Abstract
We tested whether and how the maternal environment (i.e. host species exploited by a mother), rearing conditions (i.e. host species exploited by her offspring) or both (i.e. matches and mismatches in host species exploited by a mother and her offspring) affect reproductive performance in the offspring. We experimentally manipulated maternal and rearing environments in two generalist fleas (Xenopsylla conformis and Xenopsylla ramesis) implementing a factorial cross-rearing design. Mothers exploited either the principal host (PH) or auxiliary hosts that were either closely (CAH) or distantly related (DAH) to the PH. After six generations of infesting a given host species, we cross-reared fleas within and between host species. These fleas reproduced and we measured their reproductive performance both quantitatively (i.e. egg number) and qualitatively (i.e. egg size, development time, body size of the next generation). We found that identity of the host a flea was reared on (=actual host) had the strongest effect on its performance. Individuals reared on the PH performed considerably better than those reared on either auxiliary host. Moreover, fleas reared on a CAH performed better than those reared on a DAH. Actual host identity also had a stronger effect on reproductive performance in X. ramesis than in X. conformis. Nevertheless, there was no difference in performance between match and mismatch maternal and actual host identities. We conclude that rearing environment has the strongest effect on fitness in generalist parasites. Moreover, phylogenetic distance between an auxiliary host and the PH determines the level of suitability of the former.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effect of the maternal environment on offspring performance is well-known (Rossiter 1991; Mousseau and Fox 1998; Lindström 1999; Lummaa and Clutton-Brock 2002). For example, maternal nutrition, as defined by leaf chemistry of host plants, significantly influenced reproductive potential and growth rate of the offspring in the polyphagous gypsy moth, Lymantria dispar (Rossiter et al. 1988). In another example, abiotic conditions, such as climate or soil characteristics, in the maternal environment of the maritime pine tree, Pinus pinaster, significantly affected success and timing of seed germination (Cendán et al. 2013). Obviously, the level of suitability of the maternal environment determines, to a great extent, the amount of resources that a mother could allocate to her offspring and is thus further translated into offspring fitness. Indeed, few would deny, that if a mother lives in a favourable environment and produce offspring that live in the same favourable conditions, these offspring will likely perform well (Marshall and Uller 2007). If a mother lives in an unfavourable environment and her progeny live in unfavourable conditions as well, this progeny will likely perform poorly (but see Herman et al. 2012), although a mother facing resource limitations may invest more in offspring quality by sacrificing their numbers (Parker and Begon 1986). Whether maternal and offspring environments are favourable or unfavourable, the performance of mothers and the offspring will likely match in matching environments. However, mismatches often occur between the offspring and maternal environments in nature as these environments might be separated in time and space (Bernardo 1996).
A mismatch between maternal and offspring environments could lead to alternative outcomes in terms of offspring performance and, ultimately, fitness (e.g. Giordano et al. 2014). In a mismatch scenario when a mother lives in a favourable environment and her offspring in unfavourable conditions, the offspring, nevertheless, could perform well if the mother’s good condition is carried over to them in terms of, for example, the amount of resources provided by the mother that would allow the offspring to cope with an unfavourable environment. In this scenario, offspring performance would therefore mainly be determined by the maternal environment. If, however, maternal provisioning is not sufficient enough to compensate for unfavourable conditions, offspring will perform poorly and their performance will mainly be determined by their own conditions. In an opposite scenario when a mother lives in an unfavourable environment but the offspring, for some reason, are reared under favourable conditions, they, nevertheless, could perform either poorly because of their low quality carried over from the mother or well because they would have enough resources from their own environment to compensate for poor maternal provisioning by, for example, a period of rapid growth within the favourable environment (reviewed in Metcalfe and Monaghan 2001; Ali et al. 2003). Therefore, either the maternal or the rearing environment will have a major effect on offspring performance. These scenarios suggest that the relative effects of maternal versus rearing environments on offspring performance and fitness may differ depending on the direction of mismatches between these environments.
