Abstract
Little is known of the prevalence and genetic identity of Giardia duodenalis in sheep in Algeria. The present study aimed at characterizing G. duodenalis in lambs up to 6 months of age in Djelfa, Algeria. A total of 346 fecal specimens were collected from 28 farms and screened for G. duodenalis cysts by zinc sulfate flotation microscopy, and positive specimens were confirmed using a direct immunofluorescence assay. Microscopy-positive specimens were analyzed by PCR and sequence analysis of the triosephosphate isomerase and glutamate dehydrogenase genes to determine G. duodenalis assemblages. Coprological examination indicated that the overall infection rate was 7.0% (24/346). Lambs under 3 months of age had higher infection rate (18/197, 9.0%) than older (6/149, 4.0%) animals, and animals with diarrhea (7/44, 16.0%) had higher infection rate than animals without diarrhea (17/302, 5.6%). PCR sequence analyses of the 15 G. duodenalis isolates revealed the presence of assemblages A in 6 isolates, assemblage E in 7 isolates, and both in 2 isolates. Assemblage A was only found in pre-weaned lambs with diarrhea, while assemblage E was mostly found in post-weaned lambs without diarrhea. The assemblage E isolates from sheep were genetically related to those from cattle in Algeria, while assemblage A isolates were from a well-known subtype prevalent in humans. Data generated from the study improve our understanding of the transmission of G. duodenalis in Algeria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardia duodenalis (synonyms G. intestinalis and G. lamblia) is a flagellated protozoan in humans and a wide range of animals (Thompson and Ash 2016). Giardiasis is associated with pathophysiological alterations in the intestinal mucosa (Carmena et al. 2012), resulting in the occurrence of diarrhea, malabsorption, and weight loss of the infected hosts (Aloisio et al. 2006; Vivancos et al. 2018). The health and economic impacts are especially severe in children and young farm animals (Fletcher et al. 2012; Einarsson et al. 2016). Transmission of the pathogen is through the fecal-oral route, mostly via direct contact with infected hosts and ingestion of contaminated food and water (Squire and Ryan 2017).
Livestock, especially cattle, have often been implicated as a major source of environmental contamination and potential reservoirs for zoonotic infection (Feng and Xiao 2011). The role of sheep and goats, however, is less clear (Robertson et al. 2010; Conan et al. 2017). The prevalence of G. duodenalis infection in sheep varied from 1.5 to 42% among studies conducted in different countries (Geurden et al. 2010; Feng and Xiao 2011; Tzanidakis et al. 2014; Wang et al. 2016; Wegayehu et al. 2017). Some reports have indicated that the prevalence is higher during pre-weaned period (less of 3 months) and starts to decline as the age of the animals increases (Ye et al. 2015). In contrast, other studies reported higher infection rates in post-weaned lambs and adults (Yang et al. 2014; Wang et al. 2016). Giardia cyst shedding has been associated with reduced growth, carcass weight, and dressing efficiency in sheep (Jacobson et al. 2016).
To date, eight major genotypes known as assemblages (A–H) have been identified in G. duodenalis, with assemblages A and B being recognized as major human pathogens (Feng and Xiao 2011). Molecular biologic characterizations of isolates have thus far indicated a dominance of assemblage E, with some occurrences of assemblages A and B, in sheep in most countries (Gómez-Muñoz et al. 2009; Sahraoui et al. 2019).
Algeria has one of the largest populations of sheep flocks in Africa, mainly concentrated in the steppe (Baroudi et al. 2018). However, there are scant data on the occurrence and identity of G. duodenalis in sheep in this arid country (Sahraoui et al. 2019). The present study aimed at determining the infection rate and distribution of G. duodenalis genotypes in sheep in Djelfa, the steppe of Algeria.
Materials and methods
Study area and specimen collection
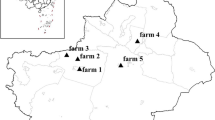
This study was carried out during the period of May 2014 to June 2016. A total of 346 fecal specimens (149 from males and 168 from females) were collected from 28 extensive sheep farms (Table 1) in the region of Djelfa in central steppe of Algeria (Fig. 1). Specimens were collected directly from the rectum from young animals up to 6 months of age (197 under 3 months and 149 over 3 months) using gloved fingers. Data on the animals including age, sex, and diarrhea status (presence or absence of diarrhea) were recorded for each specimen at the site of collection. The specimens were transported in an isotherm box to the laboratory. They were screened microscopically using zinc sulfate flotation method as described by Bartlett et al. (1978). A direct immunofluorescence assays (DFA) (MeriFluor® Cryptosporidium/Giardia, Meridian Bioscience Europe, Milano, Italy) was used in the confirmatory diagnosis of G. duodenalis infection. Positive specimens were stored in 2.5% potassium dichromate at 4 °C prior to molecular analysis.
