Abstract
Many infectious diseases including malaria, lymphatic filariasis, chikungunya and dengue are transmitted by mosquitoes. Understanding the entomological diversity in a given area is critical for defining health risks, but also to design and evaluate control measures. Our study aimed to establish an entomological database of the Culicidae fauna in two clusters (Nyalla and Kambo) in the city of Douala in Cameroon. A longitudinal study was conducted from August to December in the Nyalla and Kambo neighbourhoods in the city of Douala. Culicidae larvae were weekly collected and reared until adult emergence. Adults were then morphologically identified using reference identification keys. A total of 20 breeding sites were productive out of an approximately 90 sites prospected. Water collections, pits, swamps and cinder blocks were main categories of larval habitats. In total, 2125 mosquito larvae were collected and reared during the survey. A total of 1790 mosquito adults emerged and were further identified and distributed among three genera: Culex (73.3%); Anopheles (22.6%) and Aedes (4.1%). Eleven Culicidae species were identified: Culex duttoni, Cx. descens, Cx. poicilipes, Cx. univittatus, Cx tigripes, Cx. antennatus, Cx. simpsoni, Cx. pipiens, Anopheles gambiae sl, Aedes aegypti and Ae. albopictus. Culex duttoni (39%) and An. gambiae s.l. (22.6%) were the main species recorded. The equitability, species richness and abundance of these genera was influenced by the nature of breeding sites and physical parameters including rainfall and temperature. The present study provides insights on the entomological diversity of the Culicidae fauna in the study area. Such information will help to orientate the fight against mosquito vectors in conjunction with the species and the types of breeding sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large majority of infectious diseases are transmitted by a vector that can either be a tick, a fly or a mosquito of the family Culicidae. This family consists mainly of Aedes; vectors of Chikungunya, dengue, yellow fever, Zika virus and dengue, Culex; vectors of Japanese encephalitis, West Nile virus and lymphatic filariasis, and Anopheles; vectors of malaria parasites (WHO 2017).
Vector-borne diseases account for about one million deaths each year and are responsible for more than 17% of infectious diseases (WHO 2017). Dengue is considered a dangerous infectious disease prevalent in about 100 countries and affects more than 2.5 billion people (WHO 2017). Some viral diseases such as yellow fever, Chikungunya, Zika virus and many others affect hundreds of millions of people around the world. As regarding malaria, it was responsible for 405,000 deaths in 2018 worldwide with over 67% of deaths occurring in children under five especially in sub-Saharan African countries (WHO 2019).
In Cameroon malaria is responsible for 23.6% of all hospital consultations with half the number being represented by children under five, and 41% of hospitalizations. The disease is also responsible for 17.5% of all population morbidity and 61.9% of deaths in underfives in the country (Bulletin Epidemiologique Annuel 2018).
Knowledge on biodiversity requires a description of the Culicidae fauna, that is, the identification of mosquitoes living in this environment, define their potential role as vector agents and their importance in medical entomology. Insects are presented as indicators of biodiversity only in rare works (Schäfer et al. 2004). A recent approach on this topic proposes to classify mosquito species into functional groups according to their biological characteristics (Schäfer et al. 2004; Pradel et al. 2007). Based on life history traits of the species, this classification gives information about the biodiversity of wetlands sampled (Serandour 2007). Besides, the knowledge on entomological diversity provides data critical for the designing and evaluation of larval control measures in the field, and information on the factors that can determine the reproduction, survival, and space-time distribution of major vectors of diseases (Piyaratne et al. 2005).
In this regard, our study aimed at determining the composition of the Culicidae fauna in two areas in the town of Douala in Cameroon, namely Nyalla and Kambo, with the aim of contributing to the knowledge on the biodiversity of these two areas and establishing a primary entomological database for further vector control strategies. The specific objectives of the study were: i) to identify the Culicidae fauna from Nyalla and Kambo areas, ii) to determine the physical parameters of each breeding site, and iii) determine the ecological indices of the breeding sites and their correlation with development of Culicidae species from these two areas of the town of Douala, Cameroon.
