Abstract
Parasites should make the same decisions that every animal makes regarding fitness reward. They can maximize reproductive success by selection of those habitats that guarantee the greatest fitness output. We consider the host population as a habitat of a parasite population. Consequently, hosts (=habitats) that differ quantitatively or qualitatively will support different numbers of parasites. The nature of habitat selection can be detected by isodars, lines along which habitat selection yields equivalent fitness reward. We applied this approach to study host selection of five fleas, each infesting two desert rodents. Xenopsylla conformis, Xenopsylla ramesis, Nosopsyllus iranus theodori and Stenoponia tripectinata medialis parasitize Gerbillus dasyurus and Meriones crassus. Synosternus cleopatrae pyramidis parasitizes Gerbillus andersoni allenbyi and Gerbillus pyramidum. Three fleas (X. conformis, X. ramesis and S. c. pyramidis) were able to perceive quantitative (amount of the resource; e.g. organic matter in the nest for flea larvae) and/or qualitative (pattern of resource acquisition; e.g. host defensiveness) differences between hosts. Two other fleas did not perceive between-host differences. X. conformis was a density-dependent host selector that showed sharp selectivity at low density. X. ramesis and S. c. pyramidis were density-independent host selectors with a direct correspondence of density with habitat quality. N. i. theodori and S. t. medialis were non-selectors with no relationship at all between density and host quality. The results of the application of the isodar theory suggest that ectoparasites, like other animals, behave as if they are able to make choices and decisions that favour environments in which their reproductive benefit is maximized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A common theme in modern evolutionary ecology is that the behaviour of an individual is greatly influenced by the evolutionary motivation to maximize life-time fecundity (Lomnicki 1988). One of the mechanisms to maximize reproductive success is to select those habitats that guarantee the greatest fitness output. This statement is a keystone of the theory of habitat selection (Rosenzweig 1981, 1989, 1991) that is based on the mechanism of the ideal free distribution (IFD; Fretwell and Lucas 1970). According to this theory, fitness is a negative function of population density. Consequently, the relationships between fitness and density will be reflected in the distribution and abundance of individuals across habitats. Habitats that differ quantitatively or qualitatively will support different numbers of individuals.
Habitat selection has been studied extensively in various animal taxa. However, most studies have been conducted on free-living animals, whereas habitat selection in parasites attracted much less attention. The main reason for this deficiency from an evolutionary ecology perspective is related to the common parasitological approach in which a parasite is considered as the "object" rather than the "subject" of the environmental and evolutionary processes that influence its behaviour and habitat selection (Sukhdeo and Bansemir 1996; Poulin and Morand 2000). In other words, parasitologists rarely consider parasites as individuals that make decisions regarding foraging and reproductive success (Sukhdeo and Sukhdeo 1994). Nevertheless, parasites form a large proportion of the diversity of life. Parasitism is suggested to be more common than all other feeding strategies (Sukhdeo and Bansemir 1996) and parasites supposedly make the same decisions that every animal has to make regarding resource acquisition and fitness reward.
Parasite individuals are distributed across host individuals and, thus, the host population can be considered as a habitat of a parasite population. A habitat patch for a parasite is its individual host that provides the parasite with a place for living, foraging and mating. This is especially true for arthropod ectoparasites because their contact with the host is usually intermittent and an individual parasite during its lifetime can move from one host individual to another.
When fitness decreases with density increase, individuals should successively occupy habitats of ever declining quality as their population grows. If the form of the density-dependent relationship is known, we can infer the habitat selection response simply by examining patterns of density in different habitats using isodar analysis. Isodar theory (Morris 1987a, 1987b; 1988, 1990) allows one to test the fit of population densities across habitats to an IFD model. This theory explains the mechanisms of habitat selection and demonstrates that quantitative and qualitative differences between habitats lead to predictable and easily tested differences in population density. These differences can be expressed as characteristic isodars, which are lines of equal fitness, and are plotted in density space (N1 plotted on N2) as lines at every point of which the fitness of individuals in one habitat is equal to that of individuals in another. To the left of the isodar, there are too many individuals in the habitat with the greater fitness relative to those in the other habitat, and the fitness there is depressed. A density-dependent habitat selector should move to the alternative habitat. To the right of the isodar, fitness in the habitat with the lower fitness is depressed.
Isodars, thus, can be used to infer the nature of differences between habitats and help us to understand the form of population regulation (Morris 1987a, 1987b, Morris 1988) as follows: (1) the slopes of the isodars indicate qualitative habitat preferences as population density varies; (2) the intercepts of the isodars can be used to indicate the quantitative differences in habitat richness perceived by the consumers; and (3) non-significant regressions imply density-independent habitat selection. The isodar approach has been successfully applied to the population ecology of free-living animals (mammals — Morris 1989, 1996; Ovadia and Abramsky 1995; Abramsky et al. 1997; Shenbrot and Krasnov 2000; birds — Fernandez-Juricic 2001; fish — Rodriguez 1995). However, this approach has never been applied to the population ecology of parasites, though some are known to follow an IFD of density-dependent habitat selection (Kelly and Thompson 2000).
