Abstract
In Western Europe, the Echinococcus multilocularis lifecycle is predominantly sylvatic, typically involving red foxes (Vulpes vulpes) as the main definitive hosts with Microtus spp. and Arvicola spp. as intermediate hosts. During a 4-year surveillance study (2012–2015), Danish red foxes and raccoon dogs (n = 1345) were examined for E. multilocularis. Moreover, 134 insectivores and rodents collected in South Jutland during spring and summer 2016 were examined for the presence of metacestodes. The sedimentation and counting technique and molecular typing were used to identify E. multilocularis infections in the carnivores, while the rodent livers were examined macro- and microscopically for parasite lesions. Following morphological identification of E. multilocularis adult worms, the identity was verified by sequence analysis of the 12S rRNA gene in most cases (n = 13). Echinococcus multilocularis infection was demonstrated in 19 red foxes (Vulpes vulpes) originating from only two specific areas of South Jutland, namely Højer and Grindsted, and in two raccoon dogs (Nyctereutes procyonoides), originating from Højer. In Højer, 28.5% (CI 95% 11.7–45.3) of the examined red foxes were E. multilocularis positive per year. Moreover, positive red foxes were identified each year from 2012 to 2015, while E. multilocularis positive red foxes were only identified in Grindsted in 2013 (4.0%) and 2014 (6.4%). In contrast, all collected rodents were negative for E. multilocularis. We conclude that E. multilocularis is locally endemic in South Jutland with a high local prevalence in Højer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Echinococcus multilocularis is a small tapeworm belonging to the family Taeniidae. In Europe, the typical life cycle is sylvatic, including red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) as main final hosts (Oksanen et al. 2016), and the rodent species Microtus spp. and Arvicola spp. as main intermediate hosts (Woolsey et al. 2015, 2016; Beerli et al. 2017; Romig et al. 2017). Adult E. multilocularis are localised in the small intestine, from where eggs are excreted with faeces to the environment. The intermediate hosts become infected when ingesting infective eggs from the environment. Following hatching of the eggs, the oncospheres migrate to internal organs and develop into cyst-forming metacestodes that produce protoscolices. When the final host eat an infected rodent, the metacestode stage develops to adult intestinal tapeworms. If humans accidentally ingest infective eggs, they may develop alveolar echinococcosis, a severe zoonotic disease in Europe (Bouwknegt et al. 2018). Infections in humans are rare; nonetheless, the clinical implications are critical and cause considerable public health concern due to treatment costs and high mortality if left untreated (Torgerson et al. 2008). In Denmark, no autochthonous human cases have hitherto been reported (Statens Serum Institute 2017). However, as human echinococcosis is not notifiable in Denmark, the actual number of cases is unknown.
Echinococcus multilocularis in wildlife is endemic in large parts of Europe, and infections in red foxes are reported in countries neighbouring Denmark (Lind et al. 2011; Denzin et al. 2014). In North-Western Germany, the estimated E. multilocularis prevalence in red foxes has increased from 12 to 20% between 1991 and 2005 (Berke et al. 2008), and in Central Germany from 12 to 42% between 1990 and 2009 (Staubach et al. 2011). The increasing red fox populations probably influenced E. multilocularis expansion across Europe (Davidson et al. 2012; Liccioli et al. 2015). Furthermore, raccoon dogs are observed as final hosts for E. multilocularis throughout Europe (Schwarz et al. 2011; Bružinskaitė-Schmidhalter et al. 2012; European Food Safety Authority 2015; Laurimaa et al. 2015). The raccoon dog originates from East Asia but is now widespread in Northern and Eastern Europe including Denmark (Wilson and Reeder 2005). The presence of red foxes and raccoon dogs in Denmark and the mild and humid climate favours the survival of E. multilocularis eggs and the spread of infection (Takeuchi-Storm et al. 2015), as does the presence of intermediate hosts (Winge 1908; Baggøe and Jensen 2007).
The first wildlife E. multilocularis infection in Denmark was diagnosed in 1999 in an epidemiological study of helminth infections in 1040 red foxes (Saeed et al. 2006). The infected red fox was road killed west of Copenhagen and harboured 53 adult worms. Additional two red foxes from the Copenhagen area were diagnosed with E. multilocularis in 2000 by the sedimentation and counting technique (SCT) and confirmatory PCR (Saeed et al. 2006). Throughout the following decade, only few red foxes were screened for E. multilocularis infections, i.e. from 2006 to 2008; 118 red foxes from the greater Copenhagen area and South Jutland were examined for E. multilocularis, none were positive (Al-Sabi et al. 2014); and from 2009 to 2012, 99 raccoon dogs and 384 red foxes from all regions of Denmark were examined for E. multilocularis (Al-Sabi et al. 2013). In the latter study, one red fox shoot in November 2011 in South Jutland was E. multilocularis positive, harbouring 20 adult worms (Enemark et al. 2013; Al-Sabi et al. 2013). This finding led to a 4-year surveillance program for E. multilocularis in Danish wildlife initiated in 2012 and a survey of intermediate hosts collected in South Jutland in 2016. The results are presented in this paper.
Materials and methods
Study design
A national E. multilocularis surveillance program was implemented for wild carnivores in Denmark 2012–2015. The surveillance program was designed by the National Veterinary Institute, The Technical University of Denmark (DTU-VET) and financed by the Danish Veterinary and Food Administration. Selection of carnivores for the study was-risk based with a special focus on Mid- and South Jutland due to close proximity of this region to the endemic area of North Germany. This sampling strategy implied a variation in the number of examined animals from each region. The foxes were road-killed or hunted wild carnivores collected by the Danish Nature Agency or by local hunters. The geographical origin of the carnivores was recorded. However, if the exact location was not available, the coordinates of the region were used instead.
