Abstract
Procamallanus is a species-rich genus of parasitic nematodes of marine, brackish, and freshwater fishes, occurring also occasionally in amphibians and reptiles. In the Neotropical region, this genus is highly diverse, with species described from a wide range of fish families. In this study, we reassess the taxonomic status of Procamallanus rebecae with molecular and morphological data and describe a new species endemic to Nicaragua and Costa Rica. We analyzed all Procamallanus isolated from fish from the Nicaraguan lakes and some rivers in Costa Rica after an exhaustive analysis of their freshwater fish endoparasite fauna. Procamallanus rebecae is a host-specific parasite of Middle American cichlids, previously reported in southern Mexico, Nicaragua, and Costa Rica. We therefore compared these Central American specimens with individuals of P. rebecae collected in cichlids from southeastern Mexico using two genomic regions (28S rDNA and mitochondrial cytochrome oxidase subunit 1, COI). We found high levels of sequence divergence between Procamallanus from the two geographical regions, with up to 9.8 and 10.5% for both genetic markers, respectively. We also analyzed their morphology and found conspicuous differences in the shape of the mouth and the structure of the female cauda. We therefore describe the specimens of Procamallanus from Central American cichlids as a new species. Both Procamallanus species occur in different cichlid species and are allopatrically distributed. The host specificity and ancient association patterns between cichlids and Procamallanus and the jointly colonization of both hosts and parasites during their northern dispersal from South America are briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nematode genus Procamallanus Baylis, 1923, is a diverse group of parasites of the family Camallanidae Railliet et Henry, 1915, found in marine, brackish, and freshwater fishes, and occasionally in amphibians and reptiles (Rigby and Rigby 2013). Traditionally, species in this genus have been grouped in five subgenera mainly based on the sclerotization of the oral capsule (Moravec and Thatcher 1997), although recent molecular phylogenetic analyses of camallanids have shown inconsistencies in such classification (Ailán-Choke et al. 2020). Procamallanus is very diverse in the Neotropical biogeographical region with up to 30 species described (Ailán-Choke et al. 2018). Four of these species are exclusively found in cichlid fish, two in Middle America belonging to the subgenus Spirocamallanus Olsen, 1952, P. mexicanus Moravec, Salgado-Maldonado & Caspeta-Mandujano, 2000, and P. rebecae Andrade-Salas et al. 1994, and two in South America, belonging to the subgenus Procamallanus Baylis, 1923, P. peraccuratus Pinto, Fábio, Noronha & Rolas,1976, and P. spiculastriatus da Silva, de Vasconcelos, Monks, dos Santos & Giese, 2018. Procamallanus rebecae is supposedly distributed throughout Middle America, and has been reported in several cichlid fish species in southeastern Mexico, Nicaragua, and Costa Rica (Andrade-Salas et al. 1994; Aguirre-Macedo et al. 2001; González-Solís and Jiménez-García 2006; Sandlund et al. 2010). Due to their close association with cichlid fish, it has been considered part of their biogeographical core parasite fauna (Pérez-Ponce de León and Choudhury 2005).
The intense volcanic activity during the Pleistocene in Central America originated volcanoes that over time collapsed and formed the basis for an array of present crater lakes in this region (Kutterolf et al. 2007). Nicaraguan crater lakes have been thoroughly investigated for their fish fauna (Barlow 1976; Barlow and Munsey 1976; Waid et al. 1999; Elmer et al. 2010a, 2010b), which colonized them from the adjacent great Nicaraguan large lakes, Nicaragua and Managua, of tectonic origin (Barluenga and Meyer 2010). The crater lakes are dominantly inhabited by cichlid fish (Waid et al. 1999), and particularly by the Midas cichlid species complex Amphilophus spp. (Barlow 1976). This recent adaptive radiation has been argued to have evolved repeatedly through sympatric speciation (Barluenga et al. 2006; Barluenga and Meyer 2010; Elmer et al. 2010b; Kautt et al. 2016).
During a comprehensive survey of the metazoan endoparasite fauna of fish from Nicaraguan lakes and some rivers in Costa Rica, some specimens of the nematode genus Procamallanus were found in the digestive tract of cichlid fish. We analyzed these nematodes together with P. rebecae collected in cichlids from southeastern Mexico. In order to elucidate the phylogenetic relationships of these nematode parasites, they were compared using morphological and molecular approaches.