Fleas (Siphonaptera) are permanent satellites of higher vertebrates, being most abundant and diverse on small mammals. Fleas possess numerous traits that suggest an important role of habitat selection in their evolution and host preference. They usually alternate between periods when they occur on the body of their host and periods when they occur in its burrow or nest. In most cases, egg, larval and pupal development is entirely off-host. The larvae are not parasitic (except for a single species, Uropsylla tasmanica; Vatschenok 1988) and feed on organic debris and materials found in the burrow and/or nest of the host. The majority of rodent fleas parasitize more than one host species and the degree of association between a particular flea species and a particular host species varies (Marshall 1981). In addition, an individual flea is able to relocate from one host individual to another. This host-to-host transfer can occur either via social interactions between hosts or when a host individual visits an alien burrow or by the flea leaving its host and dispersing freely (Marshall 1981). The transfer can be between individuals of the same host species (analogous to among-patch within-habitat dispersal of free-living animals) as well as between individuals of different host species (analogous to inter-habitat dispersal).
We studied the distribution of fleas on rodents in the Negev desert, Israel and found that in habitats of non-sandy plains, each of four flea species, namely Xenopsylla conformis, Xenopsylla ramesis, Nosopsyllus iranus theodori and Stenoponia tripectinata medialis, parasitize the two most common rodent species, Gerbillus dasyurus (average adult body mass 20 g) and Meriones crassus (average adult body mass 80 g) (Krasnov et al. 1997, 1998, 1999). These two hosts co-exist in several habitats of the study area (Krasnov et al. 1996a). In sand dunes of Holot Agur and Holot Halutza, one flea species, Synosternus cleopatrae pyramidis, parasitizes two co-habitating gerbils, Gerbillus andersoni allenbyi (average adult body mass 26 g) and Gerbillus pyramidum (average adult body mass 40 g) (Krasnov et al. 1999).
We assume that the two rodent host species represent two habitats for a particular flea species. We attempt to explain flea distribution between the two hosts in the framework of the theory of habitat selection, which, in this application, can be considered as the theory of host selection. We hypothesized that fleas arrange themselves close to an IFD. We used isodars to detect the adaptive nature of their host selection. The predictions are that if fleas at various densities are able to access and choose the hosts in which their expected reproductive success is maximized, then a significant relationship will exist between the densities on the two hosts. In other words, host-selecting fleas are probably able to perceive inter-host differences and have mechanisms to select those hosts (Fretwell and Lucas 1970; Rosenzweig 1981). If fleas are unable to make a choice, then no significant isodar will be found.
Materials and methods
Study areas
The study was conducted in the Ramon erosion cirque (30°30′–30°40′N; 34°39′–35°05′E) and in Holot Agur-Holot Halutza regions (30°54′–31°00'N; 34°23′–34°42′E), in the Negev desert, Israel. The climate of the Ramon cirque is characterized by hot, dry summers (mean daily air temperature during July is 34°C) and relatively cold winters (mean daily temperature during January is 12.5°C). Annual rainfall is 100 mm on the northern rim and 56 mm in the bottom of the cirque. The dominant vegetation consists of Retama raetam, Zygophyllum dumosum, Artemisia monosperma, Anabasis articulata, Atriplex halimus, and Noaea mucronata.
Holot Agur and Holot Halutza are sandy regions situated in the western Negev desert. The landscape is represented by linear stabilized dunes, often with mobile ridges, covered by Artemisia monosperma, Echiochilon fruticosum and R. raetam. Average annual rainfall in the area is 108 mm, whereas mean daily air temperature during July is 25°C and that during January is 11.1°C.
Flea species
X. conformis and X. ramesis are common ectoparasites of gerbils and birds throughout the Middle East. These two species have a parapatric distribution in the Ramon cirque in that X. conformis is displaced by X. ramesis on M. crassus and G. dasyurus from eastern (drier) to western (more mesic) habitats (Krasnov et al. 1997, 1998). Both species are active throughout the year, although their abundances in winter are low (Krasnov et al. 1997, 2002a). N. i. theodori and S. t. medialis are winter fleas. No imago has been found from April until December (Krasnov et al. 1997, 2002a). S. c. pyramidis is widely distributed in Israel and is characteristic of sand-dwelling gerbils (Theodor and Costa 1967; Krasnov et al. 1999). This flea is active throughout the year and does not demonstrate high seasonal variation in abundance (B. R. Krasnov and G. I. Shenbrot, unpublished data). All these flea species occur also on other rodents in the area (Krasnov et al. 1997, 1999). However, extremely low abundances and sporadic occurrence of these fleas on rodents other than M. crassus, G. dasyurus, G. a. allenbyi and G. pyramidum suggest that other rodents have little effect on the host selection process.
