Abstract
Clonorchis sinensis (C. sinensis) can induce a food-borne parasitic disease (clonorchiasis). Numerous studies have analyzed functional proteins, immunologic factors, pro-inflammatory cytokines, and cell signaling transduction that promote the development of clonorchiasis. In a previous study, it was shown that C. sinensis adult-derived total protein (CsTP) might be involved in the pathogenesis and development of liver fibrosis via bringing about Th2 immune response. In the present study, further investigation of CsTP on cellular function and inflammatory effect in vitro and in vivo has been elicited. CsTP induced inflammation and autophagy as evidenced by upregulation of TNF-α, IFN-γ, and autophagic markers LC3B and P62. Exposed to CsTP upregulated the antiapoptotic gene Bcl-2 expression, diminished the apoptosis induced by H2O2, but promoted the proliferation and migration of LX-2 cells in proper concentration range. Additionally, the protein levels of p-AKT and p-mTOR were repressed in response to CsTP, suggesting a correlation of blocking the activation of mTOR/AKT signaling pathway. These results revealed that CsTP might exacerbate hepatic pathological changes by regulating cell proliferation, apoptosis, autophagy, and inflammation in the liver and LX-2 cells. Some effects might be partially involved in the mTOR and AKT pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over thirty-five million people have been suffering from C. sinensis infection worldwide with the majority of infected individuals resided in China. One third of them are from Guangdong province (Hong and Fang 2012). In addition to the few people who accompanied by the symptoms including abdominal pain, fever, eosinophilia, jaundice, chills, or diarrhea, the infection is often scarcely symptomatic. So, clonorchiasis is incorporated into one of neglected tropical diseases. There is mounting evidence that humans and animals of C. sinensis infections occur for the reason of consuming freshwater fish with live infective metacercariae (Rim 2005). It is unsettling that C. sinensis infection can cause liver atrophy, portal fibrosis, and even hepatobiliary tumor (Choi et al. 2004). The clarity for exact pathogenic mechanism to search effective control strategy is already a concern.
It is illustrated that most parasites maintain their long-lasting parasitism in the hostile environment via producing excretory-secretory products (ESPs), metabolic and disintegrated products, and bacteria accompanied by adult worm, thereby resulting in mechanical tissue injury and metabolic product (de Almeida et al. 2017; Kim et al. 2016; McDonald et al. 2013; Tweyongyere et al. 2016). All reports about the interaction between helminth and host to date are focused on the effect of ESPs (Lee et al. 2008; Won et al. 2019) or parasite itself (Chookami et al. 2016; Janssen et al. 2016), suggesting an important role in directly or indirectly aggravating inflammation and leading to fibrosis (Chung et al. 2003; Pak et al. 2009). Recently, some researchers put their attention on adult worm antigens. Previous studies have highlighted the capability of some parasite antigens in pro-inflammation (Jakobsen et al. 2019; Wangala et al. 2019) or anti-inflammation (Botelho et al. 2011; Lima et al. 2002; McConchie et al. 2006; Sun et al. 2019) activities. Trypanosoma cruzi (T. cruzi) antigens manifested that parasite antigens could induce inflammatory and angiogenesis in the sponge implanted-mouse model. It mainly increased the productions of vascular endothelial growth factor (VEGF) and inflammatory factors TNF-α, CCL2, and CCL5 on day 7 post-implant through sponges (Guedes-da-Silva et al. 2015). However, recent studies by Jeong et al. (2011), Sun et al. (2019), Matisz et al. (2017), and Lima et al. (2002) characterized distinct effectiveness of adult worm extracts against secretion of inflammatory cytokines in vitro and in vivo. Besides, minority researches have demonstrated the anti-tumourigenic effect (Botelho et al. 2009b) and angiogenic response (Zueva et al. 2019) of adult worm antigens. It increased proliferation, migration, and invasion, but inhibited apoptosis, and promoted the fibroblast-like Chinese hamster ovary (CHO) cells transforming to sarcomas after incubating with Schistosoma haematobium total antigens (Sh) (Botelho et al. 2009a).
Other groups demonstrated the critical role of parasite antigens in modulating immunological effect (Lima et al. 2002; McConchie et al. 2006; Sun et al. 2019; Wang et al. 2017a). The crude antigens of reared Echinococcus granulosus adult worms induced a higher ratio of protective immunity than that excretory-secretory (E/S) and immunodominant antigens (Rahimi et al. 2017). In peripheral blood mononuclear cells (PBMCs) stimulated with Schistosoma mansoni adult worm antigen (SWA), a Th2 immune response was observed in response to SWA, indicating that SWA could be the dominant inducer of Th2 cytokine production, such as IL-4, IL-5, and IL-13 (Tweyongyere et al. 2016). Notably, about the total protein derived from C. sinensis (CsTP), an early report by Jeong et al. clarified that CsTP might decrease the expression of CD80, CD86, and CD40 in LPS- or OA-treated DCs but increase the amounts of CD4 + CD25 + Foxp3 + Treg cell. At the end, airway inflammation was suppressed in the host (Jeong et al. 2011). Our recent study also disclosed that CsTP took a part in orchestrating Th2 immune response by upregulating the production of IL-13 and IL-4 in vitro and in vivo, and finally resulted in the occurrence of liver fibrosis in the host. It also attested to the role of the interplay between mannose receptor (MR) and CsTP (Zhao et al. 2018).
Taken together, these studies underscored the role of parasitic total proteins in the deep investigation and development of parasitic diseases. Here we sought to determine the potential effects of CsTP on the proliferation, apoptosis, cytokine production, and autophagy activation in the mouse liver and LX-2 cells. Furthermore, the probable mechanism was analyzed.
