Abstract
The n-hexane, ethyl acetate, methanol, and acetone extracts of Piper cubeba Linn. and P. retrofractum Vahl. (Piperaceae) were evaluated in vitro against promastigotes of Leishmania donovani, and all exhibited significant in vitro activity at 100 μg/ml. Two lignans, cubebin and hinokinin, were isolated from the hexane extract of P. cubeba; and one bis-epoxy lignan, (−)-sesamin, and two amides, pellitorine and piplartine, were isolated from the hexane and methanol extracts of P. retrofractum. Cubebin and piplartine showed significant antileishmanial activity in vitro at 100 μM and were further tested in vivo in a hamster model of visceral leishmaniasis. Piplartine showed activity at 30 mg/kg dose. This is the first report of antileishmanial activity of these two plants and their isolated constituents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a group of tropical diseases caused by a number of species of protozoan parasites belonging to the genus Leishmania. Leishmaniasis, along with the parasitic diseases malaria and trypanosomiasis, has a major impact on human populations all over the world, particularly in Africa, Asia, and Latin America [1]. The World Health Organization (WHO) estimates that 350 million people live at risk of infection with Leishmania parasites. There are three main manifestations of leishmaniasis caused by different species of Leishmania. Visceral leishmaniasis, which is also known as kala-azar, is caused by L. donovani. More than 90% of the world’s cases of visceral leishmaniasis (VL) are in India, Bangladesh, Nepal, Sudan, and Brazil. If untreated, it often leads to death [2]. Cutaneous and mucocutaneous leishmaniases are more prevalent in Afghanistan, Saudi Arabia, and some Latin American countries. Leishmaniasis is one of the opportunistic infections that attack HIV-infected individuals, and in the recent years, the coexistence of HIV and Leishmania species causing visceral disease has resulted in several thousand cases of dually infected individuals. Leishmaniasis patients are highly susceptible to HIV infection, and leishmaniasis accelerates the onset of AIDS by cumulative immunosuppression and by stimulation of the replication of the virus in HIV-infected patients [2]. The drugs of choice for the treatment of leishmaniasis are pentavalent antimonials such as sodium stibogluconate and meglumine antimonates. However, these present renal and cardiac toxicity. The second choice for treatment, pentamidine, is also associated with serious side effects. So there is an urgent need to discover new antileishmanial agents that are safe and cheap.
In our research program directed toward discovery of newer chemotherapeutic agents for leishmaniasis, we have selected plants that are frequently encountered in Ayurvedic polyherbal formulations that were used to treat symptoms similar to kala-azar in medieval times, as leishmaniasis was unknown during those times. We have observed strong activity in the fruits of Piper cubeba Linn. (syn. Cubeba officinalis Raf.) and in the stem bark of P. retrofractum Vahl. (syn. P. chaba Hunter, Chavica officinarum Miq., P. officinarum DC.) (Piperaceae). Piperine, a major constituent of several Piper species, is reported to show antileishmanial activity both in vitro and in vivo [3–5] (Fig. 1).
Here we describe the isolation of three lignans, hinokinin, cubebin, and (−)-sesamin; and two amides, pellitorine and piplartine, from P. cubeba and P. retrofractum and their antileishmanial activity against L. donovani promastigotes in vitro by cell cytotoxicity assay. Active compounds were evaluated in vivo in L. donovani-infected golden hamster for VL. Antileishmanial activity of these plants and compounds is reported for the first time.
Materials and methods
Plant materials were extracted using Soxhlet extractor (Perfit India Ltd., India). Extracts were concentrated using vacuum rotary evaporator (Buchi R-200, Switzerland). The melting points were determined on a melting point apparatus (Mettler Toledo FP-72, Switzerland). IR spectra were taken on a Fourier transform infrared (FTIR) spectrometer (Nicolet, USA). MS were recorded on low-resolution gas MS (GCMS) (QS-5000, Shimadzu, Japan) or LCMS (Micromass, Waters, U.S.A.). NMR was recorded on 300 MHz spectrometer (Avance DPX 300, Bruker, Germany). Precoated TLC plates having silica gel 60 F254, 0.2-mm thick (Merck, Germany), were used for TLC. Silica gel 60–120 mesh (CDH Laboratory Reagents, India) was used for column chromatography. Lignans were detected by methanolic sulphuric acid or anisaldehyde sulphuric acid reagent, and amides were detected by Dragendorff’s reagent.
Extraction of plant material
Fruits of P. cubeba and stems of P. retrofractum were purchased from the local market, and the materials were identified and authenticated by Arvind Saklani, Department of Natural Products. A voucher specimen is kept in our laboratory for future reference. Plant materials were extracted sequentially with n-hexane, ethyl acetate, and methanol. The extracts were concentrated under vacuum on rotary evaporator to yield 10.6% of n-hexane, 3.7% of ethyl acetate, and 2.2% of methanolic extract of P. cubeba fruits. Similarly, P. retrofractum stems yielded 0.6% of n-hexane, 0.9% of ethyl acetate, and 0.9% of methanolic extract. Each plant material was separately extracted with acetone. The yields were 15.2% and 2.0% for P. cubeba and P. retrofractum, respectively.