Materials and methods
Study area and period
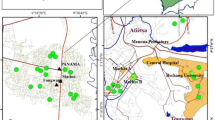
This study was carried out in the Nyalla and Kambo areas, under the Logbaba health district, Douala (Figs. 1 and 2). These areas are characterized by a dry season (October to May) and a rainy season (June to October). Average temperature ranges between 25 °C to 30 °C with high humidity (Meva’a abomo 2004; Regional coastal meteorological service). The topography of these areas consist of mostly flat terrain and humid or dry but also flat terrain which encourages close range construction with inadequate drainage systems as well as the practicing of agriculture. All these climatic conditions are favourable for the proliferation of breeding sites and mosquito development.
Potential larval breeding sites were prospected from August to December 2017.
Collection, storage and transport of larval and nymph stages
Sampling of larvae was done using the “dipping” method (Service 1993). The collected water was poured into a white or clear-bottomed dish. The larvae were collected using a Pasteur-type pipette and counted, before transferring into a polystyrene container or bottle containing water from breeding sites. Containers were labelled and transported to the insectarium of the Faculty of Sciences (The University of Douala, Douala, Cameroon).
Larval rearing
Larvae were separated with respect to subfamilies (Culicinae, Anophelinae and Toxorhynchitinae) and placed in labelled bins containing water from breeding sites and covered with mosquito net. The larvae were fed every morning with fry flour (Tetra min baby), it is larvae food which contains fish meal, dry yeast,ground brown rice, shrimp meal and protein extracts. Nymphs obtained after larval development were sorted and transferred into plastic jars placed in iron cages covered with mosquito net. A piece of cotton wool soaked with sugar water was deposited on top of the cage to ensure the nutrition of the adults which emerged.
Adult mosquito identification
All the mosquitoes collected were identified based on their morphology. Adult mosquitoes were examined with a binocular magnifying glass and identified using the identification keys (Gillies and De Meillon 1968; Gillies and Coetzee 1987; Rueda 2004; Jupp 1996).
Ecological analysis; classic ecological indexes
Biodiversity was mainly investigated through the calculation of several ecological indices of composition namely species richness and frequency or relative abundance; and of structure namely Simpson index (Is) and equitability (E) computed as follows:
Is ═ 1-∑Pi2 with Pi ═ ni/N, where ni: number of individuals of the given species; N: total number of individuals).
The equitability of a breeding site corresponds to the ratio of the Simpson index calculated for this site to the ideal Simpson index of this same site. This ideal Simpson index is calculated assuming that the species of the breeding site are fairly distributed. The calculation of the equitability was made by the following formula:
E ═ Is*S/S-1, where S is the total number of species identified in the breeding site.
The equitability (also known as regularity of the distribution of species) is an important element of diversity. A species represented abundantly or by a single individual does not have the same contribution to the ecosystem. With the same number of species, the presence of very dominant species mathematically results in the rarity of some others. It is therefore quite intuitively understandable that the maximum diversity will be achieved when the species will have a very regular distribution. This index from 0 (no uniform distribution of species) to 1 (all the species are evenly distributed) (Marcon 2018).
Other ecological parameters including the General Culicidae index (GCI), absolute Culicidae index (ACI), and relative Culicidae index (RCI) allowed us to estimate the occupancy of larval breeding sites by mosquitoes (Williams and Pinto 2012).
GCI appraises the proportion of water body colonized by mosquitoes in a given locality. It is calculated by dividing the number of mosquitoes’ larvae producing sites (n) and nymphs by the total number of prospected sites (N) as the following formula: GCI = n/N (Williams and Pinto 2012).
ACI the relative proportion of breeding sites colonized by a given vector species in a given locality. To calculate this index, larvae should be identified at the species level. It is obtained by dividing the number of sites producing this species (ne) to total number of sites prospected (N), ACI = ne/N (Williams and Pinto, 2012).
RCI indicates the abundance of a given species in a breeding site relative to the total number of all mosquitoes present in this breeding site. It is calculated by dividing the number of sites where the species “e” is found (ne) to the total number of mosquitoes’ larvae productive sites (Ne), RBI = ne/Ne (Williams and Pinto, 2012).
Data analysis
Data was entered into an Excel 2007 spreadsheet (Microsoft Office, 2007) and statistical analysis was done using StatView software version 5.0. for Windows (SAS Institute Inc., USA). The Spearman’s correlation, Mann-Whitney test and the one-way analysis of variance (ANOVA) tests were used to compare quantitative variables. Chi-square test and Fisher’s exact tests were used for comparing qualitative variables. The level of significance was set at P value <5%.