Parasites represent a convenient model for studying the effects of matches and mismatches between maternal and rearing environments on reproductive performance of the offspring. This is because (i) the main resource-providing environment of a parasite is its host and (ii) the availability, quality and pattern of acquisition of these resources vary among hosts belonging to different species (Combes 2001). As a result, abundance of even a highly host-opportunistic parasite varies among host species being highest in its principal host and lower in auxiliary hosts (Dogiel et al. 1961; Dogiel 1964). This variation in abundance reflects variation in reproductive performance of a parasite (Khokhlova et al. 2012a), so that the principal host is the most favourable environment for a given parasite, whereas the environment represented by auxiliary hosts is much less beneficial. Furthermore, if the principal host and the auxiliary hosts of a parasite co-occur in the same habitat, the parasite offspring could encounter either the same host species as the mother or a different one. Both matches and mismatches between maternal and rearing environments often occur (e.g. Krasnov et al. 1997, 1999). In particular, the offspring of a parasite exploiting the principal host could encounter either the principal or an auxiliary host and vice versa. Elucidating the relative effects of the maternal and rearing environments in terms of host species on parasite performance will give insight into the mechanisms behind successful host switching and its role in parasite evolution. This could also assist in predicting changes in distribution of parasite-borne diseases under climate change and anthropogenic environmental transformation (Clark et al. 2018).
Here, we asked whether matches and mismatches in the identity of a host species exploited by a mother and her offspring affect reproductive performance of the latter. For our purposes, reproductive performance was measured via quantity (egg number) and quality (egg size, development time, body size) of the next generation. To answer this question, we experimentally manipulated maternal and rearing environments (i.e. host species identity) in two fleas, Xenopsylla conformis and Xenopsylla ramesis, implementing a factorial cross-rearing design. Fleas are obligatory haematophagous ectoparasites, exploiting mainly small- and medium-sized mammals, which alternate periods of staying on the host with time spent in the host’s burrow/nest. Females oviposit on a host’s body or in the burrow/nest but pre-imaginal development takes place mainly off-host (reviewed in Krasnov 2008). Fleas are unequally distributed among host species and their abundance and prevalence vary strongly in dependence of host identity (Marshall 1981), suggesting that host identity is a key aspect of flea survival and successful reproduction.
Both flea species used in this study are host generalists that commonly parasitize multiple rodent species in the Negev Desert. However, these species attain the highest prevalence and abundance on their principal host, considered here as favourable environment, Meriones crassus (Krasnov et al. 1997, 1999). Their auxiliary hosts (Gerbillus nanus and Acomys russatus for X. conformis; Gerbillus dasyurus and A. russatus for X. ramesis), considered here as unfavourable environments, occur in the same habitats as the principal host. Two of these hosts, G. nanus and G. dasyurus, are from the same subfamily (Gerbillinae) as the principal host, whereas A. russatus is from another subfamily (Deomyinae). Both flea species have often been recorded on an auxiliary host closely related to the principal host but very rarely on a distantly related auxiliary host (Krasnov et al. 1997, 1999). Earlier, Khokhlova et al. (2012a) showed that flea fitness is strongly affected by host identity with highest fitness in the principal host and lower fitness in auxiliary hosts. Furthermore, Khokhlova et al. (2012a) demonstrated that phylogenetic relatedness of an auxiliary host to the principal host reflected its degree of suitability from a flea’s perspective; thus, when fleas fed on auxiliary hosts their reproductive performance decreased as phylogenetic distance between the auxiliary host and the principal host increased. In other words, an auxiliary host closely related to the principal host can be considered as a moderately unfavourable environment for a flea, whereas an auxiliary host phylogenetically distant from the principal host can be considered as a highly unfavourable environment.
We refer to the maternal host as the host exploited by a mother and to the actual host as the host that her offspring were forced to feed (=reared) on. We predicted that fleas produced by mothers exploiting the principal host and reared on the principal host would have the highest performance. We further predicted that, in case of both maternal and actual auxiliary hosts, reproductive performance will be lower in an auxiliary host phylogenetically distant to the principal host. In mismatched maternal and actual hosts, alternative outcomes indicating relative strength of the effect of either maternal or rearing environment are expected. High performance of fleas born from mothers fed on the principal host but reared on an auxiliary host will suggest a stronger effect of the maternal environment, whereas their poor performance will indicate the main role of rearing environment. In the inverse host scenario, higher performance of offspring implies an effect of rearing environment, whereas their poor performance will advocate the effect of the maternal environment. We also predicted that a decrease in offspring quality will not be accompanied with an increase in their numbers and vice versa because recent studies with these species demonstrated that no trade-off existed between offspring quantity and quality as measured by egg number and size, respectively (Kiefer et al. 2016; van der Mescht et al. 2017).