DNA extraction
All specimens positive for G. duodenalis by microscopy were washed of potassium dichromate with distilled water by centrifugation at 1000×g for 10 min. Genomic DNA was extracted from approximately 0.2 mL of the sediment using the FastDNA® Spin Kit for Soil (MP Biomedicals, Santa Ana, CA) following manufacturer’s guidelines.
PCR analysis of Giardia duodenalis
Giardia duodenalis in 24 microscopy-positive specimens was characterized by PCR and sequence analyses targeting the triosephosphate isomerase (tpi) and glutamate dehydrogenase (gdh) genes, using established procedures (Sulaiman et al. 2003; Read et al. 2004).
Sequence and phylogenetic analyses
All positive secondary PCR products were purified using Montage PCR Cleanup Filters (Millipore, Bedford, MA) and directly sequenced in both directions using the BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) on an ABI 3130 Genetic Analyzer (Applied Biosystems). The nucleotide sequences obtained were read and assembled using ChromasPro (http://technelysium.com.au/ChromasPro.html) and aligned with reference sequences of each target using ClustalX 2.1 (http://www.clustal.org/). The maximum likelihood (ML) implemented in MEGA7 (http://www.megasoftware.net/) with the general time reversible model was used in phylogenetic analysis. Bootstrap analysis with 1000 replicates was used to estimate support of the cluster formation. Unique nucleotide sequences generated in this study were deposited in GenBank under accession numbers MT321644 to MT321652.
Statistical analysis
The chi-square test was used in the comparison of infection rates between age groups, sex category, and the presence or absence of diarrhea. The analysis was conducted using XLSTAT 2014 (Microsoft®, WA, USA). Differences were considered significant at p ≤ 0.05.
Results
Prevalence of G. duodenalis by microscopy
Microscopic examination of the fecal specimens by the zinc sulfate flotation method revealed that 24/346 (7.0%) contained Giardia cysts, all of which were confirmed by direct immunofluorescence assay. Giardia cysts were detected on 9 of the 28 (32.1%) farms examined, with infection rates by farm varying from 0 to 42.9% (Table 1). Giardia infection rate was 6.7% (10/149) in specimens from males and 7.7% (13/168) in specimens from females (χ2 = 0.12; p = 0.72). In addition, the infection rate was 9.1% (18/197) in animals under 3 months of age, compared with 4.0% (6/149) in animals of over 3 months of age (χ2 = 3.14; p = 0.063). The G. duodenalis infection rate was significantly higher in lambs with diarrhea (7/44, 15.9%) than those without diarrhea (17/302, 5.6%) (χ2 = 6.28; p = 0.02).
Distribution of G. duodenalis assemblages
PCR analysis of 24 microscopy-positive specimens yielded the expected PCR products from 15 of the specimens. The failure in PCR amplification in nine microscopy-positive specimens could be due to low parasite burdens and long storage of the specimens prior to PCR analysis. Sequence analysis of the PCR products indicated the presence of both assemblages A and E. In tpi PCR, assemblage A was detected in 8 specimens and assemblage E in 7 specimens, whereas in gdh PCR, they were detected in 5 and 9 specimens, respectively. One specimen failed to yield the expected gdh PCR product. Among the PCR-positive specimens, two (42165 and 42166) were identified as having assemblage A at the tpi gene locus but assemblage E at the gdh locus (Table 2). Assemblage A was seen in younger animals (≤ 6 weeks) with diarrhea. In contrast, assemblage E was mostly seen in older asymptomatic animals (Table 2).
Phylogenetic relationship of G. duodenalis assemblages
At the tpi gene locus, the assemblage A sequences detected belonged to A1-like (n = 1) and A4 (n = 7). The sequence of subtype A1-like had an A to T substitution compared with JF792423 from sheep in Spain, while the subtype A4 had sequences identical to KR075934 from sheep in China. Furthermore, the assemblage E sequences detected showed complete identity to MK442913 from sheep in China.