Results
Nature of breeding sites
Breeding sites included in the present study consisted of water collections (temporary rain pool, land-based water and household waste water, pits, swamps and cinder blocks as shown in Fig. 1.
Culicidae biodiversity
A total of 2125 larvae were collected during the study of which 1790 had evolved to adult stage representing an emergence rate of 84%. Eleven (11) Culicidae species belonging to three genera Anopheles, Culex and Aedes were recorded (Table 1). Culex mosquitoes accounted for 73.3% of all mosquito species collected. Culex duttoni (39.0%) and Anopheles gambiae s.l. (22.6%) were the main species recorded representing approximately 62% of the total culicidae fauna.
Composition and structure of ecological indexes
Species richness
The species richness varied from one site to another ranging from 1 to 7 species (Table 2). Breeding site 3 and 11 had the highest species richness, seven species each, while sites 5 and 6 had just one species. The subfamily of Culicinae (8 Culex and 2 Aedes species) was the richest compared to Anophelinae with one species An. gambiae s.l, bearing in mind that it is a species complex that requires molecular identification (Coetzee et al. 2013) which was not carried out here – so there could be more than one species.
Abundance of breeding sites and with regard to their nature
The breeding site 11 (water collection near dump) had the highest number of larva (n = 806) (Fig. 3a). In addition, Culex (n = 1193) and Anopheles (n = 334) mosquitoes were more abundant in water collections while all Aedes (n = 74) mosquitoes were found in cinder blocks (Fig. 3b). Aedes albopictus mosquitoes was more abundant than Aedes aegypti (42 individuals versus 32 individuals).
Correlational analysis between abundance and physical parameters
No statistically significant association (P value >0.05) was found between the larval abundance and surface of breeding site and water height (Fig. 4b and c). On the other hand, a negative and statistically significant correlation was found between these two variables (z = −2; p = 0.04) between the water height and abundance of Anopheles (Fig. 4a).
Water height and distribution of species
As shown in Fig. 5, the genus Anopheles was mainly found in breeding sites whose water height ranging between 0.02 and 0.04 m.
Relationship between temperature, rainfall and Culicidae abundance in each breeding site
The relationship between temperature, rainfall, and species abundances each month of the study is depicted in Fig. 6. In fact, temperature varied between 27 °C and 36 °C in August and December respectively with abundances of 322 and 109 individuals respectively. The highest value of abundance was recorded in October (1125 individuals) while the highest monthly rainfall was recorded in August (708.08 mm) which then decreased to 248.16 mm in October.
Structure of ecological indexes
The mean of Simpson index value was 0.48 ± 0.24. The ecological diversity was highest in site 3 (Simpson index value of = 0.78). In contrast, the diversity was lowest in site 9 (Is = 0.12) as depicted in Fig. 7a. As regarding the equitability, this parameter was close to 1 in sites 2, 7, 14 and 20 thereby outlining a quite equal distribution of sampled species in these sites (Fig. 7b).
Additional ecological indexes
The proportion of water bodies colonized by mosquitoes was significantly higher in Nyalla than in Kambo (4.3% versus 17.2%, P value = 0.0001). The absolute Culicidae index (ACI) for genera Culex, Anopheles and Aedes was 19%, 15% and 1% respectively while their relative Culicidae index was 90%, 70% and 5%.
Discussion
The present study reveals that the Culicidae fauna of Nyalla and Kambo areas is rich and diverse given a high number of species identified (11 species). These species have already been reported in previous studies in Cameroon (Fontenille and Toto 2001; Awono-Ambene et al. 2004; Antonio-Nkondjio et al. 2005, 2006; Simard et al. 2005). This finding is in line with that of Mbouhom Kamga (2006) in the town of Yaounde, Cameroon who reported 10 species.
The different species found in this study were collected from a variety of breeding sites consisting of water collections, pits, swamps and cinder blocks. These sites were created mainly during due to human activity. Kambo and Nyalla are hallmarked by a large number of construction site and agricultural farming activities.
The highest numbers of species were recorded in sites 3 and 11; in contrast to sites 5 and 6 in which only one species was found. This was likely due to competition between species, a phenomenon which has been shown, if sustainably present, leads to the maintenance of one species (Degallier 1988).