Materials and methods
Sample collection
Fish were collected across three parasitological surveys carried out in Nicaragua, in November–December of three consecutive years, 2017–2019. Fish were sampled from the great Nicaraguan Lakes Nicaragua and Managua, and from five crater lakes in the surrounding region. The parasitological scanning was carried out on a total of 896 fish, including 20 cichlid species and seven species of non-cichlid fish. We studied non-cichlid fishes in the locality to further corroborate that the species is indeed a cichlid parasite in the geographic area. Additionally, we included specimens of Procamallanus from two species of cichlids (18 individuals) sampled in Costa Rica in 2015. We also included samples of Procamallanus obtained from cichlids collected between 2003 and 2014 in eight localities of southeastern Mexico (Fig. 1, Table 1). All fish were collected with gill nets, cast nets, and harpooning, then sacrificed with an overdose of tricaine (MS-222), dissected, and all organs immediately studied for parasites under a stereomicroscope. We isolated all Procamallanus specimens, rinsed them in 6.5% saline solution, and fixed in 100% EtOH or 4% hot (nearly boiling) formalin for molecular and morphological analyses, respectively.
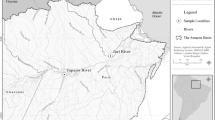
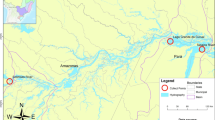
Map showing the sampling sites of Procamallanus spp. in cichlid hosts in Middle America, with a close-up to the great lakes and crater lakes of Nicaragua. Locality code according to Table 1
Molecular analyses
Genomic DNA was isolated using DNAzol Reagent (Invitrogen) or Speedtools tissue DNA extraction kit (Biotools) according to the manufacturer’s protocol, from a fragment of the middle body of individual nematodes (hologenophore sensu Pleijel et al. 2008). We amplified two molecular markers, the mitochondrial cytochrome oxidase subunit 1 (COI) and the large subunit of the 28S rDNA. The partial COI region was amplified using the primers 507 (5′-AGTTCTAATCATAARGATATYGG-3′) (Nadler et al. 2006) and HCO (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. 1994). The partial 28S rDNA region was amplified using the primers 391 (5′-AGCGGAGGAAAAGAAACTAA-3′) and 536 (5′-CAGCTATCCTGAGGGAAAC-3′) (García-Varela and Nadler 2005). The amplification and sequencing protocols followed those used in Santacruz et al. (2020). Sequences were assembled and edited using Geneious v7 (Kearse et al. 2012).
A dataset for each genetic marker was constructed including the newly generated sequences plus sequences of camallanids available in GenBank. The species Dracunculus lutrae Crichton and Beverly-Burton, 1973, was used as outgroup based on previous phylogenetic analyses (Černotíková et al. 2011; Choudhury and Nadler 2016). Sequences were aligned with the T-COFFEE platform (http://tcoffee.crg.cat; (Di Tommaso et al. 2011). The best-fit model of molecular evolution for each dataset was calculated with ModelFinder (Kalyaanamoorthy et al. 2017) using the Bayesian information criterion (BIC). Phylogenetic reconstruction for each marker was performed with maximum likelihood (ML) in IQ-TREE v.1.6.2 (http://iqtree.cibiv.univie.ac.at/), with Ultrafast bootstrap; 10,000 iterations, SH-aLTR branch test; 10,000 replicates (Nguyen et al. 2015; Hoang et al. 2017). Phylogenetic reconstruction was also run applying Bayesian inference (BI) in MrBayes v3.2.7a (Huelsenbeck and Ronquist 2001), using the CIPRES Science Gateway Web Portal v3.3 (Miller et al. 2010). Analyses were performed using two runs of four chains, each for 107 generations, sampling trees every 2000 generations, and a temperature of 0.2. The first 25% of trees were discarded as burn-in and the resulting trees were used to obtain a 50% majority-rule consensus tree. Tracer v1.7.1 (Rambaut et al. 2018) was used to assess convergence of independent runs, with effective sample sizes (ESS) > 200. The pairwise distances between groups for each dataset were calculated as uncorrected p-distances in MEGA v7 (Kumar et al. 2016).
Morphological analyses
The hologenophores and complete individuals were cleared in a glycerol-alcohol solution (1:1) to observe and measure internal structures using an Olympus BX51 light-inverted microscope equipped with differential interference contrast (DIC) optical components. To study the external ultrastructure, specimens were prepared for scanning electron microscopy (SEM). Samples were sonicated, critical point dried, mounted on a strip of carbon conductive tape, and then coated with gold. The SEM photographs were taken in a Hitachi scanning electron microscope 15 kV Hitachi Stereoscan Model SU1510. Type material was deposited in the Colección Nacional de Helmintos (CNHE), Institute of Biology, UNAM, México.