Materials and methods
Animals and cells
Female Balb/c mice (6–8 weeks old) were obtained and housed in the animal laboratory at Sun Yat-sen University, Guangzhou, China. The operation of all the animal experiments was based on the Guidelines and Regulations for Animal Use and Care of Sun Yat-sen University (permit no. SYXK (Guangdong) 2010-0107).
Human hepatic stellate cell LX-2 was cultured in high-glucose DMEM with FBS (10%, NQBB, Hong Kong, China) at the atmosphere of 5% CO2 and 37 °C.
Preparation and extract of C. sinensis adult worm total protein
C. sinensis adult worm was maintained in hepatic duct of the host. The preparation and extract of C. sinensis adult worm total protein were referring to the previous study in the lab (Zhao et al. 2018). Adult worms were freshly collected from the hepatic duct of SD rats experimentally infected with administering orally live metacercaria for 8 weeks. After being lysed with phosphate-buffered saline (PBS, PH 7.2), the soluble adult extract supernatant was harvested, and the protein was quantified by BCA Protein Assay Kit (Thermo Fisher Scientific, USA).
Administration and sensitization of CsTP
The challenge was performed according to the protocol as previously described (Xu et al. 2013). Each mouse was sensitized with 100 μg CsTP under emulsification of complete Freund’s adjuvant through subcutaneous injection at the first time. In the following process, the immunization dosage halved with incomplete Freund’s adjuvant. All mice were euthanized and the livers were collected after 0, 6, and 10 weeks respectively. The control group was injected using PBS prior to emulsify with complete Freund’s adjuvant in the same way.
Real-time PCR assay
Total hepatic RNA was extracted by Trizol reagent (Life Technology, Carlsbad, USA). The reverse transcription reaction was performed using RevertAid RT Reverse Transcription Mix (Thermo Fisher Scientific, USA) following the manufacturer’s instruction. The SYBR green kit (Applied Biosystem) was used in the following RT-PCR reaction to evaluate gene transcriptions of P62, TNF-α, and IFN-γ.
Primers for cytokine genes and P62 were as follows:
TNF-α: forward, 5′- GCGGTGCCTATGTCTCAGC-3′; reverse, 5′- CACTTGGTGGTTTGTGAGTGT-3′; IFN-γ: forward, 5′- ATGAACGCTACACACTGCATC-3′; reverse, 5′- CCATCCTTTTGCCAGTTCCTC-3′; GAPDH: forward, 5′- AAGGTCATCCCAGAGCTGAA-3′; reverse, 5′- CTGCTTCACCACCTTCTTGA-3′; P62: forward, 5′- CAGAGAAGCCCATGGACAG-3′; reverse, 5′- AGCTGCCTTGTACCCACATC-3′.
All values were normalized to the GAPDH value.
Western blot analysis
The liver or cell proteins were extracted by RIPA lysis buffer with a supplement of protease and phosphatase inhibitor according to the manual. Equal amount of protein (30 μg) per sample was separated on 10% or 15% SDS-PAGE and transferred onto PVDF membrane (Millipore, USA). After blocking with 5% skim milk (diluted with TBS) for 1 h, then membranes were incubated overnight at 4 °C by corresponding primary antibodies for mouse monoclonal TNF-α (Santa Cruz, CA), mouse polyclonal Bcl-2 (Santa Cruz, CA), rabbit monoclonal LC3B (Cell Signaling Technology, USA), rabbit monoclonal p-mTOR (Cell Signaling Technology, USA), rabbit monoclonal mTOR (Cell Signaling Technology, USA), rabbit polyclonal P62/SQSTM1 (Proteintech, Inc), rabbit monoclonal p-AKT (Cell Signaling Technology, USA), mouse monoclonal GAPDH (Cell Signaling Technology, USA), and mouse monoclonal β-actin (Sigma, St. Louis, USA), respectively. After washing three times in TBS, each membrane was probed with specific horseradish peroxidase (HRP)-conjugated secondary antibodies. The details had been presented in Table 1. One hour later, membranes were washed as previously described. The band was detected by ECL chemiluminescence detection system. The ImageJ software was used to determine band intensities.
Immunohistochemistry assessment
The collected livers were cut into 5 μm sections. Then, the liver section was pre-treated in accordance with the instruction for use. Paraffin embedded sections were cut into small pieces. Pre-treatment procedures for immunohistochemistry were described previously (Tang et al. 2016). The slides were washed with specific reagents, xylene, followed by a gradient ethanol. After that, 3% hydrogen peroxide in PBS (pH 7.4) was added to block the effect of endogenous peroxidase. The antigen was retrieved by citrate acid buffer (0.01 M) with high pressure method, followed by applying the blocking reagent (normal goat serum) at room temperature for 1 h. After rinsing with PBS for three times (5 min/time), the slides were incubated with rabbit polyclonal P62/SQSTM1 at 4 °C for overnight, and then incubated with the HRP-conjugated secondary antibody (1:400) at 37 °C for 1 h. DAB reagent was used to stain slides at 37 °C for 20 min. After staining with hematoxylin, the sections were washed in distilled water. Finally, dehydration and transparency procedures were performed and the slides were mounted. All images were acquired under the light microscope (Carl Zeiss, GA).
Cell proliferation and apoptosis assay
The procedure was performed as described previously (Shang et al. 2017). Human hepatic stellate cell LX-2 cells (5 × 103 cells/well) were plated in 96-well plate. After treating with or without different concentrations (0, 2, 4, 10, 25, 50, 100, 200 μg/ml) of CsTP for 24 h, cell viability was assessed using CCK-8 reagent. The rate of cell proliferation was shown as values at 450 nm.