Materials and methods
Study animals
We used rodents (M. crassus, G. dasyurus, G. nanus and A. russatus) and fleas (X. conformis and X. ramesis) from our laboratory colonies. Details on origin and maintenance of the colonies are published elsewhere (Khokhlova et al. 2012b; Krasnov et al. 2001a; Krasnov et al. 2001b). In brief, fleas in the colonies were reared on M. crassus kept individually in plastic cages (60 × 50 × 40 cm at 25 ± 1 °C and 12: 12 D: L) each containing a nest box above a mesh floor (5 × 5 mm) with a mixture of sand and flea larvae nutrient medium (94% dry bovine blood, 5% millet flour, 1% ground faeces of the respective host species) underneath (3–5 mm). Rodents were fed millet seed ad libitum and given fresh alfalfa as a water source. Every 2 weeks, all cage substrate was collected, placed into plastic boxes and kept at 25 °C and 90% relative humidity in an incubator (FOC225E, Velp Scientifica srl, Milano, Italy) where fleas developed. New rodents were then infested with newly emerged fleas. This study was conducted under permits from Ben-Gurion University Committee for the Ethical Care and Use of Animals in Experiments (IL-24-05-2017). All experimental procedures met the legal requirements of the State of Israel (Animal welfare law for the prevention of cruelty to animals (experiments on animals) of the State of Israel 1994).
Experimental design and measurements
The experimental design is outlined in Table 1. We established three lines of each flea species from randomly selected newly emerged fleas by assigning them to (i) a principal host (PH)-line maintained on M. crassus, (ii) closely related auxiliary host (CAH)-line maintained on an auxiliary host phylogenetically close to M. crassus (i.e. G. nanus for X. conformis and G. dasyurus for X. ramesis) or (iii) distantly related auxiliary host (DAH)-line maintained on an auxiliary host phylogenetically distant from M. crassus (i.e. A. russatus).
Prior to experiments, we maintained these lines for six generations and one generation lasts approximately 32 days. Initially, 100 male and 100 female fleas taken from a respective colony were released into plastic cages with two to three male rodents of a respective species. After 14 days, we collected substrate material from each cage into an individual plastic box and kept the box at an air temperature of 25 °C and 90% relative humidity in an incubator. Both flea species generally start laying eggs 2 days after being released into a host cage (unpublished data). Minimal duration of development from egg to a new imago is 24 days with the peak of imago emergence occurring at day 30–35 (Krasnov et al. 2001a; Khokhlova et al. 2010). We collected fleas from an incubated box on day 32 to guarantee that all fleas that emerged belonged to the same generation. Parents of each subsequent generation consisted of 100 male and 100 female newly emerged imagoes that were randomly selected from the previous generation and placed on the same host species as the previous generation. Although newly emerged fleas collected from a box could differ in their absolute age, they were of the same physiological age because they had never fed prior to experimental manipulations (Krasnov 2008). The procedures described above were repeated for six generations. The sixth generation was considered as the parental generation for the experimental trials.
Experiments were carried out on the offspring of the parental generations (i.e. F1). We randomly selected 100 male and 100 female newly emerged F1 imagoes of each species from each line and released them into cages with two to three individuals of the principal or an auxiliary host species as described above. Rodents were 6- to 8-month-old, sexually naïve males that have never been exposed to fleas prior to the experiments. The experiments with each flea species consisted of seven treatments (Table 1). In three treatments, maternal and offspring hosts matched. In other words, F1 fleas from each line were allowed to feed and reproduce (=reared) on the same host as their mothers did, namely (i) F1 fleas from the PH-line were reared on the principal host (PH-PH treatment), (ii) F1 from the CAH-line were reared on an auxiliary host closely related to the principal host (CAH-CAH treatment) and (iii) F1 fleas from the DAH-line were reared on an auxiliary host phylogenetically distant from the principal host (DAH-DAH treatment). The remaining four treatments involved cross-rearing in which the maternal and offspring hosts mismatched as follows. F1 fleas from each line were reared on a different host than their mothers did, namely (i) F1 fleas from the PH-line were reared on a closely related auxiliary host (PH-CAH treatment), (ii) F1 fleas from the PH-line reared on a distant auxiliary host (PH-DAH treatment), (iii) and (iv) F1 from the CAH-line and the DAH-line were reared on the principal host (CAH-PH treatment and DAH-PH treatment, respectively).