Results
We analyzed a total of 265 Procamallanus specimens, 231 isolated from 13 cichlid species in the Nicaraguan lakes, 18 isolated from two cichlid species from rivers in northwestern Costa Rica, and 16 specimens from five cichlid species in water bodies in Mexico.
Molecular analyses
We obtained sequences from two genomic regions. All obtained sequences were deposited in GenBank (accession numbers COI: MW487868–MW487887, 28S rDNA: MW485586–MW485594). The 28S rDNA phylogenetic reconstruction distinguished two well-supported Procamallanus lineages from Middle American cichlids, a relationship supported by moderate to high bootstrap and posterior probability values (80/0.98) (Fig. 2). One clade corresponded to previous descriptions of P. rebecae, and the second clade represented a well-differentiated lineage, arguably a new species. Both are reciprocally monophyletic and exhibit very high level of genetic divergence for this molecular marker, with an average of 9.8% (Table 2). The phylogenetic reconstruction with COI also resolved two well-differentiated lineages, P. rebecae and the new species, each forming a well-supported monophyletic clade (Fig. 3). The genetic divergence between both lineages averaged 10.5% (Table 2). Additionally, intraspecific genetic divergence within clades was very low for COI, (0.57% P. rebecae, 0.63% for the new species). The two lineages were not resolved as closely related to two other species of Procamallanus from Middle America from which sequences are available, i.e., P. neocaballeroi Caballero-Deloya, 1977, and P. gobiomori Moravec, Salgado-Maldonado & Caspeta-Mandujano, 2000. Both 28S rDNA and COI trees corroborated the findings of other studies in that the genus Procamallanus is paraphyletic. The topology of the trees is not comparable since the sequences available in GenBank for each molecular marker include different species. Since both trees revealed that the isolates of Procamallanus sampled in cichlids from Nicaragua and Costa Rica represent independent evolutionary units, a detailed morphological analysis was performed, and the new species is described next.
Consensus tree from IQ-TREE analysis of the COI matrix (a). Best-fit model according to BIC: TPM3+F+I+G4. The number in each node indicates the posterior probability from the BI analyses and the size of the circle is equivalent to the bootstrap support from the ML. b, c Anterior end of P. rebecae and P. barlowi n. sp. through SEM, respectively. The scale bar is equivalent to 15 μm
Family Camallanidae Railliet & Henry, 1915
Genus Procamallanus Baylis, 1923
Subgenus Spirocamallanus Olsen, 1952
Procamallanus barlowi n. sp.
Type host: Amphilophus chancho Stauffer, McCrary & Black
Type locality: Lake Apoyo, Nicaragua (11° 56′ 12.239″ N, 86° 2′ 58.956″ W)
Other localities: Nicaragua: Lake Apoyeque: Parachromis managuensis (Günther); Lake Apoyo: Amphilophus astorquii Stauffer, McCrary & Black, Amatitlania nigrofasciata (Günther), Amphilophus zaliosus (Barlow), P. managuensis; Lake Asososca León: Amphilophus citrinellus (Günther); Lake Managua: Amphilophus labiatus (Günther), Parachromis sp.; Lake Masaya: Cribroheros longimanus (Günther); Lake Nicaragua: Amphilophus citrinellus, Amphilophus labiatus, Cribroheros longimanus, Cribroheros rostratus (Gill), Hypsophrys nicaraguensis (Gill), and Hypsophrys nematopus (Günther). Costa Rica: River Irigaray: Cribroheros longimanus, River Las Animas: Amatitlania nigrofasciata (Günther).
Site of infection: Intestine and stomach.
Prevalence and abundance: Fig. 4.
Type material: Holotype (male) CNHE 11472, allotype (gravid females) CNHE 11473 (Lake Apoyo), and paratypes CNHE 11474 (Lake Apoyo), Nicaragua.
Representative DNA sequences: COI: MW487868–MW487887, 28S rDNA: MW485586–MW485594.
ZooBank registration: urn:lsid:zoobank.org:act: 59F70A20-1366-4AE3-81A2-E83781CC71AD
Etymology: the species is named after George Barlow who was a Professor of ichthyology and animal behavior at UC Berkeley, not only for his numerous contributions to cichlid biology but also for his passion and enthusiasm that inspired many generations of cichlid specialists.