The genus Culex accounted for the bulk of mosquitoes collected in this study and this may be explained by the fact that Culex colonises all kinds of breeding sites (Faraj et al. 2006). This suggest that Culex would have less requirements for their development in an environment, which is not the case with Anopheles and Aedes. A similar observation was recorded in the city of Yaounde, Cameroon by Mbouhom Kamga (2006) who revealed 62.3% Culex. Eight species of Culex were identified, including exclusively zoophilic species such as Cx. descens, Cx. tigripes and Cx. poicilipes (Mbouhom Kamga, 2006). Their presence could be explained by the presence of domestic animals and occasionally stray animals such as dogs and cats who pollute the environment and the breeding sites thereby creating favourable conditions for the proliferation of this species of mosquitoes. The presence of Cx. tigripes larvae at collection sites could explain why we found emergence rates of less than 100% at these sites as the larvae of this species are predators of the others mosquitoes larvae. (Manga et al. 1992; Nimpaye et al. 2001).
About 54 Anopheles species have been identified in Cameroon (Irish et al. 2020). Only An. gambiae s. l. was found in our study. This species complex contains the main vectors of malaria parasites in the country.
All individuals of the genus Aedes were found in the cinder blocks. This could be explained by the urbanization of the area characterized by many constructions sites (buildings), sometimes unfinished with clumps of unused blocks. The same observation was done by Hounkpe (2012) in Montpellier, France. During his study this author found that the construction of collective and private habitats promotes the proliferation of the mosquito Ae. albopictus through inappropriate structures. In the present study, Ae. albopictus was more abundant than Ae. aegypti. Hawley (1988) postulated that when these two species are found in the same milieu a competition phenomenon occurs whereby Ae. albopictus tends to colonize the breeding sites occupied by Ae. aegypti.
The absence of the genus Mansonia is explained by the non-eligibility of the breeding sites favorable to the development of its larvae. Indeed, these sites are for the most part large bodies of water having a large aquatic flora. Their presence has been reported in all regions of the country (Rageau and Adam 1952).
The correlation analysis of some physical parameters with abundance and species richness showed quite different results. The nature and type of site did not affect abundance and species richness (P value >0.05). In contrast, a significant and negative correlation between the water height of the breeding site and the presence of Anopheles was found (P value = 0.04). Due to the absence of a respiratory siphon, Anopheles gambiae require less deep breeding sites than others as Anopheles funestus. This is in line with studies conducted by Darriet (1998) in Burkina Faso and Cameroon (Darriet 1998). It should be noted that some Anopheles mosquitoes were found in some sites with a height of 0.411 m. Filamentous algae were also found in these sites and would probably have provided oxygen necessary for the development of these mosquitoes (Louah 1995).
Studies have shown that some parameters such as temperature, precipitation, and hygrometry are involved in the development and distribution of Culicidae species (Rodhain and Perez 1985; Mouchet et al. 2004). At the beginning of August, we observed the existence of several potential temporary larval sites following high precipitation (708.08 mm) and mosquito larvae were found in only four of them. The productivity of these sites was low as compared with that reported in October (322 individuals versus 1125 individuals). This increase is probably due to concomitant variation in temperature and rainfall as we noted that a decrease in rainfall leads to increase in temperature. These two parameters have great influence on hatching of eggs, creation of breeding sites, speed of post-embryonic development, and survival of adults. This result is consistent with the work of Poveda (2018) in Colombia, who showed a correlation between temperature, precipitation, and mosquito development (Poveda et al. 2018). Other parameters such the chemistry of water of breeding sites could also influence the abundance of mosquito larvae (Boulkenafet 2006).
One of the elements essential for any settlement is its degree of organization. The most favorable living conditions in a settlement, the higher the species diversity (Boulkenafet 2006).
In this study, breeding site 3 had the highest level of diversity (IS = 0.78) while the lowest diversity was reported in breeding site 9 (IS = 0.12), thereby suggesting site 3 offers the most favourable conditions to mosquitoes for breeding. In contrast, site 9 is the least favourable site for breeding. Equitability is an ecological parameter allowing for assessing distribution of different species living in a same ecological site. Breeding sites 2, 7, 14 and 20 had equitability values close to 1 (1.09, 0.86, 0.87 and 0.93 respectively); thus outlining a regular distribution of different mosquitoes species found in these four breeding sites.
Conclusion
This study outlines a high biodiversity in Nyalla and Kambo areas with three genera collected, namely Anopheles, Culex and Aedes. The distribution, species richness and abundance of these genera was influenced by the nature of breeding sites and physical parameters including rainfall and temperature. In the context of vector control, such information is vital as it will help orientate the fight against mosquito vectors at the larval and adult stages.