Intermediate hosts were collected in 2016 in Margrethe Kog, a marshland in the Højer area, in the Southwestern part of Jutland adjacent to the German border. The rodents were trapped alive (Uggland-special, Grahnab, Sweden) or by using mechanical traps (Topcats, Andermatt Biocontrol AG, Switzerland).
Sampling
A total of 1345 wild carnivores, collected 2012–2015, and 134 rodents and insectivores collected during spring and summer 2016 were included in the study. Of the carnivores, 1073 were red foxes (V. vulpes) and 272 raccoon dogs (N. procyonoides).
Of the rodents, eight were common voles (Microtus arvalis), 15 field voles (Microtus agrestis), 31 wood mice (Apodemus sylvaticus) and 15 harvest mice (Micromys minutus). Of the insectivores, 47 were common shrews (Sorex araneus), and 16 pygmy shrews (Sorex minutus).
Examination of wild carnivores
The dead carnivores were transported in sealed plastic bags at − 20 °C to DTU-VET and left at − 80 °C for a minimum of 5 days to inactivate potential infective parasites before necropsy. The small intestines were examined for E. multilocularis worms. When the intestine was unavailable, faeces were examined for E. multilocularis eggs by real-time PCR.
The examination for E. multilocularis worms in the small intestine was carried out as described by Hofer et al. (2000) using the sedimentation and counting technique (SCT). The sediment from the SCT was examined in small portions under a stereomicroscope, and adult tapeworms were counted and identified as E. multilocularis by morphologic features (Eckert et al. 2001) at DTU-VET. Faeces for analysis of E. multilocularis eggs was analysed by a semi-automated magnetic capture probe-based DNA extraction and real-time PCR method by the Swedish Veterinary Institute (SVA) (RT-PCR) (Isaksson et al. 2014). The number of animals analysed by SCT and RT-PCR, respectively, are listed in Table 1.
Examination of rodents and insectivores
Upon dissection, each animal liver was examined macroscopically for suspect parasite lesions by placing the liver in a plastic bag and compressing it between two microscope slides. Examination for internal lesions or cysts was performed by back-illumination of the compressed liver under a dissection microscope at × 10–40 magnification. Any white or grey spots and lesions were collected and inspected microscopically, placed in 70% ethanol and stored at 4 °C. Parasitic materials were examined by inspecting the tissue and fluid from freshly cut lesions by light microscopy. Each suspected lesion was subjected to multiplex PCR analysis initially for Echinococcus (Stieger et al. 2002) and subsequently broader for taeniids (Trachsel et al. 2007).
Molecular analysis of adult worms
DNA from adult worms collected from 11 red foxes and two raccoon dogs was isolated using Qiamp DNA mini kit according to the manufacturer’s instructions (Qiagen GmbH, Hilden, Germany). Amplification and sequencing of the 12S rRNA gene from each individual animal was done according to Stefanic et al. (2004). Using the software MEGA6 (Tamura et al. 2013), consensus sequences were established, and related sequences in GenBank were retrieved using BLASTn analysis.
Results
Wild carnivores
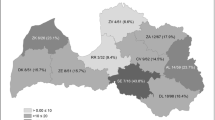
Most animals were sampled in Mid Jutland and South Jutland and only few originated from other regions, namely Funen, South-West Zealand and North-East Zealand (Fig. 1).
Most animals were examined by SCT (1234, 91.7%), while 111 (8.3%) animals were examined by RT-PCR (Table 1).
In total, 21 animals were positive for E. multilocularis. Of these, 18 were identified using the SCT and three by RT-PCR (Table 1). All E. multilocularis positive animals originated from South Jutland, bordering Germany, more specifically from the two localities Højer and Grindsted. From Højer, 28.5% (CI 95% 11.7–45.3) of the examined red foxes were E. multilocularis positive per year, and positive red foxes were identified every year of the 4-year surveillance period. Positive raccoon dogs were only identified in Højer in 2014. From Grindsted, 4.0% of the red foxes were E. multilocularis positive in 2013 and 6.4% in 2014. No positive raccoon dogs were detected in Grindsted (Table 2).
The E. multilocularis infection intensity in red foxes ranged from 2 to 1527 worms per animal (mean ± S.E 215.6 ± 383.6, median = 33), while the two positive raccoon dogs harboured 53 and 109 worms, respectively. Worm quantity in the three red foxes identified as positive by RT-PCR was not recorded.
The PCR amplification and sequencing of worms from 11 red foxes and two racoon dogs revealed a 198 bp product, which had 100% nucleotide match within the Danish isolates, and 99.5–99% nucleotide match to other E. multilocularis sequences available in GenBank (analysis date 23 July 2017). Phylogenetic analysis was inferred using the Neighbor-Joining method (Saitou and Nei 1987) and grouped the Danish isolates of E. multilocularis close to isolates from Germany, the UK and Polen (data not shown). Nucleotide sequence data from isolate no. A214 reported in this paper is available in GenBank™ under the accession number: MG593258.
Rodents and insectivores
Suspected lesions from 23 of 123 examined rodents and insectivores were negative for E. multilocularis. The PCR analysis identified five samples positive for cestodes. