Abstract
Malaria, caused by protozoa of the genus Plasmodium, is a disease that infects hundreds of millions of people annually, causing an enormous social burden in many developing countries. Since current antimalarial drugs are starting to face resistance by the parasite, the development of new therapeutic options has been prompted. The enzyme Plasmodium falciparum enoyl-ACP reductase (PfENR) has a determinant role in the fatty acid biosynthesis of this parasite and is absent in humans, making it an ideal target for new antimalarial drugs. In this sense, the present study aimed at evaluating the in silico binding affinity of natural and synthetic amides through molecular docking, in addition to their in vitro activity against P. falciparum by means of the SYBR Green Fluorescence Assay. The in vitro results revealed that the natural amide piplartine (1a) presented partial antiplasmodial activity (20.54 μM), whereas its synthetic derivatives (1m—IC50 104.45 μM), (1b, 1g, 1k, and 14f) and the natural amide piperine (18a) were shown to be inactive (IC50 > 200 μM). The in silico physicochemical analyses demonstrated that compounds 1m and 14f violated the Lipinski's rule of five. The in silico analyses showed that 14f presented the best binding affinity (− 13.047 kcal/mol) to PfENR and was also superior to the reference inhibitor triclosan (− 7.806 kcal/mol). In conclusion, we found that the structural modifications in 1a caused a significant decrease in antiplasmodial activity. Therefore, new modifications are encouraged in order to improve the activity observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a disease that constitutes a serious public health problem worldwide, with an estimated 219 million cases in 2017 (WHO 2018). The disease is caused by protozoa of the genus Plasmodium, of which five species are known to cause disease in humans (Cox 2010; Amir et al. 2018). Plasmodium falciparum, alongside Plasmodium vivax, are the species that cause most of the cases and deaths annually, making them the most relevant species both clinically and epidemiologically (Hay et al. 2010). These species can cause severe form of malaria in some cases (Mackintosh et al. 2004; Alexandre et al. 2010). For P. falciparum, this severe form may cause serious organ damage, resulting in respiratory and neurological problems, in addition to fetal loss in pregnant women (Mackintosh et al. 2004). For P. vivax, common clinical manifestations are thrombocytopenia, and renal, hepatic, cerebral, and pulmonary involvement (Zubairi et al. 2013).
The chemotherapy currently used in the treatment of malaria, which is based on artemisinin combination therapy (ACT), has started to face resistance by the parasite in some regions of the globe, especially in Southeast Asia (Ashley et al. 2014), which prompts the development of drugs targeting specific pathways of the parasite.
In this sense, dissociative or type II fatty acid synthase, named FAS II, constitutes a promising molecular target for potential antimalarial drug candidates, since it is absent in humans (Qidwai and Khan 2012). Plasmodium falciparum enoyl-ACP reductase (PfENR), which belongs to this pathway, is involved in the final step of fatty acid chain elongation, catalyzing the reduction of enoyl-ACP to acyl-ACP using NADH as an electron donor, and is also the rate-limiting enzyme in this cycle (Tasdemir 2006).
Regarding potential compounds for antiparasitic agents, natural products derived from plant secondary metabolism possess a plethora of potential antiparasitic molecules (Wink 2012). With respect to antimalarial therapy, the alkaloid quinine originated a series of analogs of great relevance for the treatment of disease (Krafts et al. 2012). In this context, plants that are rich in alkaloids—which encompasses the species of the genus Piper (Gutiérrez et al. 2013)—constitute a great potential to develop new compounds against the disease. Piplartine and piperidine, two alkaloids isolated from Piper species, have been shown to present various biological activities. Piplartine presents activity against various parasitic species (Leishmania amazonensis Lainson & Shaw, 1972; Leishmania infantum Cunha & Chagas, 1937; Leishmania donovani Laveran & Mesnil, 1902 and P. falciparum), especially Trypanosoma cruzi Chagas, 1909 (Araújo-Vilges et al. 2017; Bodiwala et al. 2007; Moreira et al. 2018; Cotinguiba et al. 2009; Gomes et al. 2014; Silva-Jardim et al. 2004). In this light, this study aimed at evaluating the in silico binding affinity of two natural amides and five synthetic analogs with PfENR, their in silico physicochemical properties, and their in vitro antiplasmodial activity.
Materials and methods
All the compounds were obtained using the following methodology
Isolation of the natural compounds (piplartine and piperine)
Roots of Piper tuberculatum Jacq, 1897 were harvested on the University of São Paulo (USP) Campus, São Paulo, Brazil. A voucher specimen (Kato-0169) has been deposited at the Institute of Biosciences Herbarium. For the isolation of piplartine, 100 g of ground roots of P. tuberculatum were extracted with a mixture of methanol/chloroform 1:2 (1 L) for 72 h. The mixture was rotary evaporated under reduced pressure to yield a white solid which, after recrystallization in MeOH, produced pure piplartine (1a) (PubChem CID: 637858), which was identified by comparison of established NMR and MS data with reported data (Araújo-Vilges et al. 2017).