Description (Figs. 5 and 6, measurements in Table 3)
Scanning electron microscopy photomicrographs of Procamallanus barlowi n. sp. a, b Anterior end, lateral and apical view, respectively. c Male apical view, the arrows indicate the pores at the edge of the mouth. d Basal ring. e Position of the deirid in a lateral view. f Deirid. g Excretory pore position indicated by the arrowhead. h Female tail with a pair of lateral phasmids indicated by the arrowheads. i Female anal aperture. j Male tail, ventrolateral view. k Male tail, dorsal view; posterior lateral papillae indicated by the arrowheads. l Male tail, ventral view; right preanal papillae and sessile adcloacal papillae indicated by the white and black arrowheads, respectively. m Spine-like structures in the male tail tip. n Postanal papillae in the mail tail indicated by the arrowheads. Abbreviations: a, b, c cephalic papillae of internal, middle, and external circle, respectively
General: Medium-sized nematodes, with slightly striated cuticle. Mouth opening oval, surrounded by 12 papillae arranged in three circles (Fig. 5c); four papillae on each circle (Fig. 6a, b). Papillae of external circle larger. Papillae of innermost circle with a pore close to the edge of the mouth (Fig. 6c). One pair of lateral amphids present. Well-developed basal ring (Fig. 6d). Orange-brown buccal capsule, with thick walls, lining internally with 13–15 spiral thickenings; first three anterior spirals incomplete (Fig. 5a). Spiral thickenings with smooth edges (Fig. 6c), limited to two-thirds of buccal capsule in both sexes (Fig. 6a). Small and simple deirids (Fig. 6e). Nerve ring surrounding first half of muscular esophagus (Figs. 5a, b). Excretory pore in last third of muscular esophagus (Figs. 5a and 6g).
Female: Vulva equatorial (Fig. 5f), vulval lips not elevated. Phasmids in ventrolateral position, half-way between anus and caudal tip (Fig. 6h). Papillae surrounding anal aperture absent (Fig. 6i). Tail rounded, with terminal digit-like projection. Caudal tip with three small cuticular extensions in gravid and mature females (Fig. 5e).
Male: Smaller than female. Spicules unequal, left spicule longer than right spicule (Fig. 5g). Gubernaculum absent. Caudal alae with nine pairs of pedunculate papillae (Figs. 5g and 6j–n). Three pairs of subventral preanal papillae, and six pairs of postanal papillae; first four pairs subventral and last two pairs lateral; last pair probably representing phasmids. Two pairs of sessile adcloacal papillae. Three cuticular spine-like extensions on tip tail (Fig. 6m). In male juveniles, walls of the buccal capsule more thickened than in mature males and caudal alae fully developed with only one spicule observed.
Differential diagnosis
The new species belongs in Procamallanus in having a buccal capsule lined with continuous walls, internally smooth or with markings in one sex or both. In total, six species of Procamallanus distributed in Middle American freshwaters are considered valid, all within the subgenus Spirocamallanus, namely P. gobiomori; P. jaliscensis Moravec, Salgado-Maldonado & Caspeta-Mandujano, 2000; P. mexicanus; P. neocaballeroi; P. pereirai (Anneraux, 1946) Olsen, 1952; and P. rebecae (see Garrido-Olvera et al. 2006). Two of these species of Procamallanus are parasites of cichlids: P. mexicanus, described from Cichlasoma gedessi (Regan) (= Herichthys gedessi) in Xalapa, Veracruz, Mexico, and P. rebecae, originally described from C. helleri (Steindachner) (= Thorichthys helleri) in Campeche, Mexico, and currently distributed in 13 cichlid species in Mexico (Garrido-Olvera et al. 2006), and four cichlid species in Nicaragua and Costa Rica (Aguirre-Macedo et al. 2001; González-Solís and Jiménez-García 2006; Sandlund et al. 2010). For comparison, Fig. 7 depicts SEM photomicrographs of P. rebecae. The new species morphologically resembles these two species by the presence of three preanal papillae; however, P. barlowi n. sp. differs in the number of spiral ridges lining the buccal capsule and a different external shape of the mouth (Figs. 6b and 7a); furthermore, the female of P. barlowi n. sp. possesses a rounded cauda bearing a digit-like projection, whereas this projection is conical in P. rebecae; the female of the new species also possesses spikes on the tip of the tail which are absent in the female of P. mexicanus.
Two additional species of Procamallanus have been reported in cichlids from Brazil, both within the subgenus Procamallanus: P. spiculastriatus and P. peraccuratus. These two species are readily distinguished from the new species by the presence of an internally smooth capsule in males and females. In addition, P. spiculastriatus exhibits a ring with tooth-like structures and lacks spine-like projections on the tip of the tail. Interestingly, in African cichlids, only one species of Procamallanus (Spirocamallanus) has been reported, P. serranochromis Moravec and Van As 2015 infecting species of Serranochromis spp. (Moravec and Van As 2015). This species shares several morphological features with P. barlowi n. sp. such as the presence of three pairs of preanal papillae, wide caudal alae, and asymmetrical spicules; nevertheless, this species can be differentiated from P. barlowi n. sp. by having a bilobed tip tail in both sexes instead of spike-like as in the new species.