The cell apoptosis was analyzed by flow cytometry (FCM). Cells were treated with 1 μM H2O2 (12 h), 10 μg/ml CsTP, 10 μg/ml CsTP, and 1 μM H2O2 (12 h), 25 μg/ml CsTP, 25 μg/ml CsTP, and 1 μM H2O2 (12 h), respectively. After 24-h post-treatment, the collected cells were analyzed by the Annexin V-FITC/PI apoptosis detection kit (Keygen Biotech, China) according to the instruction.
Analysis of cell migration
For cell migration analysis, LX-2 cells were treated or untreated with 10 or 25 μg/ml CsTP for 24 h, respectively. All the cells were resuspended in serum free culture medium (100 μl/well) and then plated in the upper compartment. DMEM containing 10% fetal bovine serum (600 μl) was added to the lower chamber as an inducer. After 24 h, cells that migrated through the membrane to the lower surface were fixed with 100% ethanol for 20–30 min and stained with 0.5% crystal violet for 15 min. A cotton swab was used to gently remove the non-migrated cells on the upper side. The adhesive cells on the reverse surface were counted by microscopy (Olympus, USA).
Autophagy detection in vitro
LX-2 cells were seeded on 6-well plates before treatment with different concentrations of CsTP (10 μg/ml and 25 μg/ml). These concentrations were chosen according to the previous study (Botelho et al. 2009a). LX-2 cells were treated with or without CsTP for 24 h. The cell total proteins were extracted by using RIPA lysis buffer and analyzed by western blot.
Statistical analysis
All data are presented as mean ± SD. Differences between multiple groups were evaluated using one-way ANOVA. Comparisons between two experimental groups were performed by independent t test. p < 0.05 was considered as significantly statistical differences. The analysis was made by SPSS version 16.0 software.
Results
Production of pro-inflammatory factor TNF-α/IFN-γ and anti-apoptosis marker Bcl-2 after exposed to CsTP
At first, RT-PCR analysis data showed that hepatic TNF-α and IFN-γ in experimental group were upregulated (Fig. 1). Western blot also demonstrated that the protein level of TNF-α in mice immunized subcutaneously with CsTP was highly induced compared with the control group (Fig. 2), confirming that CsTP could exacerbate inflammatory response in mice liver. Interestingly, we observed similar change trend of Bcl-2 expression in the experimental group (Fig. 2). It was possible that CsTP coordinated with Bcl-2 expression to suppress cell apoptosis.
mRNA expressions of inflammatory cytokines and P62 in mouse liver by RT-PCR. Balb/c mice were subcutaneously immunized with CsTP for 6 weeks and 10 weeks, respectively. RNA was extracted from livers of each CsTP-immunized group and the control group. RT-PCR was used to measure the changes of mRNA levels of TNF-α, IFN-γ, and P62. Gene expression was normalized to GAPDH. Data were showed as mean ± SD, n = 5. *p < 0.05, **p < 0.01
Effect of CsTP on TNF-α and Bcl-2 protein expressions in the subcutaneous immunized mice. Western blot analysis was conducted on protein extracts from the livers of CsTP-immunized mice for TNF-α or Bcl-2 at 6 weeks (a) and 10 weeks (d). Panels (b–c) and (e–f) represented the densitometry data as mean ± SD, n = 5. *p < 0.05, **p < 0.01 vs. control group
The initiation of autophagy by CsTP
To explore whether hepatic autophagy was activated in the mice subcutaneously immunized with CsTP, the autophagy-associated molecule P62 by CsTP exposure was evaluated via qPCR and immunohistochemical staining. As shown in Fig. 1, P62 mRNA expression in CsTP-immunized mice exhibited a marked elevation compared with the control group (p < 0.01). Also, an apparent increase occurred in the distribution of P62 in the CsTP-immunized mice liver (p < 0.05), especially in10-week group after CsTP immunization (Additional file : Fig. S1). These data suggested that CsTP contributed to the enrichment of autophagic marker P62.
LC3, as a member of the highly conserved ATG8 protein family, is the most widely used marker of autophagosomes. The change of autophagic marker LC3B in response to CsTP was quantified. As shown in Fig. 3, the ratio of LC3B-II and LC3B-I was obviously increased after treating with CsTP for 10 weeks. We also observed a notable attenuation of LC3B I expression, but a minor upregulation of LC3B II/I expression ratio in the 6 week-treated group. The results hinted that CsTP may provoke P62 expression, synergistically worked with LC3 to mediate autophagy activation.
Protein expressions of autophagy marker LC3B and related molecules of mTOR pathway in CsTP-treated group or the control group. Western blot analysis for protein levels of LC3B, p-mTOR, and mTOR in the liver tissues that had been treated with CsTP for 6 weeks (a) or 10 weeks (f). Protein expression was normalized to that of β-actin. The quantification of relative intensity was analyzed by Image J (b–e, g–j). Error bars indicated mean ± SD. p values were calculated using t test. *p < 0.05, **p < 0.01, compared with the control group
Evaluation of cell viability and cell apoptosis after CsTP treatment
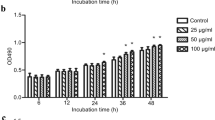
In vivo experiment confirmed the role of induced inflammation and autophagy by CsTP. The data also showed that CsTP possibly mediated apoptosis. It is hypothesized that CsTP treatment can affect cellular function. Hepatic stellate cells LX-2 were cultured within presence or not of different concentrations (0, 2, 4, 10, 25, 50, 100, 200 μg/ml) of CsTP. In the 24-h post-treatment, the proliferation of LX-2 cells was measured by CCK-8 assay, and FCM was applied to analyze the apoptosis using Annexin V-FITC/PI reagent kit. As presented in Fig. 4a, the viability of LX-2 cells was obviously increased by low concentrations of CsTP (2, 4, 10, 25 μg/ml). On the contrary, high doses of CsTP (50, 100, 200 μg/ml) attenuated cell viability.