Fruits of Piper nigrum Koehler, 1887 were purchased from a local market in São Paulo. Piperine (18a) was purified from 250 g of dried P. nigrum fruits. The fruits were ground into a powder and extracted twice with 2 L of methanol/chloroform (1:2). The extract was filtered and concentrated in a rotary vacuum evaporator. The crude extract was subjected to silica column chromatography eluted with hexanes/ethyl acetate mixtures of increasing polarity. Fractions containing piperine (18a) were pooled and recrystallized in methanol yielding a yellow crystal. Piperine (18a) (PubChem CID 638024) was identified by NMR and MS analyses and comparison with reported data (Parmar et al. 1997; Araújo-Júnior et al. 1997).
Synthesis of the synthetic analogs
Compound 1b (PubChem CID: 53440296) was obtained by the catalytic hydrogenation (4 atm of hydrogen, Pd–C) of piplartine (1a) for 4 h and was identified by comparison of NMR and MS analyses with reported data (Fokoue et al. 2018).
Compounds 1g and 1k were synthesized by adding triethylamine (3 equivalents) and the appropriate amines (i.e., pyrrolidine and n-pentylamine) to a methylene chloride solution of various acyl chlorides (1.0 equivalent). The reaction mixtures were stirred overnight at room temperature, quenched with a saturated aqueous ammonium chloride solution and then extracted with methylene chloride 3 times. Combined organic phases were washed with brine and dried over anhydrous magnesium sulfate. After filtration and concentration, the residues were purified by flash chromatography over a silica gel using hexanes-ethyl acetate (typically 30–50%) as an eluent, yielding the desired amides. Compounds 1g (PubChem CID: 121169) and 1k (PubChem 19173974) were identified by comparison of NMR and MS analyses with reported data (Campelo et al. 2018; Fokoue et al. 2018).
Compounds 1m and 14f were synthesized by adding 2E-3,4,5-trimethoxycinnamic acid and 2E-3,4-methylenedioxycinnamic acid (1 equivalent) to a solution of THF 0.9 equivalent of N,N′-dicyclohexylcarbodiimide (DCC). The reaction mixture was stirred overnight at room temperature and dried on a rotary evaporator to remove any remaining solvent. The product was dissolved in dichloromethane (DCM), a saturated solution of NaHCO3 was added, and the resulting solution was washed three times with DCM. The organic phase was combined, dried with MgSO4, filtered, and concentrated under pressure. The obtained crystalline product was then recrystallized on hot methanol or purified by chromatography over silica-gel using hexanes-ethyl acetate (typically 30–50%). Compound 1m was identified by comparison of NMR and MS analyses with reported data (Campelo et al. 2018).
Compound 14f was characterized according to the following data: 3-[(2E)-3-(2H-1,3-benzodioxol-5-yl)prop-2-enoyl]-1,3-dicyclohexylurea (Fokoue 2015).
1H NMR (300 MHz; CDCl3): δ 7.57 (d; J = 15.0 Hz; 1H, H2); 7.13 (sl, NH); 6.97 (dd; J = 8.5 and 1.5 Hz; 1H, H9); 6.95 (d; J = 1.5 Hz; 1H, H5); 6.80 (d; J = 8.0 Hz; 1H, H8); 6.55 (d; J = 15.0 Hz; 1H, H3); 6.01 (s, 2H, OCH2O); 4.13–4.06 (m; 1H, H1a); 3.78–3.73 (m; 1H, H1b); 1.99–1.19 (m; 20H, H2a, H2b, H3a, H3b, H4a, H4b, H5a, H5b, H6a, H6b). 13C NMR (75 MHz; CDCl3): δ 166.64 (C1); 154.07 (C*); 149.34 (C6); 148.23 (C7); 143.14 (C3); 129.11 (C4); 124.20 (C9); 117.26 (C2); 108.51 (C8); 106.28 (C5); 101.51 (OCH2O); 55.90 (C1a); 49.92 (C1b); 34.90 (C2b); 33.95 (C6b); 32.78 (C2a); 30.92 (C6a); 26.25 (C4b); 25.61 (C4a); 25.45 (C3b); 25.37 (C5b); 24.93 (C3a); 24.71 (C5a). EI-MS (m/z) (%): 273 (35). 257 (25). 241 (25). 190 (90). 175 (55). 145 (75). 118 (60). 98 (72). 89 (100). 63 (35). 44 (50). ESI-MS: calculated for C23H31N2O4+ [M + H]+: 399.2278; found: 399.2210. IR (KBr, ѵmáx/cm−1): 3256. 3047. 2931. 2855. 2118. 1704. 1646. 1542. 1503. 1491. 1448. 1388. 1344. 1255. 1229. 1042. 989. 936. 810.