In Middle American non-cichlid freshwater fishes, three more species of Procamallanus (Spirocamallanus) have been described, i.e., P. neocaballeroi from characids, P. gobiomori from eleotrids, and P. jaliscensis from mugilids. All these species have a buccal capsule completely filled with spiral grooves, while the new species lacks spiral ridges in the last third of the buccal capsule. The new species shares with four additional Neotropical species of Procamallanus (Spirocamallanus) the presence of spiral thickenings in the second third of the oral capsule in both females and males, namely P. belenensis Giese, Santos & Lanfredi, 2009; P. inopinatus Travassos, Artigas & Pereira, 1928; P. saofranciscensis Moreira, Oliveira & Costas, 1944; and P. pintoi Kohn & Fernandes, 1988. However, all these species are readily distinguished from the new species because they lack caudal alae and possess short spicules.
Discussion
In the present work, we describe a new species of endoparasite, the nematode Procamallanus barlowi n. sp., found in Central American cichlids. Therefore, the previous taxonomic records of P. rebecae from Nicaragua and Costa Rica (Aguirre-Macedo et al. 2001; González-Solís and Jiménez-García 2006; Sandlund et al. 2010) correspond with this newly described species. The new species is widely distributed throughout the River San Juan basin, including Nicaragua’s Great lakes, nearby crater lakes, and rivers in northwestern Costa Rica, displaying high host specificity to cichlid fish. Whether or not this species of nematode is also found in cichlids in other areas of lower and nuclear Central America requires further exploration. At the moment, both species are regarded as allopatrically distributed, and we acknowledge that there is a geographic gap in our samples to define if there is a transition area between both species and their cichlid hosts where they might occur in sympatry.
The new species herein described following an integrative taxonomy approach and P. rebecae, form a strongly supported monophyletic clade that infects specifically Middle American cichlids, and can be regarded as a part of their biogeographical core parasite fauna (sensu Pérez-Ponce de León and Choudhury 2005). The genetic divergence with both mitochondrial and nuclear markers between these two species is well above the distance threshold observed within other Procamallanus lineages (Santacruz et al. 2020), and also above that reported for other nematode species (e.g., Solórzano-García et al. 2020; Chen et al. 2020). Furthermore, the close evolutionary relationship between P. barlowi n. sp. and P. rebecae, the geographic scenario and host association, shows that the parasites of cichlids may have diversified along with their hosts, during the northward dispersal and colonization of new freshwaters from South America. Several empirical studies have demonstrated an extensive geographical range for some cichlid parasite lineages across Middle America, e.g., Crassicutis cichlasomae Manter, 1936, and Sciadicleithrum spp. (Kritsky, Thatcher, and Boeger, 1989; Mendoza-Franco and Vidal-Martínez 2005; Razo-Mendivil et al. 2010), whereas other studies have proven speciation events of both associates related with the northern dispersal after the closure of the Panama Isthmus. This pattern seems to be also common since some species pairs of helminths in cichlids reflect sister-species relationships between species found in the Central American cichlid assemblages and those of southeastern Mexico, e.g., Oligogonotylus manteri Watson, 1976–Oligogonotylus mayae Razo-Mendivil, Rosas-Valdez & Pérez-Ponce de León, 2008, and Neoechinorhynchus costarricense Pinacho-Pinacho et al. 2020–Neoechinorhynchus golvani Salado, 1978 (Razo-Mendivil et al. 2008; Pinacho-Pinacho et al. 2020). Our study provides further support for this pattern.
Our molecular phylogenetic hypotheses point out towards an ancient association between cichlids and Procamallanus which is probably older than their arrival to Middle America, since both species are not closely related with other Procamallanus from freshwater fish occurring in the same geographic area. With this data, we hypothesize that the South American Procamallanus from cichlids could be clustered with P. rebecae and the new species and form a clade that has evolved tightly linked to their cichlid hosts. However, in order to adequately test for this hypothesis, it would be required to collect additional data of Procamallanus in cichlids, and to combine this analysis with data from the only species of the genus reported in African cichlids, which would allow evaluating transcontinental dispersal. Such approach would be very useful to further understand the historical biogeography of cichlids and their parasites (Pariselle et al. 2011; Vanhove et al. 2016). Within the American continent, cichlids dispersed from South America into Middle America as a result of several waves of colonization (Říčan et al. 2013, 2016). Cichlids followed their northern dispersal and were able to expand further north, into rivers, lakes, and sinkholes (in the Yucatan Peninsula) to reach the boundaries between the Neotropical and Nearctic biogeographical zones as other fish groups such as characids (and their Procamallanus) (Ornelas-García and Pedraza-Lara 2016; Pérez-Miranda et al. 2018; Santacruz et al. 2020). Our study also evidenced that including additional data of Procamallanus from South American freshwater fishes would be required to trace the evolutionary patterns of both hosts and parasites. Likewise, the wide distribution of P. barlowi n. sp. in cichlids of the lake system of Nicaragua, a model system for the study of island-like colonization (Elmer et al. 2010a), makes the new species an ideal example to study the tempo and mode of colonization in contrast to their hosts.