At the gdh locus, the assemblage A sequences detected belonged to A1 (n = 5). The sequences of subtype A1 had 100% identity to reference sequence KR075940 from sheep in China. The assemblage E specimens generated three types of sequences. The first type included three sequences and had one difference from MK442909 from sheep in China. The second type included two sequences and had one nucleotide difference from MH621339 from goats in China. The third type of sequences included four sequences and had one nucleotide difference from MK561344 from sheep in Greece.
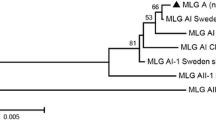
In phylogenetic analysis of the tpi sequences, the assemblage A sequences clustered with reference sequences of A4 from sheep (KR075934) and prairie dogs (KP780958 and KP780971) and humans (GQ329677) and one sequence clustered with A1 reference sequences from sheep (JF792423) and humans (GU564275 and HQ836660). The assemblage E sequences obtained clustered with reference sequences from cattle (KJ124917), sheep (MK442913 and KT922262), and pigs (MH644773) (Fig. 2).
Phylogenetic relationship among Giardia duodenalis genotypes from sheep in Algeria inferred based on sequences of the triosephosphate isomerase (tpi) gene using the maximum likelihood method implemented in MEGA7. Cluster formation support on the ML tree was calculated based on 1000 bootstrap replicates. Representative sequences obtained in this study are marked with diamonds. The sequence label includes the host animal followed by GenBank accession number and genotype identity
In phylogenetic analysis of the gdh sequences, the five assemblage A sequences clustered with numerous A1 sequences from humans and animals in the GenBank database. In contrast, the assemblage E sequences were placed in two clusters containing reference sequences from sheep in different geographical areas (Fig. 3).
Phylogenetic relationships among Giardia duodenalis genotypes from sheep in Algeria inferred based on sequences of the glutamate dehydrogenase (gdh) gene using the maximum likelihood method implemented in MEGA7. Cluster formation support on the ML tree was calculated based on 1000 bootstrap replicates. Representative sequences obtained in this study are marked with diamonds. The sequence label includes the host animal followed by GenBank accession number and genotype identity
Discussion
Results of the study have demonstrated the occurrence of G. duodenalis in sheep in Algeria. In the present study, the overall infection rate of G. duodenalis in lambs was 6.9%. Similar results have been reported in sheep in the USA (Santin et al. 2007), China (Zhang et al. 2012; Ye et al. 2015; Jin et al. 2017; Wu et al. 2018; Qi et al. 2019), Spain, Ethiopia, and Ghana (Diaz et al. 1996; Wegayehu et al. 2017; Squire et al. 2017). Younger animals and animals with diarrhea were shown in this study to have higher G. duodenalis infection rates. Previously, higher infection rates of G. duodenalis were reported in pre-weaned lambs in China, in Algeria, in Brazil, in Canada, and in Greece (Olson et al. 1997; Paz e Silva et al. 2014; Tzanidakis et al. 2014; Ye et al. 2015; Chen et al. 2019; Sahraoui et al. 2019). This could be due to the lack of acquired immunity in young animals. Similarly, previous studies had shown higher occurrence of G. duodenalis infections in lambs with diarrhea compared than in healthy animals (Olson et al. 1995; Skirnisson and Hansson 2006; Geurden et al. 2010). This is expected, as giardiasis is a known cause of outbreaks of diarrhea in pre-weaned lambs (Olson et al. 1997; Carmena et al. 2012).