Modulation of CsTP on the cell proliferation, apoptosis and migration ability in the human hepatic stellate cell LX-2. a LX-2 cells were exposed to different concentrations (0, 2, 4, 10, 25, 50, 100, 200 μg/ml) of CsTP for 24 h. CCK-8 was added to calculate cell viability after CsTP treatment. b Cell migration was examined by using transwell cell culture chambers. Ability of migration of LX-2 cells after treating with CsTP for 24 h was quantified by counting the number of five random sections of migrated cells under the microscope. The count of migrated cells per field represented the average ability of cell migration. c and d Cell apoptosis was determined by the staining of Annexin V-FITC/PI after exposing to 10 μg/ml or 25 μg/ml for 24 h. Annexin V-FITC+/PI− or Annexin V-FITC+/ PI+ presented early apoptosis or late apoptosis, respectively. Necrotic cells were showed by Annexin V-FITC−/PI +. One representative plot of three biological experiments was shown. *p < 0.05, **p < 0.01 vs. the untreated control group, #p < 0.05 vs. the H2O2 treated group
Accordingly, to determine whether CsTP was associated with abnormal hepatic stellate cell apoptosis, all treated or untreated LX-2 cells were subsequently stained with Annexin V-FITC and PI. The data showed that 25 μg/ml of CsTP treatment blocked the pro-apoptotic effect induced by H2O2, indicating that CsTP had effect of anti-apoptosis (Fig. 4c, d). This corresponded to the observation from in vivo study.
LX-2 cell migration promoted by CsTP
We next set out to address whether CsTP would impair the migration of LX-2 cells. Changes in cell migration after LX-2 cells treated with 10 or 25 μg/ml CsTP for 24 h were assessed. Compared with the control group, LX-2 cells treated with CsTP had a better migration capability, while a high dose of CsTP (25 μg/ml) showed better migration than a low dose of CsTP (10 μg/ml, Fig. 4b). These data indicated that CsTP facilitated hepatic stellate cell (HSC) migration.
Analysis of autophagy in human hepatic stellate cell LX-2 treated with CsTP
Consistent with the result in vivo, we detected the autophagic change of LX-2 treated with CsTP. LC3B II/I expression ratio in LX-2 cells was higher after exposed to 25 μg/ml CsTP. Intriguingly, the expression of autophagy-related gene P62 also displayed an upregulation in LX-2 cells after stimulating with different concentrations of CsTP (10 or 25 μg/ml) for 24 h (Fig. 5); demonstrating autophagic level was increased in the treatment of high concentration of CsTP.
Detection of autophagic change and activation of mTOR/AKT signaling pathway in CsTP-treated LX-2 cells. The protein levels of autophagic molecules LC3B, P62, and p-AKT (a–d), p-mTOR, mTOR (e–h) were determined from the lysates of LX-2 cells after stimulation for 24 h with 10 μg/ml or 25 μg/ml by Immunoblot assay. The control group was exposed to PBS. The results were representative of three independent experiments. The quantification of each band was showed as bar chart (b–d, f–h). Data were calculated and plotted as mean ± SD. *p < 0.05, **p < 0.01 vs. the control group
Activation of mTOR/AKT signaling pathway in the function process of CsTP
Our study demonstrated a regulation of cellular function and boosting inflammation by CsTP. To further address the mechanisms that regulate this important finding, we examined hepatic p-mTOR and mTOR in the immunized mice, which presented that the protein expression of p-mTOR in the CsTP-treated group was diminished at 6 weeks and 10 weeks. And the p-mTOR/mTOR expression ratio displayed a declined trend in the 6 week-treated group. In addition, immunoblot of CsTP-treated cells and the control cells proved that CsTP attenuated the protein expression of p-mTOR in the LX-2 cells stimulated with 10 μg/ml CsTP. The ratio of p-mTOR/mTOR appeared a downward trend after CsTP exposure. These data suggested that mTOR pathway might have been implicated in the action of CsTP. Moreover, detection of phosphorylation of AKT (p-AKT) presented that p-AKT expression was significantly reduced in response to CsTP treatment. The effect was more prominent in the cell treated with the higher concentration (25 μg/ml) of CsTP (Fig. 5). Together, all the results attested that mTOR/AKT pathway might be involved in the action of CsTP in vivo and in vitro.
Discussion
Infection with C. sinensis is mostly associated with periductal liver fibrosis, cholangitis, and even hepatic cirrhosis and bile duct carcinoma. ESPs derived from this parasite and its component proteins induce the inflammation and biliary fibrosis in the mouse model (Yan et al. 2015b). It has also been shown that some proteins of ESPs play a role in the regulation of immune responses, such as induction of IL-25 (Zhou et al. 2017), IL-13 (Xu et al. 2016), IL-2 (Chung et al. 2017), and IL-4/IL-10 (Xu et al. 2013, 2015). We have previously identified the capacity of CsTP to motivate Th2-type immune response via targeting MR and subsequent affected the onset or progression of hepatic fibrosis (Zhao et al. 2018). Nevertheless, limited information is available on the properties and functions of CsTP in cellular function and inflammatory effect. Current study provides more details to understand the functions of CsTP through the in vivo and in vitro model.