After three uninterrupted days in a host’s cage, F1 fleas were collected both from the cage substrate and rodents’ bodies. The latter was done by examining a rodent over a white plastic tray and brushing its hair with soft, custom-made forceps. We selected 70 engorged females from each treatment and randomly distributed them among seven Petri dishes (40-mm diameter; 10 females per dish). Petri dishes with fleas were then placed in an incubator (see above specifications) at 25 °C and 90% relative humidity. After 48 h, we checked Petri dishes for newly laid eggs and counted them under a light microscope at × 40 magnification. We measured the maximal length and width of each egg on-screen to the nearest 0.01 mm using a digital microscope camera (Moticam 2000) and Motic Images Plus 3.0 ML software (Motic, Speed Fair Cp., Ltd., Causeway Bay, Hong Kong) which was calibrated using a stage micrometre.
Eggs produced by each group of fleas in a Petri dish were checked daily starting on day 24, the minimum time of pre-imaginal development (Krasnov et al. 2001a; Khokhlova et al. 2010) until 14 days after the last imagoes emerged. We recorded the day on which each F2 adult emerged, total number of new adults produced by each group of F1 fleas and identified the sex of each new imago by examining its genitalia under a light microscope. We measured the maximal length of its left and right hind femurs to the nearest 0.01 mm with a digital microscope camera (see above specifications).
We calculated the mean number of eggs produced per F1 female as well as three variables describing quality of F2 fleas, namely (a) egg size, (b) duration of pre-imaginal development from egg to adult and (c) body size of a newly emerged adult. Mean number of eggs produced per female was calculated by dividing the total number of eggs by the total number of females per group of fleas. The size of an egg was estimated via its volume and calculated, following Berrigan (1991), as V = 1/6π × W2 × L, where V is the egg volume (=size), W is the maximal egg width and L is the maximal egg length. The duration of pre-imaginal development was calculated as the number of days from oviposition to emergence of a new imago. We used the average of the maximal length of the left and right hind femurs as a proxy of body size. High positive correlation between body size and femur length has been demonstrated earlier for both flea species (Krasnov et al. 2003a; Khokhlova et al. 2010).
Data analyses
We analysed the effects of maternal environment (i.e. identity of the maternal host species), offspring environment (i.e. identity of the actual host species) and match/mismatch in maternal and offspring environments (i.e. interaction between the identity of the maternal and actual hosts) on the response variables separately for each: (i) flea species and (ii) combination involving the principal host and either a closely or distantly related auxiliary host (hereafter referred to as a treatment group). We plotted empirical quantiles of each dependent variable against theoretical quantiles of a comparison distribution in R v3.3.2 (R Development Core Team 2017) using the “qqp” function implemented in the “car” package (Fox and Weisberg 2011). Based on the best-fit distribution of each dependent variable, we either applied log-transformations (i.e. for egg number and pre-imaginal development rate) or left data untransformed (i.e. for egg size and new imago size).
To test for the effect of maternal environment, offspring environment and match/mismatch in maternal and offspring environments on reproductive performance we used either general linear models (for egg number) or linear mixed-effects models (for egg size, development rate and new imago size) implemented in the “lme4” package (Bates et al. 2015) in R. To account for variation between replicates, we included ID number of a group of females as a random effect in each linear mixed-effects model. Data on development rate and imago size in fleas were analysed separately for each sex because these variables differ substantially between males and females (see Krasnov et al. 2001a; van der Mescht et al. 2017 for development rate and Krasnov et al. 2003a for body size). We initially constructed a model with all possible terms for each response variable and for each flea species. Then, we selected the best model based on the Akaike Information Criteria corrected for sample size (AICc) using the “model.sel” function implemented in the R package “MuMIn” (Barton 2016). We first evaluated the difference between the AICc of the best and the second best model (ΔAICc). If this value was greater than or equal to 2, we accepted the former as the best model. However, if this value was less than 2, we assessed the Akaike weights [w (AICc)] of these two models to confirm the best model (Wagenmakers and Farrell 2004). Finally, we tested whether the difference between each factor in the best model and a baseline (intercept for a best model with factors and 0 for an intercept-only best model) is significantly different using the “summary” function implemented in the “lme4” package in R.
Results
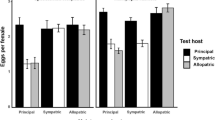
In X. conformis, the best-fit model of the relationship between the number of eggs and host species included the identity of the maternal and actual host species as explanatory variables but not their interaction (Table 2). As for maternal hosts, more eggs were produced by fleas born from mothers that fed on any auxiliary host than those from mothers fed on the PH (Fig. 1a, b; Table S1 of Electronic Supplementary Material). In contrast, more eggs were laid by fleas actually reared on the PH as compared to any auxiliary host (Fig. 2a, b; Table S1).