Molecular analysis of G. duodenalis isolates in the present study indicated the occurrence of assemblages A and E, with almost an equal distribution. These results are in contradiction to the occurrence of assemblages E and D reported recently by Sahraoui et al. (2019) in lambs in Algeria. In most studies elsewhere, assemblage E has been reported as the dominant G. duodenalis genotype in sheep (Geurden et al. 2008; Ryan et al. 2005; Yang et al. 2009; Robertson et al. 2010; Jafari et al. 2014; Minetti et al. 2014; Paz e Silva et al. 2014; Tzanidakis et al. 2014; Wegayehu et al. 2017; Qi et al. 2019). However, a common occurrence of both assemblages A and E has been reported in sheep in China (Zhang et al. 2012; Liu et al. 2014; Ye et al. 2015; Wang et al. 2016) and some European countries (Geurden et al. 2008; Gómez-Muñoz et al. 2012). Of note, assemblage E is mainly found in cloven-hoofed domestic mammals (cattle, water buffaloes, sheep, goats, and pigs) (Feng and Xiao 2011). Recently, we have identified the occurrence of both assemblages A and E in cattle in Algeria, with assemblage A as the dominant genotype (Baroudi et al. 2017), indicating that the transmission of G. duodenalis in ruminants Algeria could be different from other areas. In this study, assemblage A was only found in pre-weaned lambs with diarrhea, while assemblage E was mostly found in post-weaned lambs without diarrhea. Thus far, as assemblage E is the dominant G. duodenalis genotype in lambs, there have been no reports of age and diarrhea-associated differences in the distribution of assemblages A and E in lambs.
The assemblages A and E of G. duodenalis identified in the study have zoonotic potential. The assemblage A subtype A4 at the tpi locus and A1 at the gdh gene locus was reported in humans previously (Feng and Xiao 2011). Although assemblage E is mostly a genotype in hoofed animals, it was detected in human specimens from Egypt (Foronda et al. 2008), Brazil (Fantinatti et al. 2016), and Australia (Zahedi et al. 2017). Therefore, assemblage E can potentially infect humans and threat public health.
In conclusion, G. duodenalis appears to be a common pathogen in lambs in Djelfa, Algeria. As both assemblages A and E have been identified as common G. duodenalis genotypes in lambs, this raises questions about the public health risk of acquiring G. duodenalis infection from sheep. More epidemiological studies are needed to confirm this hypothesis and to improve our understanding of the epizootiology and epidemiology of giardiasis in small ruminants in Algeria.
References
Aloisio F, Filippini G, Antenucci P, Lepri E, Pezzotti G, Cacciò SM, Pozio E (2006) Severe weight loss in lambs infected with Giardia duodenalis assemblage B. Vet Parasitol 142:154–158
Baroudi D, Khelef D, Hakem A, Abdelaziz A, Chen X, Lysen C, Roellig DM, Xiao L (2017) Molecular characterization of zoonotic pathogens Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in calves in Algeria. Vet Parasitol Reg Stud Reports 8:66–69
Baroudi D, Hakem A, Adamu H, Amer S, Khelef D, Adjou K, Dahmani H, Chen X, Roellig D, Feng Y, Xiao L (2018) Zoonotic Cryptosporidium species and subtypes in lambs and goat kids in Algeria. Parasit Vectors 11:582
Bartlett MS, Harper K, Smith N, Verbanac P, Smith JW (1978) Comparative evaluation of a modified zinc sulfate flotation technique. J Clin Microbiol 7:524–428
Carmena D, Cardona GA, Sánchez-Serrano LP (2012) Current situation of Giardia infection in Spain: implications for public health. World J Clin Infect Dis 2:1–12
Chen D, Zou Y, Li Z, Wang SS, Xie SC, Shi LQ, Zou FC, Yang JF, Zhao GH, Zhu XQ (2019) Occurrence and multilocus genotyping of Giardia duodenalis in black-boned sheep and goats in southwestern China. Parasit Vectors 12:102
Conan A, O’Reilly CE, Ogola E, Ochieng JB, Blackstock AJ, Omore R, Ochieng L, Moke F, Parsons MB, Xiao L, Roellig D, Farag TH, Nataro JP, Kotloff KL, Levine MM, Mintz ED, Breiman RF, Cleaveland S, Knobel DL (2017) Animal-related factors associated with moderate-to-severe diarrhea in children younger than five years in western Kenya: a matched case-control study. PLoS Negl Trop Dis 11:e0005795