C. sinensis infection provokes sustained inflammation and hyperplasia, which are the main pathogenic factors of liver fibrosis (Yoo et al. 2011). To clarify the relevance of CsTP with inflammation, we examined the transcriptional levels of hepatic TNF-α and IFN-γ after subcutaneous immunization with CsTP, which all genes presented an apparent upregulation (Fig. 1). The western blot results clearly showed that TNF-α (Fig. 2) was highly expressed in the CsTP-treated group. These data demonstrated that CsTP had the potent ability to induce much more severe inflammation. It was consistent with the research by Guedes-da-Silva et al., which reported that Trypanosoma cruzi (T. cruzi) antigens led to high expression of inflammatory mediators (Guedes-da-Silva et al. 2015). Data obtained in a study by Botelho et al. (Botelho et al. 2011) also had proposed that components derived from Schistosoma haematobium (S. haematobium) elevated the inflammatory infiltrate and promoted the dysfunction of urothelium. Here, our results verified the pro-inflammatory effect by CsTP. This may explain why C. sinensis infection and many types of adult worm proteins significantly induce chronic inflammation, resulting in increasing the risk of cholestasis liver fibrosis (Xu et al. 2016; Yan et al. 2015a, 2017; Zhang et al. 2013; Zhou et al. 2017).
It is well recognized that autophagy regulates the occurrence and development of inflammatory response (Crisan et al. 2011; Mizushima et al. 2008; Saitoh et al. 2008). Saitoh et al. reported that deletion of ATG16L1 gene could disrupt autophagy, induce the IL-1β and IL-18 mediated inflammation, resulting in more severe inflammatory bowel disease (Saitoh et al. 2008). Recent data showed that autophagy-deficient Kuffer cells (KCs) enhanced hepatic inflammation, fibrosis, and tumor via NF-kB-ROS-IL-1α/β pathways (Sun et al. 2017). Another study also found that dihydroartemisinin (DHA) had an anti-inflammatory effect of hepatic fibrosis and activates autophagy by ROS-JNK1/2 signaling pathway (Zhang et al. 2016). Consistently, we observed an increase of the ratio of autophagic marker LC3B II and I in the CsTP-treated group. And the upregulation was much more noticeable at 10 weeks (Fig. 3). Immunohistochemistry result of P62 presented the same upregulation. The level and distribution were more abundant in the experimental group than that of the control group (Additional file: Fig. S1). It was likely that CsTP mediated hepatic autophagy might facilitate autophagic response by inducing the expression of LC3B and P62.
Studies have shown tight link between the autophagy and homeostasis of hepatocytes (Tu et al. 2014), chondrocytes (Xin et al. 2017), and gastric cancer cells (Chen et al. 2017). Li and his colleagues found that autophagy inhibitor promoted cleaved caspase3-dependent apoptosis in cultured rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS). Suppressing autophagy could downregulate cell proliferation and inflammation, which were mediated via the PI3K/AKT signaling pathway (Li et al. 2017). It was illustrated that inhibited autophagy by GW-H1 might enhance apoptosis in gastric cancer AGS cells (Pan et al. 2015). The study by Liu and his colleagues also identified the viewpoint of autophagy negatively regulating apoptosis (Liu et al. 2011). Here our study provided clear evidence that LX-2 cells were proliferated in the low concentration (2, 4, 10, 25 μg/ml) of CsTP, while a high concentration (50 μg/ml or greater) reversed cell proliferation (Fig. 4a). Moreover, CsTP reduced the expression of antiapoptotic marker Bcl-2 in the CsTP-immunized mice, indicating inhibition of apoptotic cell (Fig. 2). Result from FCM in CsTP-treated LX-2 cells showed that CsTP could neutralize the apoptosis caused by H2O2 (Fig. 4c, d). Additionally, CsTP provoked the migration ability of LX-2 cells (Fig. 4b).
In agreement with our observation, previous studies had identified a crucial role of mTOR/AKT signaling in the cell homeostasis, including cell apoptosis, proliferation, survival, migration, and autophagy. mTOR pathway is a typical autophagy-mediated signaling, and AKT is activated accompanying with mTOR kinase (Du et al. 2019; Li et al. 2019a, b). Wang and his colleagues (Wang et al. 2017b) demonstrated that PI3K/AKT activation and the phosphorylation of mTOR were suppressed in the process of α-mangostin reducing inflammation and inducing autophagy/apoptosis. PI3K/AKT/mTOR pathway was involved in the inhibition of cell proliferation and invasion, and activation of autophagy in curcumin stimulated melanoma cells (Zhao et al. 2016). The findings from our study showed an apparent attenuation of mTOR in the CsTP-treated LX-2 cells and mice (Figs. 3 and 5). We also found that CsTP decreased p-AKT (Fig. 5). All the results were in accordance with the previous reports.
Conclusions
In summary, we demonstrate for the first time to implement that CsTP had the potent function to suppress apoptosis, but promote migration, inflammation, and autophagy in the indicated treatment. Mechanistically, dysregulation of the mTOR/AKT signaling pathway was involved in these activities. Thus, CsTP is an effective pro-inflammatory media and an underlying pathogenic factor of liver fibrosis. It may be a promising therapeutic agent by targeting its induced autophagy.