Effect of the species identity of the maternal host on egg number (mean ± SE) in Xenopsylla conformis involving the principal host (PH) and a a closely related auxiliary host (CAH) or b a distantly related auxiliary host (DAH). Maternal host species is indicated by the colour of the bars (black bars: PH; white bars: CAH; grey bars: DAH)
Effect of the species identity of the actual host on egg number (mean ± SE) in Xenopsylla conformis involving the principal host (PH) and a a closely related auxiliary host (CAH), or b a distantly related auxiliary host (DAH) and in Xenopsylla ramesis involving the principal host (PH) and c a closely related auxiliary host (CAH), or d distantly related auxiliary host (DAH). Actual host species is indicated by the colour of the bars (black bars: PH; white bars: CAH; grey bars: DAH)
The best-fit model for the effect of host species on the size of eggs produced by X. conformis exploiting the PH and a CAH included the maternal host, the actual host and their interaction (Table 2). However, the identity of the actual host species was the only explanatory variable that had a significant effect. Fleas reared on the PH laid significantly larger eggs than those reared on a CAH (Fig. 3a; Table S1).
Neither the species of the maternal or the actual host or their interaction had an effect on development rate of either male or female F2 offspring according to the best-fit models of the effect of feeding on the PH and a CAH or a DAH (Table 2). The best-fit model for the effect of host species identity on the size of the offspring produced by fleas fed on the PH and a CAH included the maternal and actual hosts as explanatory variables for males. The size of male offspring was significantly affected by the species identity of the actual host. Larger males were produced by fleas reared on a CAH than by those reared on the PH (Fig. 4a; Table S1). The best-fit model for the effect of host species identity on the size of male and female offspring, when fleas fed on the PH and a DAH, included the actual and maternal hosts but not their interaction (Table 2). Moreover, the size of offspring of both sexes was only significantly affected by the species identity of the actual host. Thus, imagoes were larger when reared on a DAH compared to those on the PH (Fig. 4b, c; Table S1).
Effect of the species identity of the actual host on length of the hind femur (mean ± SE) of new male imagoes in Xenopsylla conformis involving the principal host (PH: black bars) and a a closely related auxiliary host (CAH: white bar) or b a distantly related auxiliary host (DAH: grey bar) and c new female imagoes involving the principal host (PH: black bar) and a distantly related auxiliary host (DAH: grey bar)
In X. ramesis, the best-fit model of the relationship between the number of eggs produced and host species identity included the maternal and actual host species (Table 3). However, actual host species identity was the only variable that had a significant effect on egg number. Females reared on the PH laid significantly more eggs than those reared on either auxiliary host (Fig. 2c, d; Table S2). Both the maternal and actual host species identities were included in the best-fit model of the effect of host species on egg size when fleas fed on the PH and a CAH (Table 3). Female fleas laid significantly larger eggs when reared on the PH than on a CAH (Fig. 3b; Table S2). Significantly larger eggs were laid by females whose mothers fed on a CAH than those from mothers fed on the PH (Table S2).
Discussion
Our results indicate that maternal and rearing environments independently affected reproductive performance in X. conformis and X. ramesis; however, the rearing environment had the strongest effect. Fleas reared on the PH performed considerably better than those reared on an auxiliary host. Moreover, as we predicted, the phylogenetic relatedness of an auxiliary host to the PH reflected the degree of its suitability so that when fleas fed on an auxiliary host their reproductive performance decreased with an increase in phylogenetic distance between this host and the PH. The effect of rearing environment was also stronger in X. ramesis than in X. conformis. Nevertheless, we found no interaction between the maternal and rearing environments. Thus, in contrast to what we predicted, there was no difference in performance between matched and mismatched environments.
The stronger effect of rearing than maternal environment on reproductive performance could be related to physiological mechanisms that affect offspring fitness during particular stages of development in holometabolous insects. Adult female fleas can directly invest in their offspring via the number of eggs, egg size and egg provisioning (Marshall 1981; Krasnov 2008). However, once the eggs hatch and larvae emerge, their nutritional status is determined by the larval diet (Silverman and Appel 1994). Larvae of the majority of flea species feed on organic debris in the burrow/nest of the host; therefore, maternal control over the larval environment, or any post-hatching components of offspring fitness, is very limited (Krasnov 2008). Nevertheless, some female fleas can indirectly invest in their offspring by voiding their gut contents and excreting faecal pellets around the clutch which larvae can feed on when they hatch (Silverman and Appel 1994). Once new imagoes emerge, their fitness is mainly determined by their first blood meal as initiation of feeding may trigger important processes that affect blood digestion rates (Filimonova 1986; Fielden et al. 2001, 2004). For example, alkaline phosphatase and acid phosphatase were mainly distributed in the midgut, nerve nuclei, testes, ejaculatory ducts, oviducts and spermathecal glands, while adenosine triphosphatase was distributed in all tissues of newly emerged Monopsyllus anisus and Leptopsylla segnis prior to the first blood meal (Xun and Qi 2004). After digesting the first blood meal, concentration of all three enzymes increased only in the midgut. Our results suggest that the actual host on which newly emerged imagoes take their first blood meal strongly affect their fitness, whereas the effect of maternal environment is likely buffered by the larval environment.