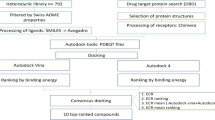
Virtual screening
OSIRIS property explorer and Molinspiration
Initially, a virtual screening of the natural compounds and their derivatives was performed. This analysis allows for a rational choice of drugs by selecting those that present adequate physicochemical properties, which might, in turn, translate into good oral bioavailability. For these analyses, we used the software OSIRIS property explorer (https://www.organic-chemistry.org/prog/peo/) and the server Molinspiration (http://www.molinspiration.com).
OSIRIS
The substances were drawn in the software OSIRIS property explorer (https://www.organic-chemistry.org/prog/peo/) and compared with a list of approximately 5300 different fragments in respect to their druglikeness properties. These fragments are obtained from 3300 commercial drugs and 15,000 chemicals available from the company Fluka®.
The software OSIRIS computes theoretical physicochemical and toxicological properties of compounds, aiming to evaluate some properties and toxicological assessments such as mutagenic, tumorigenic, irritant, and reproductive risks. The toxicological results are color-coded, wherein red indicates high risk, yellow indicates moderate risk, and green indicates no risk. The software also evaluated the druglikeness and drugscore of the respective compounds, which aim to measure whether or not a compound qualifies as a drug by considering its physicochemical properties, structural similarity with commercially available drugs, and toxicity risks.
The software also shows the druglikeness, a parameter that evaluates how structurally similar the compound is to commercially available drugs. Its values range from negative to positive values, with positive values indicating that the compound is structurally similar to commercially available drugs, whereas negative values indicate otherwise. Finally, the software calculates the drugscore, a final parameter which is based on (1) pharmacokinetic and physicochemical features, (2) toxicological prediction, and (3) druglikeness. The drugscore values range from 0 to 1, with values close to 1 indicating that the compound may qualify as a drug (https://www.organic-chemistry.org/prog/peo/).
Molinspiration
The evaluated compounds were also analyzed in the server Molinspiration, which calculated the number of violations to the Lipinski’s rule of 5 according to their: molecular weight (≤ 500 μM), clogP (≤ 5), hydrogen bond acceptors (≤ 10), and hydrogen bond donors (≤ 5) (Lipinski 2004). The server also evaluated two additional properties: the total polar surface area (TPSA) and the number of rotatable bonds. According to Veber et al. (2002), it is desirable that drug candidates should have a TPSA ≤ 140 Å2 as well as a number of rotatable bonds ≤ 10.
Molecular modeling and molecular docking studies
The molecular modeling study was conducted in partnership with the Laboratory of Molecular Modeling and Computational Simulation of the Federal University of Alfenas – Minas Gerais, Brazil. All computer applications were run on OpenSUS Tumbleweed. Structures of the ligands 1a, 1b, 1g, 1k, 1m, 14f, 18a, and triclosan (ligand used in the redocking analysis) were constructed using the software Maestro 10.2.010. The software LigPrep 3.4 with OPLS_3 force field and ionization state for pH 7.0 ± 2.0 was used to prepare the ligands involved in the studies. The crystallographic structures of PfENR with triclosan (PDB ID: 1UH5) from P. falciparum were obtained from the Protein Data Bank (PDB) database and the software Protein Preparation Wizard was used for the preparation of these receptors. Co-crystallized NAD and triclosan present in 1UH5 were removed from the structure and chain A alone was selected for docking studies. The OPLS3 force field in MacroModel 9.9 was used for optimization. Molecular docking studies between PfENR and the ligands were performed using the Induced Fit Docking protocol, in which the program Prime was used for refinement of the compounds and the program Glide provided the scoring considering the proteins and the flexible ligand. The grid box area was defined with the amino acids Tyr 277, Ile 323 and Phe 368. All computer programs used belong to the Schrödinger suite (Pidugu et al. 2004; Schrödinger, Small-Molecule Drug Discovery Suite 2015; Schrödinger release 2015a, b, c).
In vitro culture of P. falciparum
Erythrocytic forms of the W2 strain of P. falciparum were used in the antiplasmodial assays. The parasites were cultured in O+ red blood cells in RPMI 1640 medium (Gibco) supplemented with 25 mM HEPES, 300 μM hypoxanthine, 11 mM glucose, 40 μg/ml gentamicin 10% (v/v), and O+ plasma or 5% albumax (Gibco) under the conditions established by Trager and Jensen (1976). The erythrocytes added to the culture originated from a single volunteer donor, with a final hematocrit of 4%. The culture flasks were kept in an incubator at 37 °C, with the addition of a composite gaseous mixture (5% CO2, 5% O2, and 90% N2). The medium was changed every 48 h. The project was approved by the local Ethics and Research Committee (CEP) under number: 83791418.8.0000.5300, case: 2.541.143.
Determination of parasite growth inhibition (IC50)
The substances were diluted in dimethylsulfoxide (DMSO), at concentrations up to 0.5%. The serial dilution started from an initial concentration of 2000 μM in the first well (20 μL). Cultures were synchronized until they reached the desired parasitemia of 8% young trophozoites, as described by Lambros and Vanderberg (1979). Subsequently, the parasite culture with a predominance of ring forms was adjusted to 0.5% parasitemia and 2% hematocrit. Thus, all compounds were diluted 10×, at concentrations from 200 to 1.56 μM. Three controls were used: artemisinin (170 nM) was used as the positive control, untreated red blood cells infected with P. falciparum constituted the negative control, and uninfected red blood cells were used as a blank (whose fluorescence was discounted from all values obtained).