Abbreviations
- CsTP:
-
Clonorchis sinensis adult-derived total protein
- ESPs:
-
excretory-secretory products
- s.c:
-
subcutaneously
- FBS:
-
fetal bovine serum
- ELISA:
-
enzyme-linked immunosorbent assay
- DAPI:
-
4′, 6-Diamidino-2-Phenylindole
- TNF-α:
-
tumor necrosis factor-alpha
- IFN-γ:
-
Interferon-gamma
- DCs:
-
dendritic cell
- TI-TP:
-
total protein of the adult worm Toxascaris leonina
- OVA:
-
ovalbumin
- PBMC:
-
peripheral blood mononuclear cells
- SWA:
-
Schistosoma mansoni adult worm antigen
- Sh:
-
S. haematobium total antigens
- CHO:
-
Chinese hamster ovary
- VEGF:
-
vascular endothelial growth factor
- NAG:
-
N-acetyl-β-D-glucosaminidase
- scFv:
-
B cell single-chain antibody Fv
- LC3B:
-
microtubule-associated proteins 1A/1B light chain 3B
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- Bcl-2:
-
B cell lymphoma 2
- p-mTOR:
-
phospho-mTOR
- IL-1β:
-
interleukin-1β
- HRP:
-
horseradish peroxidase
- CCK-8:
-
cell count kit-8
- HBV:
-
hepatitis B virus
- HSC:
-
hepatic stellate cell
- KCs:
-
Kuffer cells
- DHA:
-
dihydroartemisinin
- RA-FLS:
-
rheumatoid arthritis fibroblast-like synoviocytes
References
Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, da Costa JM (2009a) Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol 39:1083–1091
Botelho M, Oliveira P, Gomes J, Gartner F, Lopes C, da Costa JM, Machado JC (2009b) Tumourigenic effect of Schistosoma haematobium total antigen in mammalian cells. Int J Exp Pathol 90:448–453
Botelho MC, Oliveira PA, Lopes C, Correia da Costa JM, Machado JC (2011) Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol Oncol 29:809–814
Chen L, Li Z, Zhang Q, Wei S, Li B, Zhang X, Zhang L, Li Q, Xu H, Xu Z (2017) Silencing of AQP3 induces apoptosis of gastric cancer cells via downregulation of glycerol intake and downstream inhibition of lipogenesis and autophagy. Onco Targets Ther 10:2791–2804
Choi BI, Han JK, Hong ST, Lee KH (2004) Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev 17:540–552 table of contents
Chookami MB, Sharafi SM, Sefiddashti RR, Jafari R, Bahadoran M, Pestechian N, Yousofi Darani H (2016) Effect of two hydatid cyst antigens on the growth of melanoma cancer in C57/black mice. J Parasit Dis 40:1170–1173
Chung EJ, Jeong YI, Lee MR, Kim YJ, Lee SE, Cho SH, Lee WJ, Park MY, Ju JW (2017) Heat shock proteins 70 and 90 from Clonorchis sinensis induce Th1 response and stimulate antibody production. Parasit Vectors 10:118
Chung YB, Yang HJ, Hong SJ, Kang SY, Lee M, Kim TY, Choi MH, Chai JY, Hong ST (2003) Molecular cloning and immunolocalization of the 17 kDa myoglobin of Clonorchis sinensis. Parasitol Res 90:365–368
Crisan TO, Plantinga TS, van de Veerdonk FL, Farcas MF, Stoffels M, Kullberg BJ, van der Meer JW, Joosten LA, Netea MG (2011) Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS One 6:e18666
de Almeida T, Fernandes JS, Lopes DM, Andrade LS, Oliveira SC, Carvalho EM, Araujo MI, Cruz AA, Cardoso LS (2017) Schistosoma mansoni antigens alter activation markers and cytokine profile in lymphocytes of patients with asthma. Acta Trop 166:268–279
Du H, Zhang X, Zeng Y, Huang X, Chen H, Wang S, Wu J, Li Q, Zhu W, Li H, Liu T, Yu Q, Wu Y, Jie L (2019) A novel phytochemical, DIM, inhibits proliferation, migration, invasion and TNF-alpha induced inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal pathway. Front Immunol 10:1620
Guedes-da-Silva FH, Shrestha D, Salles BC, Figueiredo VP, Lopes LR, Dias L, Barcelos Lda S, Moura S, de Andrade SP, Talvani A (2015) Trypanosoma cruzi antigens induce inflammatory angiogenesis in a mouse subcutaneous sponge model. Microvasc Res 97:130–136
Hong ST, Fang Y (2012) Clonorchis sinensis and clonorchiasis, an update. Parasitol Int 61:17–24
Jakobsen SR, Myhill LJ, Williams AR (2019) Effects of Ascaris and Trichuris antigens on cytokine production in porcine blood mononuclear and epithelial cells. Vet Immunol Immunopathol 211:6–9
Janssen L, Silva Santos GL, Muller HS, Vieira AR, de Campos TA, de Paulo Martins V (2016) Schistosome-derived molecules as modulating actors of the immune system and promising candidates to treat autoimmune and inflammatory diseases. J Immunol Res 2016:5267485
Jeong YI, Kim SH, Ju JW, Cho SH, Lee WJ, Park JW, Park YM, Lee SE (2011) Clonorchis sinensis-derived total protein attenuates airway inflammation in murine asthma model by inducing regulatory T cells and modulating dendritic cell functions. Biochem Biophys Res Commun 407:793–800
Kim TS, Pak JH, Kim JB, Bahk YY (2016) Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep 49:590–597
Lee KH, Park HK, Jeong HJ, Park SK, Lee SJ, Choi SH, Cho MK, Ock MS, Hong YC, Yu HS (2008) Immunization of proteins from Toxascaris leonina adult worm inhibits allergic specific Th2 response. Vet Parasitol 156:216–225
Li J, Yang R, Yang H, Chen S, Wang L, Li M, Yang S, Feng Z, Bi J (2019a) NCAM regulates the proliferation, apoptosis, autophagy, EMT, and migration of human melanoma cells via the Src/Akt/mTOR/cofilin signaling pathway. J Cell Biochem 121:1192–1204