In vitro antileishmanial evaluation
In vitro promastigote cell cytotoxicity assay using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide] cell proliferation assay was used to test the antileishmanial activity in vitro. L. donovani (0.125 × 106) promastigotes from logarithmic phase culture was allowed to grow for 48 h before the treatment of samples. After addition of samples, the cells were further allowed to grow for 48 h. MTT to a final concentration of 400 μg/ml was added and incubated for 3 h at 24°C. The cells were centrifuged at 6,000×g, and pellets were dissolved in dimethyl sulfoxide before taking the absorbance at 540 nm. The mean percentage of posttreatment viable cells was calculated relative to control, and results were expressed as the concentration inhibiting the parasite growth [6]. The standards miltefosine and pentamidine were used at reported IC50 values [6, 7].
In vivo antileishmanial evaluation
A hamster model of VL was used for in vivo screening. VL was produced by intracardiac injection of L. donovani amastigotes (2 × 107) in golden hamsters. Twenty days after infection, hamsters were administered the compound for a period of 10 days, with an interval of 5 days. Hamsters were sacrificed after 2 days of the last dose to examine spleen weight and spleen parasitic burden. Spleen parasitic burden was assessed from Giemsa-stained impression smears [8].
Statistical analysis
All the results are expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) was used for statistical analysis. When ANOVA showed significant difference, post hoc analysis was performed with Tukey’s test. P < 0.05 was considered statistically significant. Statistical analysis was carried out using Jandel Sigma Stat Version 2, software.
Results and discussion
Preliminary investigation on various extracts prepared from P. cubeba and P. retrofractum revealed that n-hexane extract of P. cubeba showed more than 90% inhibition of promastigotes of L. donovani in vitro at a concentration of 100 μg/ml, whereas methanol as well as acetone extracts from P. retrofractum showed more than 75% inhibition at a concentration 20 μg/ml. The IC50s of all the extracts were determined, and three from P. retrofractum were found to show high activity, their IC50s being less than 7.5 μg/ml. Extracts from P. cubeba showed comparatively weaker activity. The results are shown in Table 1.
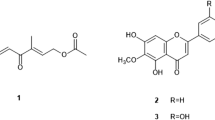
n-Hexane extract of P. cubeba (10 g) was subjected to column chromatography on silica gel using hexane–ethyl acetate. Based on the thin-layer chromatography (TLC) pattern, fractions were pooled into four main fractions (1–4). Concentration of fraction 2 (25% ethyl acetate) yielded viscous oil (385 mg), which was identified as a lignan, hinokinin (1) [9–12]. Solvent evaporation of fraction 3 (30% ethyl acetate) yielded a colorless crystalline compound (780 mg) that was identified from the spectral data as cubebin (2) [9–11].
Chromatographic separation of the n-hexane extract of P. retrofractum (6 g) with the hexane–ethyl acetate gradient resulted in the isolation of the two compounds. Compound 3 (20% ethyl acetate, 22 mg) was characterized as (−)-sesamin [12, 13]. Compound 4 (25% ethyl acetate, 12 mg) gave a positive test with Dragendorff’s reagent. The infrared (IR) spectrum indicated the presence of an amide group (3,372, 3,019, 1,670, and 1,522 cm−1) as well as an olefinic double bond (1,630 and 927 cm−1). The ultraviolet (UV) spectrum showed λ max at 257 nm. This, along with the IR spectrum, suggested the presence of conjugated dienoic acid amide derivative. Compound 4 was identified as pellitorine by comparison of spectral data with literature values [14, 15].
Methanol extract (40 g) of P. retrofractum was subjected to vacuum liquid chromatography with the hexane–ethyl acetate gradient to yield five major fractions (Fr. 1–5). Chromatographic separation of fraction 4 (80% ethyl acetate, 3.5 g) with the hexane–ethyl acetate gradient on silica gel resulted in the isolation of compound 5 (50% ethyl acetate, 1.4 g), which gave a positive test with Dragendorff’s reagent. The IR spectrum showed strong absorption at 1,684 and 1,620 cm−1, which indicated the presence of conjugated amide. The 1H-NMR spectra showed characteristic peak of cinnamoyl protons at δ 7.68 (d, J = 15 Hz) and 7.42 (d, J = 15 Hz). The doublet at δ 6.04 indicated an α–β unsaturated carbonyl moiety. Literature comparison of spectral data showed compound 5 was a pyridone amide piplartine [16].