Plates were incubated at 37 °C with 5% CO2 for 48 h. Thereafter, the supernatant was discarded without suspending the red cells. To the latter, 1× PBS was added (200 μL/well) and centrifuged at 1500 rpm for 10 min. In parallel, 200 μL of PBS 1× was added to a flat-bottom 96-well culture plate for fluorescence reading. After centrifugation of the plate, the supernatant was discarded again. In the plate containing the culture, 200 μL of lysis buffer (20 mM Tris-HCl, 5 mM EDTA, 0.08% Triton X-100, 0.008% saponin in 1× PBS, pH 7.5) containing SYBR Green I (Invitrogen) was added in the following ratio: 2 μL of SYBR Green I dye to 10 ml of the lysis buffer solution. The mixture was homogenized and 200 μL of it was transferred to the reading plate where 200 μL of PBS 1× was previously added. The plate was incubated for 30 min at room temperature. Subsequently, a UV/visible spectrophotometer reading (Synergy HT (BioTek)) with an excitation of 485 nm and emission of 535 nm was performed. The concentration at which the compounds caused 50% of parasite growth inhibition (IC50) was determined by the dose-response curve of the triplicate mean for each evaluated compound (Smilkstein et al. 2004).
Cultivation of mammal cell lines
VERO cells (renal cells isolated from the kidneys of the African monkey Cercopithecus aethiops) and HepG2 cells (cell line derived from human hepatocarcinoma) were used for the cytotoxicity assays. These cell lines are related to the excretion of substances and to drug metabolism, respectively. Cells were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS) (Gibico) and 40 mg/L gentamicin (complete medium) in an incubator with 5% CO2 at 37 °C. The culture was monitored daily by optical microscopy, and the medium was changed whenever necessary until 90% confluence was reached, indicating favorable conditions for the cytotoxicity assay (Calvo-Calle et al. 1994).
Cytotoxicity assay using the MTT method
After reaching the desired confluence, the mammal cell lines were detached from the bottom of the flasks with the aid of trypsin 1× (Sigma-Aldrich) and centrifuged (1500 rpm for 10 min). The supernatant was removed and the pellet resuspended in 1 ml of complete medium. An aliquot of 10 μL of this volume was removed so that the cells could be counted in a Neubauer chamber using an optical microscope. Afterwards, the culture was adjusted to 1 × 104 cells/well. The cells were then plated in 96-well plates and incubated for 24 h at 36 °C. All compounds were distributed separately on the plate, in triplicate, using the serial dilution method (200 to 1.56 μM). The culture was incubated again (48 h). Untreated cells were constituted as the positive control, whereas the cells treated with lysis buffer containing Tris at 20 mM (Sigma-Aldrich), EDTA at 5 mM (Dinâmica), saponin at 0.008%, and Triton X-100 at 0.008% (v/v) (Sigma-Aldrich) constituted the negative control.
After the treatment period, the plates were revealed using the MTT technique ([3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide]) (Mossmann 1983). This technique evaluates cell viability through mitochondrial dehydrogenase, present only in metabolically viable cells. After the addition of MTT (Sigma-Aldrich) at a concentration of 5 mg/ml, the plate was incubated for 4 h, the supernatant discarded immediately, and 100 μL of dimethyl sulfoxide (DMSO) (Dynamic) was added to dissolve the formazan crystals (violet coloration). A spectrophotometer (Biochrom, Asys Expert Plus) was used to read the absorbance (570 nm) of the test plate. Afterwards, the software Origin 9.1 (Origin Lab Corporation, Northampton, MA, USA) was used to determine the cytotoxic concentration for 50% of the cell population (CC50).
Selectivity index (SI)
The SI of the evaluated compounds was obtained using the ratio: CC50/IC50. Compounds whose SI were ≥ 10 were considered to be selective, whereas compounds with SI < 10 were considered non-selective (Katsuno et al. 2015; Gomes et al. 2014; Nogueira and Rosário 2010).
Results and discussion
Piplartine, piperine, and analogs
For the present study, five analogs (1b, 1g, 1k, 1m, and 14f) were synthesized from the natural amide piplartine following previously published literature, without major changes, and characterized by spectrometric and spectroscopic data. The yields were not optimized since we focused on obtaining a sufficient amount of pure compounds for the assays.
In silico prediction of physicochemical and toxicological properties of the evaluated compounds.
Due to the importance of some physicochemical features for the biological activity of chemicals (Lipinski 2004), the virtual prediction of parameters such as molecular weight, hydrogen bond acceptors and donors and cLogP (computed partition coefficient), as well as possible biological activities (drugscore), were calculated using the software OSIRIS Property explorer and the server Molinspiration.