References
Aguirre-Macedo ML, Scholz T, González-Solís D et al (2001) Some adult endohelminths parasitizing freshwater fishes from the Atlantic drainages of Nicaragua. Comp Parasitol 68:190–195
Ailán-Choke LG, Ramallo G, Davies D (2018) Further study on Procamallanus (Spirocamallanus) pintoi (Kohn et Fernandes, 1988) (Nematoda: Camallanidae) in Corydoras paleatus and Corydoras micracanthus (Siluriformes: Callichthyidae) from Salta, Argentina, with a key to congeneric species from Neotrop. Acta Parasitol 3:595–604
Ailán-Choke LG, Tavares LER, Luque JL, Pereira FB (2020) An integrative approach assesses the intraspecific variations of Procamallanus (Spirocamallanus) inopinatus, a common parasite in Neotropical freshwater fishes, and the phylogenetic patterns of Camallanidae. Parasitology 147:1752–1764. https://doi.org/10.1017/S0031182020001687
Andrade-Salas O, Pineda-López RF, García-Magaña L (1994) Spirocamallanus rebecae sp. n. (Nematoda: Camallanidae) from freshwater fishes in south-eastern Mexico. Folia Parasitol (Praha) 41:259–270
Barlow GW (1976) The Midas Cichlid In Nicaragua. In: Thorson TB (ed) Investigations of the ichthyofauna of Nicaraguan lakes. University of Nebraska, Press, Lincoln, NE, pp 333–358
Barlow GW, Munsey JW (1976) The red devil-Midas-arrow cichlid species complex in Nicaragua. In: Thorson TB (ed) Investigations of the ichthyofauna of Nicaraguan lakes. University of Nebraska, Press, Lincoln, NE, pp 359–369
Barluenga M, Meyer A (2010) Phylogeography, colonization and population history of the Midas cichlid species complex (Amphilophus spp.) in the Nicaraguan crater lakes. BMC Evol Biol 10:326. https://doi.org/10.1186/1471-2148-10-326
Barluenga M, Stölting K, Salzburger W et al (2006) Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439:719–723. https://doi.org/10.1038/nature04325
Černotíková E, Horák A, Moravec F (2011) Phylogenetic relationships of some spirurine nematodes (Nematoda : Chromadorea : Rhabditida : Spirurina) parasitic in fishes inferred from SSU rRNA gene sequences. Folia Parasitol (Praha) 5683:135–148
Chen HX, Zhang LP, Feng YY, Li L (2020) Integrated evidence reveals a new species of Cosmocerca (Ascaridomorpha: Cosmocercoidea) from the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura). Parasitol Res 119:1795–1802. https://doi.org/10.1007/s00436-020-06687-3
Choudhury A, Nadler SA (2016) Phylogenetic relationships of Cucullanidae (Nematoda), with observations on Seuratoidea and the monophyly of Cucullanus, Dichelyne and Truttaedacnitis. J Parasitol 102:87–93. https://doi.org/10.1645/15-806
Di Tommaso P, Moretti S, Xenarios I et al (2011) T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res 39:W13–W17. https://doi.org/10.1093/nar/gkr245
Elmer KR, Kusche H, Lehtonen TK, Meyer A (2010a) Local variation and parallel evolution : morphological and genetic diversity across a species complex of neotropical crater lake cichlid fishes. Philos Trans R Soc B Biol Sci 365:1763–1782. https://doi.org/10.1098/rstb.2009.0271
Elmer KR, Lehtonen TK, Kautt AF, Harrod C, Meyer A (2010b) Rapid sympatric ecological differentiation of crater lake cichlid fishes within historic times. BMC Biol 8:1–15. https://doi.org/10.1186/1741-7007-10-70
Folmer O, Black M, Hoeh W et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299. https://doi.org/10.1071/ZO9660275
García-Varela M, Nadler SA (2005) Phylogenetic relationships of palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. J Parasitol 91:1401–1409. https://doi.org/10.1645/GE-523R.1
Garrido-Olvera L, García-Prieto L, De León GPP (2006) Checklist of the adult nematode parasites of fishes in freshwater localities from Mexico. Zootaxa 1201:1–45. https://doi.org/10.11646/zootaxa.1201.1.1
González-Solís AD, Jiménez-García MI (2006) Parasitic nematodes of freshwater fishes from two Nicaraguan crater lakes. Comp Parasitol 73:188–192. https://doi.org/10.1654/4195.1