Rodent trapping and flea collection
Rodents were trapped on 23 plots of 1 ha in the Ramon cirque and on 16 plots of 1 ha in Holot Agur-Holot Halutza region over 1998–2002. Each plot in the Ramon cirque was sampled during 1--5 consecutive days at 6-month intervals, using 50 Sherman live-traps placed in at 5×5 grid (two traps per station with an interval of 20 m between stations). Individual lifespan of the imago of the studied fleas is <2 months (B. R. Krasnov and N. V. Burdelova, unpublished data). Each sample is, thus, an independent estimate of flea abundance. The overall density of rodents was evaluated as the minimum number of individuals known to be alive. Plots in Holot Agur-Holot Halutza were sampled once using 50 Sherman live-traps placed in a line (interval of 20 m between traps). Rodent abundances estimated using grid versus transect sampling do not differ significantly (G. I. Shenbrot and B. R. Krasnov, unpublished data).
We collected fleas from each individual rodent only when captured the first time. The animal's fur was combed thoroughly, using a toothbrush, over a white plastic pan and fleas were carefully collected. The fur was brushed several times, until no additional fleas were recovered. Fleas were stored in 70% alcohol. After this, each rodent was marked by toe-clipping and released.
We estimate the density as the mean number of a particular parasite per individual of a particular host for each trapping session and a grid. This parameter is more appropriate in this study than the traditional parameter of a parasite burden (mean number of parasites per infested host individual) because it incorporates all available habitat (=host) patches rather than only those patches being used.
We also calculated flea density per 1 cm2 of rodent body surface. Fleas are usually found over all the body of a rodent except for the head and the tail, partly because these body parts are most easily accessible for self-grooming. The body surface of a rodent was calculated as that of a cylinder (TB-TL-GSL)×BD×π, where TBL is the average total body length from the tip of the snout to the tip of the tail, TL is the average tail length, GSL is the average greatest anterior-posterior length of the skull, BD is the diameter of the body, and π=3.14. Linear measurement of living animals does not allow accurate estimates to be made. Consequently, we used average values calculated for each species. We conducted measurements of TBL, TL, GSL and BD (±1 mm) on dead adult animals of each species and found low individual within-species variation in body surface. Coefficient of variation of body surface was 3.1% for M. crassus (n=20), 4.2% for G. dasyurus (n=69), 3.8% for G. a. allenbyi (n=26) and 2.9% for G. pyramidum (n=9). In total, 944 rodents were captured and 4,428 fleas were collected.
Isodar analysis
Interpretation of the isodar analysis follows Morris (1987a, 1987b, 1988). Situations where habitats differ quantitatively only, result in isodars with a non-zero intercept and slope equal to 1.0 (parallel population regulation), whereas situations where habitats differ qualitatively only, result in isodars with zero intercept and a slope >1.0 (divergent population regulation). If habitat 1 is qualitatively and quantitatively more suitable than habitat 2, the resulting isodar has a non-zero intercept and a slope >1.0 (divergent population regulation maintained) (Morris 1988). If a quantitatively superior habitat is also the one with the lower foraging efficiency, the slope of the fitness function with density is steeper in the habitat with the greater resource, and the fitness curves can cross (Morris 1988). This situation produces an isodar with a non-zero intercept and a slope <1.0 (crossover population regulation). Finally, if habitat 2 is still qualitatively less suitable but quantitatively superior so that its carrying capacity is greater than that of habitat 1, the resulting isodar also has a non-zero intercept and a slope <1.0 (convergent population regulation), but only for crossovers does the isodar pass through N 2=N 1 before reaching carrying capacity. Note that in the two latter cases the plot is N 2 versus N 1 (Morris 1988). Substantiation of the above statements is explicitly discussed in Morris (1987a, 1987b; 1988).
We performed isodar analysis by plotting flea density estimates (per 1 cm2 of body surface) for each replicate of the host species whose infestation was higher (analogous to high density treatment of Morris 1987a) against the corresponding (i.e. calculated for the same grid) estimates for the host species whose infestation was lower (analogous to low density treatment of Morris 1987a) and by analysing the data by model II regression. Linear regressions were generated for each of the significant correlations, and the slope of the reduced major axis (Sokal and Rolf 1995; Legenre and Legendre 1998) and its confidence interval were calculated to allow comparison with the predicted slope of 1.0. Following Morris (1987a, 1987b, 1988), we assumed that density-dependent feedback on population growth rate is linear with increasing density. We also looked for curvilinear effects in isodars (continuous input model, Morris 1994). To differentiate between linear and curvilinear effects, we plotted flea density estimates for a pair of host species on both arithmetic and logarithmic scales and generated model II regressions. We did not test for non-linear effects in isodars because there was no hint of non-linearities in the data (pre-emptive habitat selection model, Morris 1994). Points with zero density on one of the hosts were omitted from the analyses as they could inflate the error about the regression because quantitative differences between host species allow fleas to occupy a variety of densities on the alternative host while still behaving optimally with respect to habitat selection.