The information obtained during this study could allow the development of new strategies to optimize vector control at the larval level. It would therefore be important:
-
study the impact of urbanization and agriculture on culicidae density and specific wealth;
-
characterize chemically the waters of the breeding grounds;
-
study the variations in Culicidal densities according to the seasons;
-
study the risk of vector transmission of malaria in these areas;
-
assess the knowledge and practical skills of populations on vector ecology and the vector control methods used;
At the end of this study, it is advisable to recommend that the populations clean up their living environment in order to limit the presence of breeding sites;
Abbreviations
- ACI:
-
Absolute Culicidae index
- ANOVA:
-
Analysis of variance
- GCI:
-
General Culicidae index
- GPS:
-
Global Positioning System
- Minsanté:
-
Ministère de la Santé publique (Ministry of Public Health)
- RCI:
-
relative Culicidae index (RCI)
- RBM:
-
Roll Back Malaria
- USA:
-
The United States of America
- WHO:
-
World Health Organization
References
Awono-Ambene HP, Kengne P, Simard F, Antonio-Nkondjio C, Fontenille D (2004) Description and bionomics of Anopheles (Cellia) ovengensis (Diptera: Culicidae), a new malaria vector species of the Anopheles nili group from South Cameroon. J Med Entomol 41:561–568
Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, Fontenille D (2006) Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol 43:1215–1221
Antonio-Nkondjio C, Simard F, Awono-Ambene P, Ngassam P, Toto JC, Tchuinkam T, Fontenille D (2005) Malaria vectors and urbanization in the equatorial forest region of South Cameroon. Trans Royal Soc Tropical Med Hygiene 99:347–354
Bulletin Epidemiologique Annuel (2018) du Paludisme au Cameroun 2018: Ministry of public Health
Boulkenafet F (2006) Contribution à l’étude de la biodiversité des Phlébotomes (Diptera: Psychodidae) et appréciation de la faune Culicidienne (Diptera : Culicidae) dans la région de Skikda. Mémoire ès Sciences, Université de Mentouri, Algérie
Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ (2013) Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619:246–274
Darriet F. (1998) La lutte contre les moustiques nuisants et vecteurs de maladies: l'évaluation de nouveaux insecticides utilisables contre les moustiques en Afrique tropicale. Consulted on 14/06/2016 upon http://www.documentation.ird.fr/hor/fdi:010015251
Degallier N (1988) Aedes aegypti (L.): Importance de sa bioécologie dans la transmission de la dengue et des autres arbovirus. Bull de la Société de Pathologie Exotique 81:97–110
Faraj C, Elkohli M, Lyagoubi M (2006) Cycle gonotrophique de Culex pipiens (Diptera : Culicidae), vecteur potentiel du virus West Nile, au Maroc : estimation de la durée en laboratoire. Bull de la Société de Pathologie Exotique 99:119–121
Fontenille D, Toto JC (2001) Aedes (Stegomyia) albopictus(Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis 7:1066–1067
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara, 2nd edn. Publication of The South African Institute for Medical Research, Johannesburg
Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region), 2nd edn. Publication of The South African Institute for Medical Research, Johannesburg
Hawley WA (1988) The biology of Aedes albopictus. J Am Mosq Control Assoc 4:1–40
Hounkpe J (2012) Gîtes larvaires d’Aedes albopictus dans le bâti et les ouvrages de gestion des eaux pluviales : état des lieux et enjeux en termes de stratégie de contrôle. Mémoire d'Ingénieur du Génie sanitaire, Ecole des Hautes Etudes en Santé Publique (E.H.E.S.P), Rennes
Irish SR, Kyalo D, Snow RW, Coetzee M (2020) Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical region and associated islands. Zootaxa 4747(3):401–449
Jupp PG (1996) Mosquitoes of southern Africa: Culicinae and Toxorhynchitinae, Ekogilde Pubs.,156 pages
Louah MA (1995) Ecologie des Culicidae (Diptera) et état du paludisme dans la péninsule de Tanger. Thèse d’Etat es sciences, Faculté des Sciences de Tétouan, Maroc