Hoang DT, Chernomor O, von Haeseler A et al (2017) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. https://doi.org/10.5281/zenodo.854445
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Kautt AF, Machado-Schiaffino G, Torres-Dowdall J, Meyer A (2016) Incipient sympatric speciation in Midas cichlid fish from the youngest and one of the smallest crater lakes in Nicaragua due to differential use of the benthic and limnetic habitats? Ecol Evol 6:5342–5357. https://doi.org/10.1002/ece3.2287
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kutterolf S, Freundt A, Pérez W, Wehrmann H, Schmincke HU (2007) Late Pleistocene to Holocene temporal succession and magnitudes of highly-explosive volcanic eruptions in west-central Nicaragua. J Volcanol Geotherm Res 163:55–82. https://doi.org/10.1016/j.jvolgeores.2007.02.006
Mendoza-Franco EF, Vidal-Martínez VM (2005) Phylogeny of species of Sciadicleithrum (Monogenoidea: Ancyrocephalinae), and their historical biogeography in the Neotropics. J Parasitol 91:253–259. https://doi.org/10.1645/ge-3389
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Gateway Computing Environments Workshop, GCE 2010. pp 1–8
Moravec F, Thatcher VE (1997) Procamallanus (Denticamallanus subgen. n.) dentatus n. sp. (Nematoda: Camallanidae) from the characid fish, Bryconops alburnoides, in the Brazilian Amazon. Parasite 4:239–243. https://doi.org/10.1051/parasite/1997043239
Moravec F, Van As LL (2015) Procamallanus (Procamallanus) spp. (Nematoda: Camallanidae) in fishes of the Okavango River, Botswana, including the description of P. (P.) pseudolaeviconchus n. sp. parasitic in Clarias spp. (Clariidae) from Botswana and Egypt. Syst Parasitol 90:137–149. https://doi.org/10.1007/s11230-014-9541-0
Nadler SA, Bolotin E, Stock SP (2006) Phylogenetic relationships of Steinernema Travassos, 1927 (Nematoda: Cephalobina: Steinernematidae) based on nuclear, mitochondrial and morphological data. Syst Parasitol 63:161–181. https://doi.org/10.1007/s11230-005-9009-3
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Ornelas-García CP, Pedraza-Lara C (2016) Phylogeny and evolutionary history of Astyanax mexicanus. In: Biology and evolution of the Mexican cavefish. Elsevier Inc., pp 77–90
Pariselle P, Boeger WA, Snoeks J, Billon Bilong CF, Morand S, Vanhove MPM (2011) The monogenean parasite fauna of cichlids: a potential tool for host biogeography. International J Evol Biol. 2011:1–15. https://doi.org/10.4061/2011/471480
Pérez-Miranda F, Mejía O, Soto-Galera E, Espinosa-Pérez H, Piálek L, Říčan O (2018) Phylogeny and species diversity of the genus Herichthys (Teleostei: Cichlidae). J Zool Syst Evol Res 56:223–247. https://doi.org/10.1111/jzs.12197
Pérez-Ponce De León G, Choudhury A (2005) Biogeography of helminth parasites of freshwater fishes in Mexico: the search for patterns and processes. J Biogeogr 32:645–659. https://doi.org/10.1111/j.1365-2699.2005.01218.x
Pinacho-Pinacho CD, Sereno-Uribe AL, García-Varela M, Pérez-Ponce de León G (2020) A closer look at the morphological and molecular diversity of Neoechinorhynchus (Acanthocephala) in Middle American cichlids (Osteichthyes: Cichlidae), with the description of a new species from Costa Rica. J Helminthol 94:E23. https://doi.org/10.1017/S0022149X18001141
Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogenet Evol 48:369–371. https://doi.org/10.1016/j.ympev.2008.03.024
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Razo-Mendivil U, Rosas-Valdez R, Pérez-Ponce de León G (2008) A new cryptogonimid (Digenea) from the Mayan cichlid, Cichlasoma urophthalmus (Osteichthyes: Cichlidae), in several localities of the Yucatán Peninsula, Mexico. J Parasitol 94:1371–1378. https://doi.org/10.1645/ge-1546.1
Razo-Mendivil U, Vázquez-Domínguez E, Rosas-Valdez R, de León GPP, Nadler SA (2010) Phylogenetic analysis of nuclear and mitochondrial DNA reveals a complex of cryptic species in Crassicutis cichlasomae (Digenea: Apocreadiidae), a parasite of Middle-American cichlids. Int J Parasitol 40:471–486. https://doi.org/10.1016/j.ijpara.2009.10.004