Most of the compounds in Tables 1 and 2 are within the limits established by Lipinski’s rule of five, with some observations. Compounds 1m and 14f presented a red alert for mutagenicity and reproductive toxicity (the latter was also presented by compounds 14f and 18a), which can be explained by the presence of groups with a large volume in their structures. Compounds 1m and 14f presented one violation to the Lipinski’s rule of five. It is important to stress, however, that these alerts do not necessarily exclude compounds 14f and 18a from being safe drugs, but rather indicate a probable risk.
The molecular weights of the compounds averaged 303.36 ± 58.15 g/mol; the cLogP values averaged 2.87 ± 1.09 and were below 5 for all compounds. This parameter is related to lipophilicity, in that compounds with cLogP values < 5 present inadequate oral bioavailability, owing to the difficulty of passing through biological membranes (Oliveira 2013).
According to Freitas (2015), high and positive cLogP values indicate a hydrophobic and lipophilic nature of the compounds, while negative values indicate a hydrophilic nature. Compounds 14f and 1m presented cLogP values = 4.7 and 4.38, respectively, indicating that these molecules are hydrophobic/lipophilic. The cLogP of compounds 1a and 18a were = 1.78 and 3.6, which means that these compounds are hydrophobic/lipophilic. The compounds also presented desirable values for druglikeness (0.115 ± 3.30), drugscore (0.385 ± 0.19), and solubility (− 3.04 ± 1.35). The drugscore values can range from 0 to 1 (a compound that completely qualifies as a drug). The positive values of druglikeness observed mean that the evaluated compounds possess fragments that are commonly found in commercially available drugs (Magalhães 2009).
The S (solubility—Table 1) scale classifies the compounds’ solubility in water as being insoluble for values lower than 10; slightly soluble < 6; moderately soluble < 4; soluble < 2; very soluble < 0 (Guerra 2019). The S (solubility) values for the natural compounds 1a (piplartine) and 18a (piperine) were 2.39 and − 3.61, respectively. These results underpin their hydrophobic/lipophilic attributes. Since they are poorly soluble in water, these compounds have limited biological applicability. Aqueous solubility has a determinant role in the absorption and distribution process to reach the blood circulation. Most commercial compounds have S (mol/L) greater than − 4.00 (OSIRIS Property explorer). In general, poorly water-soluble compounds are incompletely absorbed in the body. However, water solubility can be increased and/or improved by the addition of hydrochlorides, sodium salts, etc.
The total polar surface area (TPSA), hydrogen bond donors, hydrogen bond acceptors and rotatable bonds were within the desired thresholds for all compounds in Table 2, except 14f, which presented more than five hydrogen bond donors. Compounds 1g and 1a presented the best druglikeness and drugscores (Tables 1 and 2), indicating that these compounds are the ones that best qualify as drugs. Therefore, we infer that the modifications made at the dihydropiperidinone moiety of the ring of compound 1a, substituted by a piperidinyl ring (1g) contributed to these results.
Inhibitory concentration (IC50) and cytotoxic concentration (CC50)
The compounds 1a and 1m obtained IC50 = 20.54 μM and 104.45 μM respectively. The compounds 1b, 1g, 1k, 14f, and 18a obtained IC50 values > 200 μM (Table 3). The control with the solvent DMSO did not affect cell viability.
The cytotoxicity assays demonstrated that piplartine was toxic on the evaluated mammal cell lines and on P. falciparum, which suggests that the compound might be exerting its effects through non-specific mechanisms, involving enzymes, receptors and/or pathways that are present in both protozoa and mammal cells. Some studies show that the biological and toxicological activity of piplartine may be attributed to the presence of α,β-unsaturated carbonyl groups, in which the substitution of any of these unsaturated groups led to a loss of biological activity as well as a decrease in cytotoxicity (Campelo et al. 2018; Bezerra et al. 2013; Bezerra et al. 2008; Ponte-Sucre et al. 2015).
Selectivity Indexes were not obtained for the compounds 1b, 1g, 1k, 14f, and 18a, since neither the CC50 nor the IC50 values could be determined for these compounds.
Molecular docking studies on a series of molecules against P. falciparum P fENR
The molecular docking results revealed that the compounds that presented the highest binding affinities for the enzyme were 1m and 14f (Supplementary material). However, the rest of the evaluated compounds also obtained binding energies that were higher than the reference inhibitor triclosan.
All the evaluated compounds presented superior affinity values for PfENR (GScore (Glide Score)) when compared to the reference inhibitor triclosan. This might be explained by the higher volume of the compounds when compared to triclosan, along with the contribution of steric effects, thus increasing their affinity for the enzyme, which indicates that the molecules may present higher inhibitory activities than the reference compound (Table 4).
The compound 14f (Table 4) presented the highest affinity value (GScore = − 13.047 kcal mol−1), with 2 interactions per hydrogen bond (HBond) between the carbonyl oxygen atoms and the Tyr111 and Tyr277 residues, in addition to displaying a high number of favorable van der Waals (vdW) contacts (483). No parasite growth inhibition was observed for the compound 14f at 200 μM, which might be explained by steric effects relative to the molecule’s size or other physicochemical properties.