Results
Flea distribution on G. dasyurus and M. crassus
The average density of X. conformis on M. crassus was 13.78±0.11 per host individual, ranging from 4 to 37. The density of this flea on G. dasyurus was significantly lower and averaged 0.71±0.11 fleas per host individual, ranging from 0 to 4 (Wilcoxon matched pairs test, Z=4.22, P<0.001). Similarly, density of X. ramesis on M. crassus was 11.12±2.21 per host individual, ranging from 3 to 36, whereas on G. dasyurus the density of this species attained 0.28±0.03 per host individual only within the range from 0 to 6 (Wilcoxon matched pairs test, Z=3.53, P<0.001).
In contrast to Xenopsylla species, the density of N. i. theodori did not differ significantly between M. crassus and G. dasyurus (1.72±0.29 per host individual, range 1–8 and 2.13±0.47, range 1–10, respectively, Wilcoxon matched pairs test, Z=0.22, P=0.83). A non-significant difference between densities on different hosts was also the case for S. t. medialis. The density of this flea was 1.57±0.43 per host individual on M. crassus (range 1–3) and 0.82±1.18 per host individual on G. dasyurus (range 0–2) (Wilcoxon matched pairs test, Z=0.56, p=0.57).
M. crassus is 4 times heavier than G. dasyurus. At a first glance, this can explain why M. crassus supported more fleas than G. dasyurus. However, it is obvious that body surface rather than body mass is of primary importance in relation to the number of supported ectoparasites. Average body surface of M. crassus exceeds that of G. dasyurus by 2.3 times only (125.05 cm2 versus 55.01 cm2). When the parameters of flea density were recalculated per 1 cm2 of body surface, the higher density of X. conformis and X. ramesis on M. crassus compared with that on G. dasyurus remained significant (Wilcoxon matched pairs tests, Z=4.28 and Z=3.72, respectively, P<0.001). The absence of a difference in N. i. theodori and S. t. medialis density between M. crassus and G. dasyurus also remained after this recalculation (Wilcoxon matched pairs tests, Z=1.34 and Z=1.27, respectively, P>0.2).
Flea distribution on G. a. allenbyi and G. pyramidum
Average density of S. c. pyramidis on G. a. allenbyi was 3.93±0.58 (range 1–9), whereas it was significantly higher on G. pyramidum, attaining 18.51±3.65 (range 3–50) (Wilcoxon matched pairs tests, Z=2.19, P<0.02). G. pyramidum is almost twice as heavy as G. a. allenbyi, but the body surface of G. pyramidum exceeds that of G. a. allenbyi by 1.3 times only (79.99 cm2 versus 59.63 cm2). The significance of a between-host difference in flea density remained also after recalculation of flea density per 1 cm2 of body surface (Wilcoxon matched pairs tests, Z=2.22, P<0.02).
Isodar analysis
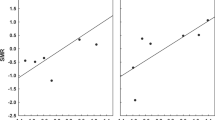
We performed isodar analyses for each of five flea species with flea density on M. crassus (for X. conformis, X. ramesis, N. i. theodori, and S. t. medialis) or G. pyramidum (for S. c. pyramidis) as the dependent variable and flea density on G. dasyurus (for all fleas except S. c. pyramidis) or G. a. allenbyi (for S. c. pyramidis) as the independent variable. The results of linear regression analyses (model II) are presented in Table 1; the isodars are illustrated in Fig. 1. Briefly, both the intercept and slope of the isodar of X. conformis were significant and the slope was >1.0. Isodars of X. ramesis and S. c. pyramidis had significant slopes (>1.0 for both species), but their intercepts did not differ significantly from zero. Finally, the analyses failed to produce significant isodars for N. i. theodori and S. t. medialis.