Manga L, Robert V, Messi J, Desfontaine M, Carnevale P (1992) Le paludisme urbain à Yaoundé, Cameroun. 1. Etude entomologique dans deux quartiers centraux. Mémoire de la Société Royale Belge d'Entomologie 35:155–162
Marcon E. (2018) Mesure de la biodiversité. PDF version 2018; 283 p,Consulted on 13/07/2019 upon www.ecofog.gf/IMG/pdf/mesures_de_la_biodiversite.pdf
Mbouhom Kamga DB (2006) Dynamique de la faune culicidienne sur le campus de l’Université de Yaoundé (Cameroun). Mémoire de DEA. Université de Yaoundé, Cameroun
Meva’a Abomo D (2004) De l’abondance des ressources en eau à la rareté de l’eau potable dans les villes littorales du Sud; un indicateur pertinent de la crise managériale de ces espaces urbains : l’exemple de Douala au Cameroun. Mémoire de Maitrise. Université de Douala, Cameroun
Mouchet J, Carnevale P, Cooseman SM, Julvez J, Manguin S (2004) Biodiversité du paludisme dans le monde. Editions John Libbey, Montrouge
Nimpaye H, Van Der Kolk M, Fontenille D, Boudin C (2001) Le paludisme urbain à Yaoundé au Cameroun : Etude entomologique dans le quartier central à « Dakar ». Bull de Liaison Documentaire OCEAC 34:11–14
Piyaratne MK, Amerasinghea FP, Amerasinghea PH, Konradsen F (2005) Physico-chemical characteristics of Anopheles culicifacies and Anopheles varuna breeding water in a dry zone stream in Sri Lanka. J Vector Borne Diseases 42:61–66
Poveda G, Graham NE, Epstein PR, Rojas W, Quiñones ML, Darío Vélez I et al (2018) Climate and ENSO variability associated with vector-bornediseases in Colombia. Consulted on 16/08/2018 upon http://www.clas.ufl.edu/users/prwaylen/geo3280articles/Poveda.pdf
Pradel J, Rey D, Foussadier R, Bicoud D (2007) Etude écologique des moustiques (Diptera, culicidae) vecteurs potentiels d’arboviroses dans la région Rhône-Alpes. Epidémiologie et Santé Animale 51:81–94
Rageau J, Adam J-P (1952) Culicinae du Cameroun. Ann Parasitol Hum Comp 27:610–635
Rodhain F, Perez C (1985) Précis d'Entomologie Médicale et Vétérinaire. Maloine, Paris
Rueda (2004) Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. (Zootaxa 589) 60pp
Schäfer ML, Lundström JO, Pfeffer M, Lundkvist E, Landin J (2004) Biological diversity versus risk for mosquito nuisance and disease transmission in constructed wetlands in southern Sweden. Med Vet Entomol 18:256–267
Serandour J (2007) Contribution à l’étude des moustiques anthropophiles de France: le cas particulier du genre Coquillettidia. Thèse de Doctorat. Université Joseph Fourier, France
Service MW (1993) Mosquito ecology, field sampling methods, 2nd edn. Chapman & Hall, London
Simard F, Nchoutpouen E, Toto JC, Fontenille D (2005) Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: culicidae) in Cameroon, Central Africa. J Med Entomol 42:726–731
WHO (2017) Vector-borne diseases, Accessed 19/03/2019. available at https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
WHO (2019) World malaria report. The World health organization, Geneva, Switzerland 2018; 210 p. Available at www.who.int. Accessed 05/01/2020
Williams J, Pinto J. (2012) Manuel de formation à l’entomologie du paludisme. A l’intention des techniciens en entomologie et lutte antivectorielle (Niveau de base)
Acknowledgments
The authors are grateful to the populations of Nyalla and Kambo areas in Cameroon for their collaboration in the measurement of physical characteristics of breeding sites.
Author information
Authors and Affiliations
Contributions
Designing study: AAN, LGL.
Data collection: AAN, LPK, JMN.
Write-up: AAN, LPK.
Revision: FEM, LPK, WEE, GWB, JMN, LGL.
Data analysis: FEM, LPK.
Supervision: LGL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare having no competing interests.
Consent for publication
All authors gave their consent for the publication of the present paper.
Ethical statement
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ntoumba, A.A., Kojom Foko, L.P., Ekoko, W.E. et al. Entomological characteristics of mosquitoes breeding sites in two areas of the town of Douala, Cameroon. Int J Trop Insect Sci 41, 1313–1323 (2021). https://doi.org/10.1007/s42690-020-00324-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00324-3