The molecule with the second highest binding affinity was 1m (− 10.981 kcal mol−1) (Table 4), also presenting a hydrogen bond interaction with the residue Ala319 and a superior number of favorable vdW contacts (546) when compared to 14f. These data indicate that trimethoxyphenyl groups favor more hydrophobic interactions. The compound 1m presented the second best IC50 value (104.45 μM), which may be related to its molecular volume and to its access to the enzyme’s active site. Other features that could possibly explain such differences between 1m and 14f are the number of hydrogen bond acceptors and rotatable bonds, which can influence the flexibility of the molecule and consequently, its interaction with the enzyme’s active site (Veber 2002).
The compound that presented the third highest GScore value was 1a (− 10.762 kcal mol−1) (Table 4), with the presence of one hydrogen bond with the Tyr277 residue and 404 favorable vdW contacts, which are related to the small size of this molecule when compared to the derivatives 14f and 1m. Despite the satisfactory binding affinity values of these two compounds, they did not display in vitro antiplasmodial activity.
When comparing the in vitro IC50 values, it was observed that 1a presented the best results (20.54 μM) against P. falciparum owing to its molecular volume, which may facilitate its access to the active site of the enzyme. Regarding the in silico results displayed in Table 1, compound 1a presented a completely different hydrophobicity value (cLogP) when compared to the analogs. All structural modifications performed in 1a were shown to substantially decrease its antiplasmodial activity. Fokoue (2015) reported that the solubility of drugs in a hydrophobic medium decreases their biological activities, which was observed in the present study for the compound’s analogs.
It is important to stress that the IC50 values of 1a and 1k against P. falciparum were previously reported in a study by Araújo-Vilges et al. (2017), being 19.5 μM and 79.1 μM, respectively (48 h of incubation). The strain and the culture used in the study are different from those of the present study, which might explain the differences between the results found in each study.
Gomes et al. (2014) when citing Ryckmans et al. (2009) state that it is desirable that the compounds evaluated for antiplasmodial activity present low IC50 values in in vitro assays. In this perspective, Boechat et al. (2014) consider compounds with IC50 < 10 μM to be active; with IC50 > 10 and < 50 μM to be partially active; and compounds with IC50 > 50 μM to be inactive. It is also noteworthy that substances with high molecular weights and high lipophilicity are more likely to present inadequate oral bioavailability. These features may minimize the risk for lack of specificity of the drug for its respective target, besides enabling one to administer lower doses, thus reducing the occurrence of adverse effects (Lipinski 2004).
The structural modifications of piplartine (1a) were performed at the dihydropiridinone moiety, generating the compounds 1b, 1g, 1k, and 1m. These alterations caused changes in the physicochemical properties of the in silico study, as well as in the in vitro growth inhibition assay against P. falciparum, rendering the molecules inactive (according to Boechat et al. 2014) against the parasite at concentrations up to 200 μM when compared to the natural compound piplartine (1a). In 14f, the modifications occurred both at the dihydropiridinone and trimethoxybenzene moieties, which significantly altered its antiplasmodial activity and physicochemical properties.
Significantly, in the molecular docking studies, only affinity values were observed, i.e., whether or not energetically favorable interactions (electronic and steric effects) between ligand and enzyme were formed. Favorable affinity values may lead to intrinsic activity by the molecule, wherein the intrinsic activity depends on the affinity. The opposite, however, is not true: one can find favorable affinity values without the desired biological activity, since this activity is also related to other factors, such as bioavailability, metabolic stability, and others (Piccirillo and Amaral 2018).
It is important to highlight that the theoretical data (in silico) that present some violation do not eliminate the possibility of being evaluated in in vitro or in vivo tests; they only indicate which substances are valid and can guide investigators on whether or not to proceed with experimental analyses. In relation to toxicity characteristics (mutagenic, tumorigenic, genotoxic, etc.), there are in vitro and in vivo tests that allow for the evaluation of these properties.
This study reported for the first time the in silico affinity of the investigated amides against PfENR. The compounds obtained higher binding affinities than the known ligand triclosan, among which 1m and 14f presented the highest binding affinities.
However, the modifications caused a significant decrease in in vitro antiplasmodial activity in all analogs and even caused one compound (14f) to generate a toxicological alert. Therefore, new modifications on 1a are prompted in a continuous attempt to enhance its antiplasmodial activity and/or toxicity.
Change history
21 May 2020
The original version of the article above contained an error in the text.