From an ecological standpoint, the response of newly emerged imagoes to heterogeneous environments could be key as the same host species might not always return to the burrow/nest. Therefore, environmental heterogeneity, from a parasite’s perspective, consists of spatiotemporal variation in co-occurring host species. In the majority of fleas, newly emerged individuals are found in the host burrow/nest and as such interspecific transfer is possible when host species other than the maternal host enter the shelter (reviewed in Krasnov 2008). For example, the maternal host could abandon its burrow/nest, which can then be occupied by a different host species. Alternatively, other host species might occasionally visit the nest/burrow of the maternal host. In both these scenarios, newly emerged imagoes could be forced to feed on a host species other than the maternal host. Each host species represents environmental conditions of differing quality as determined by the availability, quality and pattern of resource acquisition (Combes 2001). As mentioned above, the PH represents the most favourable conditions overall (Krasnov et al. 2004a; Khokhlova et al. 2012a). This explains why the offspring reared on the PH performed better than those reared on either auxiliary host. Nevertheless, decreasing phylogenetic distance between an auxiliary host and the PH is generally associated with increasing ecological, physiological and/or immunological similarity (Krasnov et al. 2004a; Khokhlova et al. 2012a). This would explain why an auxiliary host closely related to the PH represents a moderately unfavourable environment, whereas an auxiliary host phylogenetically distant to the PH represents the most unfavourable environment to a flea.
A parasite’s preference for a particular host is related to the quality of resources gained from this host (Combes 2001). However, the host develops immunological defences against a specific parasite which could inhibit a parasite’s feeding (Fielden et al. 1992; Walker et al. 2003) so that the parasite itself had to develop adaptations to suppress or evade the defences of the PH during their common evolutionary history (Singh and Girschick 2003; Maizels et al. 2004). These adaptations can be costly and adaptations to a given host and its defences can reduce the ability of a parasite to exploit other hosts (Møller et al. 2005). Therefore, traits targeted at a specific host are expected to lose their adaptive value if this host is not encountered or exploited for an extended period (Poulin 2007). Offspring reared on the PH in our study performed the best regardless of the maternal host being either the principal (matched environment) or an auxiliary (mismatched environment) host maintained for six generations. Therefore, our results suggest that flea traits targeting the PH did not lose their adaptive value after six generations of not exploiting this host.
The higher performance of both flea species reared under the most favourable conditions could facilitate host switching and/or widening of host spectra that would then lead to expansion of their geographic ranges. This ability of host generalists to overcome poor maternal provisioning (i.e. mother raised on an auxiliary host) by exploiting a rich resource (i.e. the PH) might form the functional basis for evolutionary events such as ecological fitting (Janzen 1985; Brooks et al. 2006) and co-evolutionary alternation (Nuismer and Thompson 2006). In particular, the process of ecological fitting, whereby a parasite specialises on a particular resource (e.g. blood) that is widespread among multiple host species rather than its representation (i.e. a given host species), allows a parasite to exploit novel hosts (Brooks et al. 2006). Co-evolutionary alternation is the process whereby a parasite evolves with multiple hosts in its native habitat (Nuismer and Thompson 2006). Our results suggest that generalist parasites could persist in sub-optimal hosts for extended periods and could thus colonise novel hosts through a “stepping-stone” process (see Araujo et al. 2015).
Furthermore, we found differences between flea species in the effect of rearing environment on reproductive performance, such as when fleas fed on the PH and a CAH, which are likely associated with differences in host selection strategies. Although both flea species have the ability to perceive qualitative differences (e.g. between host variation in the absolute amount of blood obtained during a single blood meal) between host species, X. conformis also perceives quantitative differences (e.g. absolute amount of blood available for a flea imago) between hosts (Krasnov et al. 2003b). Therefore, X. conformis quantitatively perceives M. crassus as a superior host over the closely related host species G. dasyurus. This could then explain the stronger effect of rearing environment on performance in X. conformis than in X. ramesis (Krasnov et al. 2004b).