Říčan O, Piálek L, Dragová K, Novák J (2016) Diversity and evolution of the Middle American cichlid fishes (Teleostei: Cichlidae) with revised classification. Vertebr Zool 66:1–102
Říčan O, Piálek L, Zardoya R, Doadrio I, Zrzavý J (2013) Biogeography of the Mesoamerican Cichlidae (Teleostei: Heroini): colonization through the GAARlandia land bridge and early diversification. J Biogeogr 40:579–593. https://doi.org/10.1111/jbi.12023
Rigby MC, Rigby E (2013) Order Camallanida: Superfamilies Anguillicoloidea and Camallanoidea. In: Schmidt-Rhaesa A (ed) Handbook of zoology, Gastrotricha, vol 2. Cycloneuralia and Gnathifera. Nematoda. Deutsche Nationalbibliothek, Hamburg, pp 637–659
Sandlund OT, Daverdin R, Choudhury A et al (2010) A survey of freshwater fishes and their macroparasites in the Guanacaste Conservation Area (ACG), Costa Rica. Trondheim, NO
Santacruz A, Ornelas-García CP, Pérez-Ponce de León G (2020) Incipient genetic divergence or cryptic speciation? Procamallanus (Nematoda) in freshwater fishes (Astyanax). Zool Scr 49:768–778. https://doi.org/10.1111/zsc.12443
Solórzano-García B, Ospina A, Rondón S, Pérez-Ponce de León G (2020) Pinworms of the red howler monkey (Alouatta seniculus) in Colombia: gathering the pieces of the pinworm-primate puzzle. Int J Parasitol Parasites Wildl 11:17–28. https://doi.org/10.1016/j.ijppaw.2019.11.007
Vanhove MPM, Hablützel PI, Pariselle A, Šimková A, Huyse T, Raeymaekers JAM (2016) Cichlids : a host of opportunities for evolutionary parasitology. Trends Parasitol 32:820–832. https://doi.org/10.1016/j.pt.2016.07.002
Waid RM, Raesly RL, Mckaye KR, McCrary JK (1999) Zoogeografía íctica de lagunas cratéricas de Nicaragua. Encuentro-UCA 51:65–80
Acknowledgements
We thank Brenda Solórzano, Mariana Leal Cardín, Jhonatan Cabañas, Yanet Velázquez, Carlos Lozano, and the Universidad Centroamericana in Managua, Nicaragua, for their help in the field. Martin García-Varela, Jesus Hernández-Orts, and Leopoldo Andrade Gómez for donating specimens of Procamallanus from Costa Rica. We also thank Berenit Mendoza Garfías for her help taking SEM photomicrographs; Laura Márquez and Nelly López (Instituto de Biología, UNAM), Maria Luisa del Pozo and Iván Acevedo (MNCN, Madrid) helped with molecular work. Thanks also to Luis García for the loan of specimens from the Colección Nacional de Helmintos (CNHE, Mexico City). The Ministry of Natural Resources (MARENA) in Nicaragua provided permission for collection permits (No. 001-012015). Specimens from Costa Rica were collected under the collecting permit issued by the Costa Rican Government to Arturo Angulo; specimens in Mexico were collected under the Cartilla Nacional de Colector Científico FAUT 059 issued by the Secretaría del Media Ambiente y Recursos Naturales (SEMARNAT). This paper is part of the fulfillments of the first author to complete her PhD degree in the Posgrado en Ciencias Biológicas-UNAM. AS thanks the Consejo Nacional de Ciencia y Tecnología for granting a scholarship to complete her PhD program.
Funding
The present study was funded by grants of the Spanish Ministerio de Ciencia e Innovación (grant CGL2013-42462-P and CGL2017-82986-C2-1-P) to MB, and partially by a grant from the Consejo Nacional de Ciencia y Tecnología (CONACyT A1-S-21694) to GPPL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Federica Marcer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santacruz, A., Barluenga, M. & Pérez-Ponce de León, G. Taxonomic assessment of the genus Procamallanus (Nematoda) in Middle American cichlids (Osteichthyes) with molecular data, and the description of a new species from Nicaragua and Costa Rica. Parasitol Res 120, 1965–1977 (2021). https://doi.org/10.1007/s00436-021-07148-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07148-1