Li J, Zhai DS, Huang Q, Chen HL, Zhang Z, Tan QF (2019b) LncRNA DCST1-AS1 accelerates the proliferation, metastasis and autophagy of hepatocellular carcinoma cell by AKT/mTOR signaling pathways. Eur Rev Med Pharmacol Sci 23:6091–6104
Li S, Chen JW, Xie X, Tian J, Deng C, Wang J, Gan HN, Li F (2017) Autophagy inhibitor regulates apoptosis and proliferation of synovial fibroblasts through the inhibition of PI3K/AKT pathway in collagen-induced arthritis rat model. Am J Transl Res 9:2065–2076
Lima C, Perini A, Garcia ML, Martins MA, Teixeira MM, Macedo MS (2002) Eosinophilic inflammation and airway hyper-responsiveness are profoundly inhibited by a helminth (Ascaris suum) extract in a murine model of asthma. Clin Exp Allergy 32:1659–1666
Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang J, Qu X, Liu Y (2011) Beta-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer 11:183
Matisz CE, Faz-Lopez B, Thomson E, Al Rajabi A, Lopes F, Terrazas LI, Wang A, Sharkey KA, McKay DM (2017) Suppression of colitis by adoptive transfer of helminth antigen-treated dendritic cells requires interleukin-4 receptor-alpha signaling. Sci Rep 7:40631
McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF Jr, Keane-Myers AM (2006) Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function. Infect Immun 74:6632–6641
McDonald EA, Kurtis JD, Acosta L, Gundogan F, Sharma S, Pond-Tor S, Wu HW, Friedman JF (2013) Schistosome egg antigens elicit a proinflammatory response by trophoblast cells of the human placenta. Infect Immun 81:704–712
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Pak JH, Kim DW, Moon JH, Nam JH, Kim JH, Ju JW, Kim TS, Seo SB (2009) Differential gene expression profiling in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory-secretory products. Parasitol Res 104:1035–1046
Pan WR, Chen YL, Hsu HC, Chen WJ (2015) Antimicrobial peptide GW-H1-induced apoptosis of human gastric cancer AGS cell line is enhanced by suppression of autophagy. Mol Cell Biochem 400:77–86
Rahimi HR, Mohammadzadeh T, Sadjjadi SM, Sarkari B, Zahabiun F (2017) BALB/c mice immunity to hydatidosis induced by in-vitro reared Echinococcus granulosus adult worm antigens. Iran J Immunol 14:123–133
Rim HJ (2005) Clonorchiasis: an update. J Helminthol 79:269–281
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456:264–268
Shang M, Xie Z, Tang Z, He L, Wang X, Wang C, Wu Y, Li Y, Zhao L, Lv Z, Wu Z, Huang Y, Yu X, Li X (2017) Expression of Clonorchis sinensis GIIIsPLA2 protein in baculovirus-infected insect cells and its overexpression facilitating epithelial-mesenchymal transition in Huh7 cells via AKT pathway. Parasitol Res 116:1307–1316
Sun K, Xu L, Jing Y, Han Z, Chen X, Cai C, Zhao P, Zhao X, Yang L, Wei L (2017) Autophagy-deficient Kupffer cells promote tumorigenesis by enhancing mtROS-NF-kappaB-IL1alpha/beta-dependent inflammation and fibrosis during the preneoplastic stage of hepatocarcinogenesis. Cancer Lett 388:198–207
Sun S, Li H, Yuan Y, Wang L, He W, Xie H, Gao S, Cheng R, Qian H, Jiang H, Wang X, Zhan B, Fang Q, Yang X (2019) Preventive and therapeutic effects of Trichinella spiralis adult extracts on allergic inflammation in an experimental asthma mouse model. Parasit Vectors 12:326
Tang Z, Shang M, Chen T, Ren P, Sun H, Qu H, Lin Z, Zhou L, Yu J, Jiang H, Zhou X, Li X, Huang Y, Xu J, Yu X (2016) The immunological characteristics and probiotic function of recombinant Bacillus subtilis spore expressing Clonorchis sinensis cysteine protease. Parasit Vectors 9:648
Tu QQ, Zheng RY, Li J, Hu L, Chang YX, Li L, Li MH, Wang RY, Huang DD, Wu MC, Hu HP, Chen L, Wang HY (2014) Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol Sin 35:504–512
Tweyongyere R, Namanya H, Naniima P, Cose S, Tukahebwa EM, Elliott AM, Dunne DW, Wilson S (2016) Human eosinophils modulate peripheral blood mononuclear cell response to Schistosoma mansoni adult worm antigen in vitro. Parasite Immunol 38:516–522
Wang F, Ma H, Liu Z, Huang W, Xu X, Zhang X (2017b) Alpha-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed Pharmacother 92:672–680
Wang Y, Bai X, Zhu H, Wang X, Shi H, Tang B, Boireau P, Cai X, Luo X, Liu M, Liu X (2017a) Immunoproteomic analysis of the excretory-secretory products of Trichinella pseudospiralis adult worms and newborn larvae. Parasit Vectors 10:579
Wangala B, Gantin RG, Vossberg PS, Vovor A, Poutouli WP, Komlan K, Banla M, Kohler C, Soboslay PT (2019) Inflammatory and regulatory CCL and CXCL chemokine and cytokine cellular responses in patients with patent Mansonella perstans filariasis. Clin Exp Immunol 196:111–122
Won J, Cho Y, Lee D, Jeon BY, Ju JW, Chung S, Pak JH (2019) Clonorchis sinensis excretory-secretory products increase malignant characteristics of cholangiocarcinoma cells in three-dimensional co-culture with biliary ductal plates. PLoS Pathog 15:e1007818