In conclusion, we demonstrate that rearing environment has the strongest effect on fitness in generalist parasites. Furthermore, the PH represent the best rearing conditions for a parasite and the level of suitability of an auxiliary host increases with an increase in phylogenetic relatedness to the PH. Surprisingly, we found no difference in flea fitness between matched and mismatched maternal and rearing environments. Thus, more studies are needed to observe how these effects might change over evolutionary time by increasing the number of generations fleas are maintained on a particular maternal host species.
References
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190
Animal welfare law for the prevention of cruelty to animals (experiments on animals) of the State of Israel (1994) http://www.sviva.gov.il/English/env_topics/AnimalWelfare/Pages/Animal-Welfare-Legislation.aspx. Accessed 15 April 2019
Araujo SB, Braga MP, Brooks DR, Agosta SJ, Hoberg EP, von Hartenthal FW, Boeger WA (2015) Understanding host-switching by ecological fitting. PLoS One 10:e0139225
Barton K (2016) MuMIn: multi-model inference. Version 1.15. 6. URL: https://cranr-project.org/web/packages/MuMIn/index.htm.
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bernardo J (1996) The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am Zool 36:216–236
Berrigan D (1991) The allometry of egg size and number in insects. Oikos 60:313–321
Brooks DR, León-Règagnon V, McLennan DA, Zelmer D (2006) Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 87:S76–S85
Cendán C, Sampedro L, Zas R (2013) The maternal environment determines the timing of germination in Pinus pinaster. Environ Exp Bot 94:66–72
Clark NJ, Seddon JM, Šlapeta J, Wells K (2018) Parasite spread at the domestic animal-wildlife interface: anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit Vectors 11(8)
Combes C (2001) Parasitism. The ecology and evolution of intimate interactions. University of Chicago Press, Chicago
Dogiel VA (1964) General Parasitology. Oliver and Boyd, Edinburgh-London
Dogiel VA, Petrushevski GK, Polyanski YI (1961) Parasitology of fishes. Oliver and Boyd, Edinburgh-London
Fielden LJ, Rechav Y, Bryson NR (1992) Acquired immunity to larvae of Ablyomma marmoreum and A. hebraeum by tortoises, Guinea-pigs and Guinea-fowl. Med Vet Entomol 6:251–254
Fielden LJ, Krasnov B, Khokhlova I (2001) Respiratory gas exchange in the flea Xenopsylla conformis (Siphonaptera: Pulicidae). J Med Entomol 38:735–739
Fielden LJ, Krasnov BR, Khokhlova IS, Arakelyan MS (2004) Respiratory gas exchange in the desert flea Xenopsylla ramesis (Siphonaptera: Pulicidae): response to temperature and blood-feeding. Comp Biochem Physiol A Mol Integr Physiol 137:557–565
Filimonova SA (1986) Changes in the ultra-structure of the intestinal epithelium of Xenopsylla cheopis (Siphonaptera) after emerging from cocoons and beginning of feeding. Parazitologiya 20:99–105 in Russian
Fox J, Weisberg S (2011) An {R} companion to applied regression. SAGE publishing, Thousand Oaks, CA
Giordano M, Groothuis TG, Tschirren B (2014) Interactions between prenatal maternal effects and posthatching conditions in a wild bird population. Behav Ecol 25:1459–1466
Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C (2012) Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr Comp Biol 52:77–88
Janzen DH (1985) On ecological fitting. Oikos 45:308–310
Khokhlova IS, Serobyan V, Degen AA, Krasnov BR (2010) Host gender and offspring quality in a flea parasitic on a rodent. J Exp Biol 213:3299–3304
Khokhlova IS, Fielden LJ, Degen AA, Krasnov BR (2012a) Ectoparasite fitness in auxiliary hosts: phylogenetic distance from a principal host matters. J Evol Biol 25:2005–2013
Khokhlova IS, Fielden LJ, Degen AA, Krasnov BR (2012b) Digesting blood of an auxiliary host in fleas: effect of phylogenetic distance from a principal host. J Exp Biol 215:1259–1265
Kiefer D, Warburton EM, Khokhlova IS, Krasnov BR (2016) Reproductive consequences of female size in haematophagous ectoparasites. J Exp Biol 219:2368–2376
Krasnov BR (2008) Functional and evolutionary ecology of fleas: a model for ecological parasitology. Cambridge University Press, Cambridge
Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, Khokhlova IS (1997) Host–habitat relations as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev Desert. Parasitology 114:159–173