References
Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MPG, Lacerda MV, Alecrim MDGC (2010) Severe plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16(10):1611–1614
Amir A, Cheong FW, de Silva JR, Liew JWK, Lau YL (2018) Plasmodium knowlesi malaria: current research perspectives. Infect Drug Resist 11:1145–1155
Araújo-Júnior JX, da Cunha EVL, Chaves MCO, Alexander IG (1997) Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 44:559–561
Araújo-Vilges KM, de Oliveira SV, Couto SCP, Fokoue HH, Romero GAS, Kato MJ, Romeiro LAS, Leite JRSA, Kuckelhau SAS (2017) Effect of piplartine and cinnamides on Leishmania amazonensis, Plasmodium falciparum and on peritoneal cells of Swiss mice. Pharm Biol 55:601–1607
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien N, Thanh NV, Phu NH, Htut Y, Han K, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, Macinnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WR, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ (2014) Spread of artemisinin resistance in Plasmodium falciparum malaria. New Eng J Med 371:411–423
Bezerra DP, de Castro FO, Alves AP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, de Alencar NM, Mesquita RO, Lima MW, Costa-Lotufo LV (2008) In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol 28:156–163
Bezerra DP, Pessoa C, Moraes MO, Saker-Neto N, Silveira ER, Costa Lotufo LV (2013) Overview of the therapeutic potential of piplartine (Piperlongumine). Eur J Pharm Sci 48:453–463
Bodiwala HS, Singh G, Singh R, Singh CS, Dey SS, Sharma KK, Bhutani IP (2007) Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J Nat Med 61:418–421
Boechat N, Ferreira MDEL, Pinheiro LC, Jesus AM, Leite MM, Júnior CC, Aguiar AC, de Andrade IM, Krettli AU (2014) New compounds hybrids 1h-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem Biol Drug Des 84:325–332
Calvo-Calle JM, Moreno A, Eling WM, Nardin EH (1994) In vitro development of infectious liver stages of P. yoelii and P. berghei malaria in human cell lines. Exp Parasitol 79:362–373
Campelo Y, Ombredane A, Vasconcelos AG, Albuquerque L, Moreira DC, Plácido A, Rocha J, Fokoue HH, Yamaguchi L, Mafud A, Mascarenhas YP, Delerue-Matos C, Borges T, Joanitti GA, Arcanjo D, Kato MJ, Kuckelhaus S, Silva M, Moraes J, Leite JRSA (2018) Structure-activity relationship of piplartine and synthetic against Schistosoma mansoni and cytotoxicity to mammalian cells. Int J Mol Sci 19:1802–1818
Cotinguiba F, Regasini LO, Bolzani VS, Debonsi HM, Passerini GD, Sicarelli RMB, Kato MJ, Furlan M (2009) Piperamides and their derivatives as potential anti-trypanosomal agents. Med Chem Res 18:703–711
Cox FE (2010) History of the discovery of the malaria parasites and their vectors. Parasit Vectors 3:5–13
Fokoue HH (2015) Síntese, atividades biológicas e estudo de relação de estrutura-atividade de piperamidas. Doctoral thesis. Univerisity of São Paulo
Fokoue HH, Marques JV, Correia MV, Yamaguchi LFXQU, Aires-de-Sousa J, Scotti MT, Lopes NP, Kato MJ (2018) Fragmentation pattern of amides by EI and HRESI: study of protonation sites using DFT-3LYP data. RSC Adv 8:21407–21413
Freitas RP (2015) Avaliação da atividade esquistossomicida de análogos sintéticos da piplartina em vermes adultos de Schistosoma mansoni. Dissertação de Mestrado apresentada ao Programa de Pós-Graduação Interunidades em Biotecnologia USP/IPT/Instituto Butantan. USP
Gomes PR, Miguel FB, de Oliveira ME, Ferreira VV, Guimarães DSM, de Lima AB, Barbosa CS, de Oliveira MA, de Almeida MV, Viana GHR, Couri MRC, Varotti FP (2014) Síntese e avaliação da atividade antimalárica de compostos derivados da curcumina. Quim Nova 37(3):492–496
Guerra TM (2019) Estudos de docking molecular de derivados da tiazolidina como potenciais inibidores da enzima cruzaína de Trypanosoma cruzi. Dissertation. Federal Rural University of Pernambuco
Gutiérrez RMP, Gonzalez AMN, Hoyo-Vadillo C (2013) Alkaloids from piper: a review of its phytochemistry and pharmacology. Mini Rev Med Chem 13:163–193
Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW (2010) Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med 7:e1000290. https://doi.org/10.1371/journal.pmed.1000290
Katsuno K, Burrows JN, Duncan K, van Huijsduijnen RH, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT (2015) Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14:751–758
Krafts K, Hempelmann E, Skórska-Stania A (2012) From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol Res 111:1–6
Lambros C, Vanderberg J (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol:418–420
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341
Mackintosh CL, Beeson JG, Marsh K (2004) Clinical features and pathogenesis of severe malaria. Trend Parasitol 20:597–603