Isodars of five flea species parasitizing rodents in the Negev desert. Solid lines represent the (model II) best-fit regression, dashed curves are the 95% confidence intervals about the regression. X. conformis Xenopsylla conformis, G. dasyurus Gerbillus dasyurus, X. ramesis Xenopsylla ramesis, N. i. theodori Nosopsyllus iranus theodori, S. t. medialis Stenoponia tripectinata medialis, S. c. pyramidis Synosternus cleopatrae pyramidis, G. a. allenbyi Gerbillus andersoni allenbyi
Isodars for X. conformis, X. ramesis and S. c. pyramidis gave a better fit to linear models than to curvilinear ones (arithmetic scale: r 2=0.31, n=20, P<0.01; r 2=0.30, n=17, P<0.01 and r 2=0.64, n=15, P<0.001, respectively, and logarithmic scale: r 2=0.12, n=20, P=0.11; r 2=0.02, n=17, P=0.28 and r 2=0.22, n=15, P=0.06, respectively). No significant logarithmic isodars were produced for N. i. theodori and S. t. medialis.
Discussion
Host (=habitat) selection in fleas: an isodar analysis
The isodar analysis of X. conformis host selection with a slope significantly >1.0 and an intercept significantly >0 confirms the perception of M. crassus as both quantitatively (differences in the amount of the resource) and qualitatively (differences in the pattern of resource acquisition) superior host compared with G. dasyrus (Morris 1987a, 1987b, 1988). Isodars for X. ramesis and S. c. piramidis also have slopes significantly >1.0, also indicating divergent population regulation of these fleas. However, intercepts of these isodars do not differ significantly from zero. This shows that M. crassus and G. pyramidum were perceived as qualitatively more suitable hosts for X. ramesis and S. c. pyramidis, respectively, than G. dasyurus and G. a. allenbyi, but quantitatively these hosts were identical for these fleas. Finally, no isodars were produced for N. i. theodori and S. t. medialis, which means that these fleas did not perceive the differences between M. crassus and G. dasyurus.
An example of a quantitative between-host difference from a flea "viewpoint" can be the amount of organic matter in the nest chamber available as food for flea larvae. This amount can depend on the material of the nest, the time that a host spends in the nest, and the behaviour of a host. M. crassus and G. dasyurus differ in their pattern of burrow use, although the evidence for this is circumstantial. Areas inhabited by X. conformis (but not X. ramesis) are characterized by a sandy-gravel substrate (Krasnov et al. 1998). Mark-recapture and radiotracking of M. crassus demonstrated that this species was strongly territorially conservative both in relation to its home range within a season and in relation to its burrow within a daily period of activity (Krasnov et al. 1996b). This pattern of burrow use presumably increases the amount of organic matter in the burrow, which is especially important for flea larvae in an organic-poor sandy-gravel substrate. In contrast, G. dasyurus in these areas is less territorially conservative. A high proportion of individuals tend to be transients and do not possess permanent burrows (Khokhlova et al. 2001; Krasnov et al. 2002b).
As mentioned above, X. ramesis did not perceive quantitative between-host differences. Areas inhabited by X. ramesis are characterized by organic-rich loess substrate (Krasnov et al. 1998). Both M. crassus and G. dasyurus here are territorially conservative and both possess permanent burrows with well-constructed nests (Krasnov et al. 1996b; Shenbrot et al. 1997). Thus, there was no sharp between-species difference in the amount of organic matter in the burrow.
No information on the burrow structure of G. a. allenbyi and G. pyramidum is available. Nevertheless, we suppose that the mobile sand in their habitats does not allow a great variety of burrow constructions. G. a. allenbyi and G. pyramidum demonstrated temporal partitioning of habitat use (e.g. Kotler et al. 1993). However, the overall amount of time spent out of the burrow seems to be similar [calculation from data published by Kotler et al. (1993); mean time period that a species was foraging at a station multiplied by the number of foraging stations visited per night]. This suggests no quantitative between-host difference for S. c. pyramidis.
Examples of qualitative differences are between-host variation in the amount of blood that an individual insect obtains during a bloodmeal (e.g. Webber and Edman 1972) and the efficiency of blood digestion by a flea (e.g. Vatschenok 1988). Hosts also vary in their skin structure (relative ease of blood sucking), fur density (relative ease of flea movement) and defensiveness against bites (e.g. grooming). For example, smaller species with a larger body surface:body mass ratio can supposedly afford fewer fleas per unit surface area than larger species and, thus, should be more defensive. Indeed, body size has been shown to be the determinant of the level of defensiveness and, as a result, of ectoparasite infestation (Gallivan and Horak 1997; Mooring et al. 2000). However, at present, we have no data on qualitative difference between either pair of studied hosts. Many questions are in need of experimental tests and remain to be answered. For example, the coevolutionary predictions on the tolerance of different rodent species to their flea burdens can be tested.