All of the isolated compounds were tested for in vitro antileishmanial activity against promastigotes of L. donovani at 100 μM concentration. Lignan cubebin (2) showed 79.4 ± 5.5% inhibition of promastigotes; (−)-sesamin (3) and pellitorine (4) were inactive, whereas piplartine (5) showed 89.1 ± 2.9% inhibition at 100 μM. Further experiments were conducted to determine the IC50 values, and results are shown in Table 1. Piplartine and cubebin showed IC50 values at 7.5 and 28.0 μM, respectively (Table 1).
Cubebin (2) and piplartine (5), which showed significant in vitro activity, were evaluated in vivo in golden hamsters against amastigotes of L. donovani. Ten days’ treatment with piplartine (5) at 30 mg/kg/10 ml i.p. significantly reduced parasitic burden and spleen weight (Table 2). A 36% reduction in parasitic burden and 50% reduction in spleen weight were observed on piplartine (5) treatment. It was toxic at a dose of 300 mg/kg per 10 ml i.p. Standard drugs such as sodium stibogluconate and miltefosine produced 80% and 95% reduction in parasitic burden at 50 and 12.5 mg/kg doses, respectively.
References
Chan-Bacab MJ, Peña-Rodríguez LM (2001) Plant natural products with leishmanicidal activity. Nat Prod Rep 18:674–688
Carvalho PB, Ferreira EI (2001) Leishmaniasis phytotherapy. Nature’s leadership against an ancient disease. Fitoterapia 72:599–618
Kapil A (1993) Piperine: a potent inhibitor of Leishmania donovani promastigotes in vitro. Planta Med 59:474
Veerareddy PR, Vobalabonia V, Nahid A (2004) Formulation and evaluation of oil-in-water emulsions of piperine in visceral leishmaniasis. Pharmazie 59:194–197
Raay B, Medda S, Mukhopadhyay S, Basu MK (1999) Targeting of piperine intercalated in mannose-coated liposomes in experimental leishmaniasis. Indian J Biochem Biophys 36:248–251
Verma NK, Dey CS (2004) Possible mechanism of Miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother 48:3010–3015
Prasad V, Kaur J, Dey CS (2000) Arsenite resistant Leishmania donovani promastigotes express an enhanced membrane P-type adenosine triphosphatase activity that is sensitive to verapamil treatment. Parasitol Res 86:661–664
Ghosh AK, Bhattacharya FK, Ghosh DK (1985) Leishmania donovani: amastigote inhibition and mode of action of berberine. Exp Parasitol 60:404–413
Koul SK, Taneja SC, Dhar KL, Atal CK (1983) Lignans of Piper clusii. Phytochemistry 22:999–1000
Rehnburg N, Magnusson G (1988) Total synthesis of the lignans (−)- and(+)- Burseran, (−)-Cubebin and (−)-Hinokinin by diastereoselective conjugate addition of benzylanions to 2-(R)- and (S)-benzyloxy-2,5-dihydro-4-(3,4)-methylenedioxy benzoylfuran. Tetrahedron Lett 29:3599–3602
Rehnburg N, Magnusson G (1990) General conjugate addition method for synthesis of enantiomerically pure lignans. Total synthesis of (−)- and (+)-Burseran, (−)-Dehydro cubebin, (−)-Trichostin, (−)-Cubebin, (−)-5′′-methoxyhinokinin and (−)-Hinokinin. J Org Chem 55:4340–4349
Atal CK, Girothra RN, Dhar KL (1966) Occurrence of sesamin in Piper longum Linn. Indian J Chem 4:252
Lin RW, Tsai IL, Duh CY, Lee KH, Chen IS (2004) New lignans and cytotoxic constituents of Wilkstroemia lanceolata. Planta Med 70:234–238
Chen LC, Kang IJ, Wang HM (1999) Ene reaction with pummerer-type reaction intermediate of α-(methylthio)isobutyl acetamide: a new synthesis of pellitorine. J Chin Chem Soc 46:963–966
Saadali B, Boriky D, Blaghen M, Vanhaelen M, Talbi M (2001) Alkamides from Artemisia dracunculus. Phytochemistry 58:1083–1086
Duh CY, Wu YC, Wang SK (1990) Cytotoxic pyridone alkaloids from Piper aborescens. Phytochemistry 29:2689–2691
Acknowledgments
We express our sincere thanks to Prof. P. Ramarao, director, NIPER, for continuous support for this work. HSB is thankful to NIPER for fellowship. Technical assistance from Mr. Rakesh Kumar and Mr. K.S. Singh is gratefully acknowledged. GS is thankful to CSIR, New Delhi for fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
NIPER Communication No. 395.
Rights and permissions
About this article
Cite this article
Bodiwala, H.S., Singh, G., Singh, R. et al. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum . J Nat Med 61, 418–421 (2007). https://doi.org/10.1007/s11418-007-0159-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0159-2