing, Athens
Acknowledgements
Special thanks are due to Dr Antonios Michaelakis, Head Research Scientist in Benaki Phytopathological Institute, for his useful comments and suggestions.
Funding
This project was financially supported for postdoctoral studies (SSA) by the Research Committee of the Aristotle University of Thessaloniki (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Helge Kampen
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spanoudis, C.G., Pappas, C.S., Savopoulou-Soultani, M. et al. Composition, seasonal abundance, and public health importance of mosquito species in the regional unit of Thessaloniki, Northern Greece. Parasitol Res 120, 3083–3090 (2021). https://doi.org/10.1007/s00436-021-07264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07264-y