The divergent fitness-density curves suggest that there is a divergence in habitat suitability with density and that, at any given density, consumers in one habitat are more efficient at converting resources into offspring than they are in another habitat (Morris 1987a, 1987b, 1988). The effect of density on reproductive success is greater in those habitats where the foraging is less efficient. In our case, the fitness rewards for individuals of two Xenopsylla species and S. c. pyramidis parasitizing M. crassus or G. pyramidum, respectively, appeared to be higher than for those parasitizing G. dasyurus or G. a. allenbyi, respectively. Furthermore, at low density, X. conformis parasitized the "best" hosts only, whereas with an increase in flea population size, the additional host was also parasitized. In contrast, X. ramesis and S. c. pyramidis at low density appeared to parasitize both hosts equally because they seemed to be able to achieve similar maximum fitness under such conditions. However, with an increase of the flea population size, parasite pressure on the "high-quality" host increased at a higher rate than that on the "low-quality" host. In conclusion, X. conformis appeared to be a density-dependent host (=habitat) selector. It showed sharp selectivity at low density (Rosenzweig and Abramsky 1985; Morris 1988). In contrast, X. ramesis and S. c. pyramidis appeared to select hosts (=habitats) randomly at very low densities. They showed density-dependent habitat selection with a preference for the largest species at high densities. Following Morris (1988), these species can be considered to be density-independent habitat selectors with a direct correspondence of density with habitat quality. Data that would allow us to evaluate the difference in reproductive success of a flea species parasitizing different hosts and, thus, to justify supposed fitness-density relations are scarce. Future experiments and observations on the differences in fitness of fleas exploiting different hosts are needed. For example, egg production in a flea Parapulex chephrenis declined sharply when it fed on a gerbil Gerbillus dasyurus than when it fed on a spiny mouse Acomys cahirinus (Krasnov at al. 2002c).
Isodar analyses that included N. i. theodori and S. t. medialis were non-significant. This means that that there was no relationship between the densities of fleas on the hosts. These fleas can be called non-selectors (sensu Rosenzweig and Abramsky 1985) or density-independent selectors with no relationship at all between density and host quality (sensu Morris 1988). What is so special about these fleas that they seem to be unable to distinguish between two host species? First, in contrast to Xenopsylla and Synosternus, fleas of Nosopsyllus and Stenoponia genera possess combs (ctenidia) that permit the flea to anchor itself within the host's fur and to resist grooming (e.g. Traub 1972). Consequently, host defensiveness (qualitative between-host difference) is much less important for them than for fleas without ctenidia. Second, N. i. theodori and S. t. medialis differ from other fleas of this study in that their activity period is very short and limited to winter, when the availability of plant material for nest construction as well as seeds for storage by rodent hosts is highest. We suggest that the difference between M. crassus and G. dasyurus burrows are "smoothed" under such conditions and, thus, the quantitative between-host differences disappear. These two fleas oversummer mainly as cocooned stages (Krasnov et al. 2002a), so the most critical period when the availability of organic matter is of crucial importance is winter. An alternative explanation can be that the short period of activity during which fleas have to feed, copulate, oviposit and the restricted time that their offspring have to hatch, moult 3 times, and spin a cocoon, does not provide fleas with enough time for host selection decisions. This corresponds with Morris' (1988) conclusion that the isodar regression is statistically non-significant in those cases when population size is limited by extrinsic factors below which intraspecific density affects fitness.
In conclusion, the results of the application of the isodar theory to flea data suggest that ectoparasites, like other animals, behave as if they are able to make choices and decisions that favour environments in which their reproductive benefit is maximized. Moreover, this implies that a flea should have information on average host quality. It is generally unknown how fleas make these cost/benefit decisions and by which means they evaluate quantitative and qualitative between-host differences. Nevertheless, evolution of host specificity in fleas suggests that they have evolved host choice in an IFD-like way. On one hand, their fitness reward differs when they parasitize different hosts (e.g. Prasad 1969; Krasnov et al. 2002c). On the other hand, they are able to discriminate between different hosts and to select the better one using, for example, odour cues (Krasnov et al. 2002c)
Isodar theory and flea censuses
There are some complexities associated with the application of isodar analysis to census data (Morris 1988). However, they seem to be easily overcome in the analysis of a rodent-flea system. For example, habitat patches and discrete borders need to be unambiguously defined. This is exactly the case if we consider a host as a habitat patch. Another complexity is that the spatial scale of the habitat patches should be large enough to obtain the required density estimates but small enough to ensure adequate replication. Again, this is exactly the case for fleas and rodents. Additionally, the patch distribution should guarantee that individuals have the opportunity to migrate between patch types while they jointly assess density and fitness and that each individual has a choice between two different habitats (Morris 1988). Otherwise, the isodar signal will be difficult to detect. Indeed, fine-scale spatial distribution of desert rodents is characterized by aggregations of individuals of different species on certain patches (Shenbrot et al. 1999), which increases the probability of between-host contacts and supposedly allows fleas to evaluate relative between-host quality and to choose the preferable host. Furthermore, fleas can transfer from host to host by dispersal or during between-host contact. Individual fleas can disperse rather long distances (Bates 1961; Marshall 1981; Kuznetzov et al. 1999). Direct contact between different host species has been shown to ensure host-to-host flea transfer (Krasnov and Khokhlova 2001).