Krasnov BR, Hastriter MW, Medvedev SG, Shenbrot GI, Khokhlova IS, Vatschenok VS (1999) Additional records of fleas (Siphonaptera) on wild rodents in the southern part of Israel. Isr J Zool 45:333–340
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001a) Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med Vet Entomol 15:249–258
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001b) Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 38:629–637
Krasnov BR, Burdelov SA, Khokhlova IS, Burdelova NV (2003a) Sexual size dimorphism, morphological traits and jump performance in seven species of desert fleas (Siphonaptera). J Zool 261:181–189
Krasnov BR, Khokhlova IS, Shenbrot GI (2003b) Density dependent host selection in ectoparasites: an application of isodar theory to fleas parasitizing rodents. Oecologia 134:365–372
Krasnov BR, Shenbrot GI, Khokhlova IS (2004a) Relationships between parasite abundance and the taxonomic distance among a parasite’s host species: an example with fleas parasitic on small mammals. Int J Parasitol 34:1289–1297
Krasnov BR, Khokhlova IS, Burdelov NV, Mirzoyan NS, Degen AA (2004b) Fitness consequences of density-dependent host selection in ectoparasites: testing reproductive patterns predicted by isodar theory in fleas parasitizing rodents. J Anim Ecol 73:815–820
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Lummaa V, Clutton-Brock T (2002) Early development, survival and reproduction in humans. Trends Ecol Evol 17:141–147
Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE (2004) Helminth parasites—masters of regulation. Immunol Rev 201:89–116
Marshall AG (1981) The ecology of ectoparasitic insects. Academic Press, USA
Marshall DJ, Uller T (2007) When is a maternal effect adaptive. Oikos 116:1957–1963
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Møller AP, Christe P, Garamszegi LZ (2005) Coevolutionary arms races: increased host immune defense promotes specialization by avian fleas. J Evol Biol 18:46–59
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Nuismer SL, Thompson JN (2006) Coevolutionary alternation in antagonistic interactions. Evolution 60:2207–2217
Parker GA, Begon M (1986) Optimal egg size and clutch size – effects of environment and maternal phenotype. Am Nat 128:573–592
Poulin R (2007) Evolutionary ecology of parasites: from individuals to communities. Princeton University press, Princeton
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Rossiter MC (1991) Environmentally-based maternal effects: a hidden force in insect population dynamics? Oecologia 87:288–294
Rossiter MC, Schultz JC, Baldwin IT (1988) Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69:267–277
Silverman J, Appel AG (1994) Adult cat flea (Siphonaptera: Pulicidae) excretion of host blood proteins in relation to larval nutrition. J Med Entomol 31:265–271
Singh SK, Girschick HJ (2003) Tick-host interactions and their immunological implications in tick-borne diseases. Curr Sci 85:1284–1298
van der Mescht L, Khokhlova IS, Warburton EM, Krasnov BR (2017) Parasite performance and host alternation: is there a negative effect in host-specific and host-opportunistic parasites. Parasitology 144:1107–1116
Wagenmakers EJ, Farrell S (2004) AIC model selection using Akaike weights. Psychon Bull Rev 11:192–196
Walker M, Steiner S, Brinkhof MW, Richner H (2003) Induced responses of nestling great tits reduce hen flea reproduction. Oikos 102:67–74
Xun H, Qi Y-M (2004) Histochemistry of three enzymes in newly emerged and engorged adults of rat fleas Monopsyllus anisus (Rothschild) and Leptopsylla segnis (Schönherr). Acta Entomol Sin 47:444–448 in Chinese
Funding
This study was supported by the Israel Science Foundation (Grant number 149/17 to BRK and ISK). LVDM received financial support from the Blaustein Center for Scientific Cooperation and the French Associates Institute for Agriculture and Biotechnology of Drylands. EMW received financial support from the Blaustein Center for Scientific Cooperation. This is publication no. 1018 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures met the requirements of the 1994 Law for the Prevention of Cruelty to Animals (Experiments on Animals) of the State of Israel.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
van der Mescht, L., Khokhlova, I.S., Surkova, E.N. et al. Reproductive performance in generalist haematophagous ectoparasites: maternal environment, rearing conditions or both?. Parasitol Res 118, 2087–2096 (2019). https://doi.org/10.1007/s00436-019-06353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06353-3