Diaz V, Campos M, Lozano J, Manas I, Gonzalez J (1996) Aspects of animal giardiosis in Granada province (southern Spain). Vet Parasitol 64:171–176
Einarsson E, Ma’ayeh S, Svärd SG (2016) An up-date on Giardia and giardiasis. Curr Opin Microbiol 34:47–52
Fantinatti M, Bello AR, Fernandes O, Da-Cruz AM (2016) Identification of Giardia lamblia assemblage E in humans points to a new anthropozoonotic cycle. J Infect Dis 214:1256–1259
Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24:110–140
Fletcher SM, Stark D, Harkness J, Ellis J (2012) Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev 25:420–449
Foronda P, Bargues MD, Abreu-Acosta N, Periago MV, Valero MA, Valladares B, Mas-Coma S (2008) Identification of genotypes of Giardia intestinalis of human isolates in Egypt. Parasitol Res 103:1177–1181
Geurden T, Thomas P, Casaert S, Vercruysse J, Claerebout E (2008) Prevalence and molecular characterization of Cryptosporidium and Giardia in lambs and goat kids in Belgium. Vet Parasitol 155:142–145
Geurden T, Vercruysse J, Claerebout E (2010) Is Giardia a significant pathogen in production animals? Exp Parasitol 124:98–106
Gómez-Muñoz MT, Navarro C, Garijo-Toledo MM, Dea-Ayuela MA, Fernández-Barredo S, Pérez-Gracia MT, Domínguez-Márquez MV, Borrás R (2009) Occurrence and genotypes of Giardia isolated from lambs in Spain. Parasitol Int 58:297–299
Gómez-Muñoz MT, Cámara-Badenes C, Martínez-Herrero Mdel C, Dea-Ayuela MA, Pérez-Gracia MT, Fernández-Barredo S, Santín M, Fayer R (2012) Multilocus genotyping of Giardia duodenalis in lambs from Spain reveals a high heterogeneity. Res Vet Sci 93:836–842
Jacobson C, Williams A, Yang R, Ryan U, Carmichael I, Campbell AJ, Gardner GE (2016) Greater intensity and frequency of Cryptosporidium and Giardia oocyst shedding beyond the neonatal period is associated with reductions in growth, carcase weight and dressing efficiency in sheep. Vet Parasitol 228:42–51
Jafari H, Jalali MH, Shapouri MS, Hajikolaii MR (2014) Determination of Giardia duodenalis genotypes in sheep and goat from Iran. J Parasit Dis 38:81–84
Jin Y, Fei J, Cai J, Wang X, Li N, Guo Y, Feng Y, Xiao L (2017) Multilocus genotyping of Giardia duodenalis in Tibetan sheep and yaks in Qinghai, China. Vet Parasitol 247:70–76
Liu A, Yang F, Shen Y, Zhang W, Wang R, Zhao W, Zhang L, Ling H, Cao J (2014) Genetic analysis of the Gdh and Bg genes of animal-derived Giardia duodenalis isolates in northeastern China and evaluation of zoonotic transmission potential. PLoS One 9:e95291
Minetti C, Taweenan W, Hogg R, Featherstone C, Randle N, Latham SM, Wastling JM (2014) Occurrence and diversity of Giardia duodenalis assemblages in livestock in the UK. Transbound Emerg Dis 61:e60–e67
Olson ME, McAllister TA, Deselliers L, Morck DW, Cheng KJ, Buret AG, Ceri H (1995) Effects of giardiasis on production in a domestic ruminant (lamb) model. Am J Vet Res 56:1470–1474
Olson ME, Thorlakson CL, Deselliers L, Morck DW, McAllister TA (1997) Giardia and Cryptosporidium in Canadian farm animals. Vet Parasitol 68:375–381
Paz e Silva FM, Lopes RS, Bresciani KDS, Amarante AFT, Araujo JP (2014) High occurrence of Cryptosporidium ubiquitum and Giardia duodenalis genotype E in sheep from Brazil. Acta Parasitol 59:193–196
Qi M, Zhang Z, Zhao A, Jing B, Guan G, Luo J, Zhang L (2019) Distribution and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi amongst grazing adult sheep in Xinjiang, China. Parasitol Int 71:80–86
Read CM, Monis PT, Thompson RCA (2004) Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol 4:125–130
Robertson LJ, Gjerde BK, Furuseth HE (2010) The zoonotic potential of Giardia and Cryptosporidium in Norwegian sheep: a longitudinal investigation of 6 flocks of lambs. Vet Parasitol 171:140–145
Ryan UM, Bath C, Robertson I, Read C, Elliot A, Mcinnes L, Traub R, Besier B (2005) Sheep may not be an important zoonotic reservoir for Cryptosporidium and Giardia parasites. Appl Environ Microbiol 71:4992–4997
Sahraoui L, Thomas M, Chevillot A, Mammeri M, Polack B, Vallée I, Follet J, Ain-Baaziz H, Adjou KT (2019) Molecular characterization of zoonotic Cryptosporidium spp. and Giardia duodenalis pathogens in Algerian sheep. Vet Parasitol Reg Stud Reports 16:100280