Finally, IFD is the most important assumption of the isodar analysis. Parasites are considered to respond according to IFD predictions (e.g. Kelly and Thompson 2000), albeit with some reservations (see Sukhdeo and Bansemir 1996). A negative fitness-density relationship in parasites has been shown for both endoparasites (Croll et al. 1982) and ectoparasites (Tripet and Richner 1999). Kelly and Thompson (2000) developed an IFD model of host choice by blood-sucking insects based on the argument that insect vectors must have evolved to choose the least defensive hosts in order to maximize their feeding success. They argue that an individual blood-sucking insect can improve its feeding success (and, consequently, its fecundity considered as fitness reward) by choosing a host with a higher intrinsic quality, a lower defensiveness and a smaller number of blood-sucking competitors (Kelly and Thompson 2000). This model has been applied to parasite individuals distributed both within host species and between host species. Moreover, the application of the IFD model to parasite distribution over host populations explains the overdispersion of a parasite population across a host population (Sutherland 1996; Kelly and Thompson 2000). Thus, we had a good theoretical basis on which to assume that the distribution of fleas across the populations of their hosts conforms to the IFD pattern.
References
Abramsky Z, Rosenzweig ML, Subach A (1997) Gerbils under threat of owl predation: isoclines and isodars. Oikos 78:81–90
Bates JK (1961) Field studies on the behaviour of bird fleas. I. Behaviour of the adults of three species of bird fleas in the field. Parasitology 52:113–132
Croll NA, Anderson RM, Gyerkos TW, Ghardian E (1982) The population biology and control of Ascaris lumbricoides in a rural community in Iran. Trans R Soc Trop Med Hyg 76:187–197
Gallivan GJ, Horak IG (1997) Body size and habitat as determinants of tick infestations of wild ungulates in South Africa. S Afr J Wildl Res 27:63–70
Fernandez-Juricic E (2001) Density dependent habitat selection of corridors in a fragmented landscape. Ibis 143:278–287
Fretwell SD, Lucas HL (1970) On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor 19:16–36
Kelly DW, Thompson CE (2000) Epidemiology and optimal foraging: modeling the ideal free distribution of insect vectors. Parasitology 120:319–327
Khokhlova IS, Krasnov BR, Shenbrot GI, Degen A (2001) Body mass and environment: a study in Negev rodents. Isr J Zool 47:1–14
Kotler BP, Brown JS, Subach A (1993) Mechanisms of species coexistance of optimal foragers: temporal partitioning by two species of sand dune gerbils. Oikos 67:548–556
Krasnov BR, Khokhlova IS (2001) The effect of behavioural interactions on the exchange of flea (Siphonaptera) between two rodent species. J Vector Ecol 26:181–190
Krasnov BR, Shenbrot GI, Khokhlova IS, Ivanitskaya EI (1996a) Spatial structure of rodent community in the Ramon erosion cirque, Negev highlands (Israel). J Arid Environ 32: 319–327
Krasnov BR, Shenbrot GI, Khokhlova IS, Degen AA, Rogovin KA (1996b) On the biology of Sundevall's jird (Meriones crassus Sundevall) in Negev Highlands, Israel. Mammalia 60:375–391
Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, Khokhlova IS (1997) Host-habitat relation as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev Desert. Parasitology 114:159–173
Krasnov BR, Shenbrot GI, Medvedev SG, Khokhlova IS, Vatschenok VS (1998) Habitat-dependence of a parasite-host relationship: flea assemblages in two gerbil species of the Negev Desert. J Med Entomol 35:303–313
Krasnov BR, Hastriter M, Medvedev SG, Shenbrot GI, Khokhlova IS, Vaschenok VS (1999) Additional records of fleas (Siphonaptera) on wild rodents in the southern part of Israel. Isr J Zool 45:333–340.
Krasnov BR, Burdelova NV, Shenbrot GI, Khokhlova IS (2002a) Annual cycles of four flea species (Siphonaptera) in the central Negev desert. Med Vet Entomol 16:266–276
Krasnov BR, Khokhlova IS, Shenbrot GI (2002b) The effect of host density on ectoparasite distribution: an example with a desert rodent parasitized by fleas. Ecology 83:164–175
Krasnov BR, Khokhlova IS, Oguzoglu I, Burdelova NV (2002c) Host discrimination by two desert fleas using an odour cue. Anim Behav 64:33–40
Kuznetzov AA, Matrosov AN, Chyong LTV, Dat DT (1999) Movements of the synantropous rats and their fleas in the settlements of the southern Vietnam. Probl Osobo Opasnyh Infekt 79:59–65 (in Russian)
Legendre P, Legendre L (1998) Numerical ecology, 2nd English edn. Elsevier Science, Amsterdam
Lomnicki A (1988) Population ecology of individuals. Princeton University Press, Princeton, N.J.