Xin W, Yu Y, Ma Y, Gao Y, Xu Y, Chen L, Wan Q (2017) Thyroid-stimulating hormone stimulation downregulates autophagy and promotes apoptosis in chondrocytes. Endocr J 64:749–757
Xu Y, Chen W, Bian M, Wang X, Sun J, Sun H, Jia F, Liang C, Li X, Zhou X, Huang Y, Yu X (2013) Molecular characterization and immune modulation properties of Clonorchis sinensis-derived RNASET2. Parasit Vectors 6:360
Xu Y, Liang P, Bian M, Chen W, Wang X, Lin J, Shang M, Qu H, Wu Z, Huang Y, Yu X (2016) Interleukin-13 is involved in the formation of liver fibrosis in Clonorchis sinensis-infected mice. Parasitol Res 115:2653–2660
Xu Y, Lin J, Bian M, Chen W, Liang P, Wang X, Shang M, Qu H, Wu Z, Huang Y, Yu X (2015) CsRNASET2 is an important component of Clonorchis sinensis responsible for eliciting Th2 immune response. Parasitol Res 114:2371–2379
Yan C, Li B, Fan F, Du Y, Ma R, Cheng XD, Li XY, Zhang B, Yu Q, Wang YG, Tang RX, Zheng KY (2017) The roles of toll-like receptor 4 in the pathogenesis of pathogen-associated biliary fibrosis caused by Clonorchis sinensis. Sci Rep 7:3909
Yan C, Wang L, Li B, Zhang BB, Zhang B, Wang YH, Li XY, Chen JX, Tang RX, Zheng KY (2015a) The expression dynamics of transforming growth factor-beta/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis. Parasit Vectors 8:70
Yan C, Wang YH, Yu Q, Cheng XD, Zhang BB, Li B, Zhang B, Tang RX, Zheng KY (2015b) Clonorchis sinensis excretory/secretory products promote the secretion of TNF-alpha in the mouse intrahepatic biliary epithelial cells via toll-like receptor 4. Parasit Vectors 8:559
Yoo WG, Kim DW, Ju JW, Cho PY, Kim TI, Cho SH, Choi SH, Park HS, Kim TS, Hong SJ (2011) Developmental transcriptomic features of the carcinogenic liver fluke, Clonorchis sinensis. PLoS Negl Trop Dis 5:e1208
Zhang F, Liang P, Chen W, Wang X, Hu Y, Liang C, Sun J, Huang Y, Li R, Li X, Xu J, Yu X (2013) Stage-specific expression, immunolocalization of Clonorchis sinensis lysophospholipase and its potential role in hepatic fibrosis. Parasitol Res 112:737–749
Zhang Z, Guo M, Zhao S, Shao J, Zheng S (2016) ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic Biol Med 101:272–283
Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J, Sun Z, Qiao S, Song Z (2016) Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol Rep 35:1065–1074
Zhao L, Shi M, Zhou L, Sun H, Zhang X, He L, Tang Z, Wang C, Wu Y, Chen T, Shang M, Zhou X, Lin Z, Li X, Yu X, Huang Y (2018) Clonorchis sinensis adult-derived proteins elicit Th2 immune responses by regulating dendritic cells via mannose receptor. PLoS Negl Trop Dis 12:e0006251
Zhou L, Shi M, Zhao L, Lin Z, Tang Z, Sun H, Chen T, Lv Z, Xu J, Huang Y, Yu X (2017) Clonorchis sinensis lysophospholipase A upregulates IL-25 expression in macrophages as a potential pathway to liver fibrosis. Parasit Vectors 10:295
Zueva T, Morchon R, Carreton E, Montoya-Alonso JA, Santana A, Bargues MD, Mas-Coma S, Rodriguez-Barbero A, Simon F (2019) Angiogenic response in an in vitro model of dog microvascular endothelial cells stimulated with antigenic extracts from Dirofilaria immitis adult worms. Parasit Vectors 12:315
Acknowledgments
We appreciate Ph.D Danika Bakke, Ph.D Zhiyu Dai, and Ph.D Xiaoyun Wang for helping to improve the writing of this final manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Clonorchis sinensis Genome Database repository, [http://fluke.sysu.edu.cn/CsinGD/].
Funding
This work was supported by grants from Guangdong Natural Science Foundation (No. 2019A1515010583), the National Natural Science Foundation of China (No. 81641094), National Key Research and Development Program of China (Nos. 2016YFC1202003, 2016YFC1202005), and Guangdong Natural Science Foundation (No. 2019A1515010583, S2012010008504) to XL. The national key research and development program of China (No. 2017YFD0501300), Guangdong marine economy promotion projects fund (GDOE[2019]A29), and the science and technology planning project of Guangdong province (No. 2014B020203001) to XY. The National Natural Science Foundation of China (Nos. 81,101,270, 31,372,263) to YH and YW, respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All Balb/c mice used in the study were purchased from Animal Center of Sun Yat-sen University and raised with caution based on animal care and the ethical guidelines of the National Institutes of Health. The operation of whole animal studies was approved by the Sun Yat-sen University Institutional Animal Care and Use Committee (Permit Numbers: SYXK (Guangdong) 2010–0107).
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Section Editor: Xing-Quan Zhu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Shang, M., Sun, H., Wu, Y. et al. In vivo and in vitro studies using Clonorchis sinensis adult-derived total protein (CsTP) on cellular function and inflammatory effect in mouse and cell model. Parasitol Res 119, 1641–1652 (2020). https://doi.org/10.1007/s00436-020-06651-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06651-1