Santin M, Trout JM, Fayer R (2007) Prevalence and molecular characterization of Cryptosporidium and Giardia species and genotypes in sheep in Maryland. Vet Parasitol 146:17–24
Skirnisson K, Hansson H (2006) Causes of diarrhoea in lambs during autumn and early winter in an Icelandic flock of sheep. Icel Agric Sci 19:43–57
Squire SA, Ryan U (2017) Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors 10:195
Squire SA, Yang R, Robertson I, Ayi I, Ryan U (2017) Molecular characterization of Cryptosporidium and Giardia in farmers and their ruminant livestock from the coastal Savannah zone of Ghana. Infect Genet Evol 55:236–243
Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao L (2003) Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis 9:1444–1452
Thompson RCA, Ash A (2016) Molecular epidemiology of Giardia and Cryptosporidium infections. Infect Genet Evol 40:315–323
Tzanidakis N, Sotiraki S, Claerebout E, Ehsan A, Voutzourakis N, Kotsiopoulos D, Stijn C, Vercruysse J, Geurden T (2014) Occurrence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in sheep and goats reared under dairy husbandry systems in Greece. Parasite 21:45–52
Vivancos V, González-Alvarez I, Bermejo M, Gonzalez-Alvarez M (2018) Giardiasis: characteristics, pathogenesis and new insights about treatment. Curr Top Med Chem 18:1287–1303
Wang H, Qi M, Zhang K, Li J, Huang J, Ning C, Zhang L (2016) Prevalence and genotyping of Giardia duodenalis isolated from sheep in Henan Province, central China. Infect Genet Evol 39:330–335
Wegayehu T, Karim MR, Li J, Adamu H, Erko B, Zhang L, Tilahun G (2017) Prevalence and genetic characterization of Cryptosporidium species and Giardia duodenalis in lambs in Oromia Special Zone, Central Ethiopia. BMC Vet Res 13:22
Wu Y, Chang Y, Chen Y, Zhang X, Li D, Zheng S, Wang L, Li J, Ning C, Zhang L (2018) Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect Genet Evol 64:46–51
Yang R, Jacobson C, Gordon C, Ryan U (2009) Prevalence and molecular characterization of Cryptosporidium and Giardia species in pre-weaned sheep in Australia. Vet Parasitol 161:19–24
Yang R, Jacobson C, Gardner G, Carmichael I, Campbell AJ, Ryan U (2014) Development of a quantitative PCR (qPCR) for Giardia and analysis of the prevalence, cyst shedding and genotypes of Giardia present in sheep across four states in Australia. Exp Parasitol 137:46–52
Ye J, Xiao L, Wang Y, Guo Y, Roellig DM, Feng Y (2015) Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Vet Parasitol 210:235–239
Zahedi A, Field D, Ryan U (2017) Molecular typing of Giardia duodenalis in humans in Queensland - first report of assemblage E. Parasitology 144:1154–1161
Zhang W, Zhang X, Wang R, Liu A, Shen Y, Ling H, Cao J, Yang F, Zhang X, Zhang L (2012) Genetic characterizations of Giardia duodenalis in sheep and goats in Heilongjiang Province, China and possibility of zoonotic transmission. PLoS Negl Trop Dis 6:e1826
Acknowledgments
We thank Safia Zenia from École Nationale Supérieure Vétérinaire of Algiers for assistance in statistical analysis and the deceased Professor Said Amer from Kafr El Sheikh University Egypt for his precious contribution in this work.
Funding
This work was supported by National Natural Science Foundation of China (31820103014) and the 111 Project (D20008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Faculty of Nature and Life Sciences, University of Djelfa, Algeria (Ref: AT04/E.V.E.S/2017). All specimen collection was performed by the licensed veterinarians. Informed written consent was obtained from farm owners or managers, and animals were handled in compliance with the established regulations and guidelines for laboratory animal research of the People’s Democratic Republic of Algeria.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benhassine, S., Baroudi, D., Hakem, A. et al. Occurrence and molecular characterization of Giardia duodenalis in lambs in Djelfa, the central steppe of Algeria. Parasitol Res 119, 2965–2973 (2020). https://doi.org/10.1007/s00436-020-06808-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06808-y