Marshall AG (1981) The ecology of ectoparasite insects. Academic Press, London
Mooring MS, Benjamin JE, Harte CR, Herzog NB (2000) Testing the interspecific body size principle in ungulates: the smaller they come, the harder they groom. Anim Behav 60: 35–45
Morris DW (1987a) Ecological scale and habitat use. Ecology 68:362–369
Morris DW (1987b) Spatial scale and the cost of density-dependent habitat selection. Evol Ecol 1:379–388
Morris DW (1988) Habitat-dependent population regulation and community structure. Evol Ecol 2:253–269
Morris DW (1989) Density-dependent habitat selection: testing the theory with fitness data. Evol Ecol 3:80–94
Morris DW (1990) Temporal variation, habitat selection and community structure. Oikos 59:303–312
Morris DW (1994) Habitat matching: alternatives and implications to populations and communities. Evol Ecol 8:387–406
Morris DW (1996) Temporal and spatial population dynamics among patches connected by habitat selection. Oikos 75:207–219
Ovadia O, Abramsky Z (1995) Density-dependent habitat selection: evaluation of the isodar method. Oikos 73:86–94
Poulin R, Morand S (2000) The diversity of parasites. Quart Rev Biol 75:277–293
Prasad RS (1969) Influence of host on fecundity of the Indian rat flea, Xenopsylla cheopis (Roths.). J Med Entomol 6:443–447
Rodriguez MA (1995) Habitat-specific estimates of competition in stream salmonids — a field-test of the isodar model of habitat selection. Evol Ecol 9:169–184
Rosenzweig ML (1981) A theory of habitat selection. Ecology 62:327–335
Rosenzweig ML (1989) Habitat selection as a source of biological diversity. Evol Ecol 1:315–330
Rosenzweig ML (1991) Habitat selection and population interactions: the search of mechanism. Am Nat 137:5–28
Rosenzweig ML, Abramsky Z (1985) Detecting density-dependent habitat selection. Am Nat 126:405–417
Shenbrot GI, Krasnov BR (2000) Habitat selection along an environmental gradient: theoretical models with an example of Negev Desert rodents. Evol Ecol Res 2:257–277
Shenbrot GI, Krasnov BR, Khokhlova IS (1997) On the biology of Wagner's gerbil Gerbillus dasyurus (Wagner, 1842) (Rodentia: Gerbillidae) in the Negev Highlands, Israel. Mammalia 61:467–486
Shenbrot GI, Krasnov BR, Rogovin KA (1999) Spatial ecology of desert rodent communities. Springer, Berlin Heidelberg New York
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Sukhdeo MVK, Bansemir AD (1996) Critical resources that influence habitat selection decisions by gastrointestinal helminth parasites. Int J Parasitol 26:483–498
Sukhdeo MVK, Sukhdeo SC (1994) Optimal habitat selection by helminthes within the host environment. Parasitology 109:S41-S56
Sutherland WJ (1996) From individual behaviour to population ecology. Oxford University Press, Oxford
Theodor O, Costa M (1967) A survey of the parasites of wild mammals and birds in Israel. I. Ectoparasites. Israel Academy of Science and Humanities, Jerusalem
Traub R (1972) The relationship between the spines, combs and other skeletal features of fleas (Siphonaptera) and the vestiture, affinities and habits of their hosts. J Med Entomol 9:601
Tripet F, Richner H (1999) Density-dependent processes in the population dynamics of a bird ectoparasite Ceratophyllus gallinae. Ecology 80:1267–1277
Vatschenok VS (1988) Fleas — vectors of pathogens causing diseases in humans and animals. Nauka, Leningrad (in Russian)
Webber LA, Edman JD (1972) Anti-mosquito behaviour of ciconiiform birds. Anim Behav 20:228–232
Acknowledgements
We greatly appreciate the helpful comments and suggestions of Douglas Morris (Canada), Zvika Abramsky (Israel), and David Ward (South Africa) who read an earlier version of the manuscript. We thank two anonymous referees for their most helpful comments on the manuscript. This study was partly supported by the Israel Science Foundation (grant no. 663/01–1 to B. R. K., I. S. K. and G. I. S.). This is publication no. 140 of the Ramon Science Centre and no. 363 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krasnov, B.R., Khokhlova, I.S. & Shenbrot, G.I. Density-dependent host selection in ectoparasites: An application of isodar theory to fleas parasitizing rodents. Oecologia 134, 365–372 (2003). https://doi.org/10.1007/s00442-002-1122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-002-1122-2