Magalhães UO (2009) Modelagem Molecular e Avaliação da Relação Estrutura-Atividade Acoplados a Estudos Físico-Químicos, Farmacocinéticos e Toxicológicos In Silico de Derivados Heterocíclicos com Atividade Leishmanicida. Dissertation. Federal University of Rio de Janeiro
Moreira F, Riul T, Moreira M, Pilon A, Dias-Baruffi M, Araújo M, Lopes N, de Oliveira A (2018) Leishmanicidal Effects of Piperlongumine (Piplartine) and Its Putative Metabolites. Planta Med 84:1141–1114
Mossmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nogueira F, Rosário E (2010) Métodos para avaliação da atividade antimalárica nas diferentes fases do ciclo de vida do Plasmodium Methods. Revista Pan-Amaz Saúde 1:109–124.v.1 n.3 Ananindeua set. 2010. https://doi.org/10.5123/S2176-62232010000300015
Oliveira CS (2013) Síntese, caracterização e atividade antimicrobiana de compostos heterocíclicos da classe 2,3-diidro-1,3,4-oxadiazol derivados de N-acilhidrazonas. Doctoral thesis. Federal Univesity of Paraíba
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OM, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper. Phytochemistry 46:597–673
Piccirillo E, Amaral AT (2018) Busca virtual de compostos bioativos: conceitos e aplicações. Quím Nova, São Paulo 41:662–677
Pidugu LS, Kapoor M, Surolia N, Surolia A, Suguna K (2004) Structural basis for the variation in triclosan affinity to enoyl reductases. J Mol Biol 343:147–155
Ponte-Sucre A, Bruhn H, Schirmeister T, Cecil A, Albert CR, Buechold C, Tischer M, Schlesinger S, Goebel T, Fuß A, Mathein D, Merget B, Sotriffer CA, Stich A, Krohne G, Engstler M, Bringmann G, Holzgrabe U (2015) Anti-trypanosomal activities and structural chemical properties of selected compound classes. Parasitol Res 114:501–512
Qidwai T, Khan F (2012) Antimalarial drugs and drug targets specific to fatty acid product discovery. Chem Biol Drug Des 80(2):155–172. https://doi.org/10.1111/j.1747-0285.2012.01389.x
Ryckmans T, Edwards MP, Horne VA, Correia AM, Owen DR, Thompson LR, Tran I, Tutt MF, Young T (2009) Rapid assessment of a novel series of selective CB2 agonists using parallel synthesis protocols: a lipophilic efficiency (LipE) analysis. Bioorg Med Chem Lett 19:4406–4409
Schrödinger; SCHRÖDINGER RELEASE (2015–2): LigPrep, version 3.4; Schrödinger, LLC, New York, 2015a
Schrödinger; SCHRÖDINGER RELEASE(2015–2): Maestro, version 10.2.010; Schrödinger, LLC, New York, 2015b
Schrödinger; SCHRÖDINGER RELEASE (2015–2): Schrödinger Suite 2015–2 Protein Preparation Wizard; Epik version 3.2; Schrödinger, LLC, New York, NY, 2015; Impact version 6.7, Schrödinger, LLC, New York, NY, 2015; Prime version 4.0, Schrödinger, LLC, New York, NY, 2015c
Schrödinger; Small-Molecule Drug Discovery Suite(2015–2): Schrödinger Suite 2015–2 Induced Fit Docking protocol; Glide version 6.7, Schrödinger, LLC, New York, NY, 2015; Prime version 4.0; Schrödinger, LLC, New York, NY, 2015
Silva-Jardim I, Horta MF, Ramalho-Pinto FJ (2004) The Leishmania chagasi proteasome: role in promastigotes growth and amastigotes survival within murine macrophages. Acta Trop 91:121–130
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806
Tasdemir D (2006) Type II fatty acid biosynthesis, a new approach in antimalarial natural metabolic pathway of Plasmodium falciparum. Phytochem Rev 5:99–108. https://doi.org/10.1007/s11101-005-5297-0
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem Jun 45:2615–2623
WHO, World Malaria Report (2018) Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1. Acess 29 November
Wink M (2012) Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules 17:12771–12791
Zubairi ABS, Nizami S, Raza A, Mehraj V, Rasheed AF, Ghanchi NK et al (2013) Severe Plasmodium vivax malaria in Pakistan. Emerg Infect Dis 19:1851
Acknowledgments
We are thankful to the Instituto Federal de Rondônia, campus of Porto Velho – Calama; Plataforma de Bioensaios de Malária e Leishmaniose – FIOCRUZ-RO; Institute of Chemistry of the Federal University of São Paulo – USP; the Instituto Nacional de Ciência e Tecnologia em Fármacos e Medicamentos (INCT-INOFAR), Rede Mineira de Química (RQ-MG), FAPEMIG and CNPq; and Programa de Pós-Graduação em Biologia Experimental – PGBIOEXP/UNIR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Tobili Sam-Yellowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1.16 MB)
Rights and permissions
About this article
Cite this article
da Silva, M.A., Veloso, M.P., de Souza Reis, K. et al. In silico evaluation and in vitro growth inhibition of Plasmodium falciparum by natural amides and synthetic analogs. Parasitol Res 119, 1879–1887 (2020). https://doi.org/10.1007/s00436-020-06681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06681-9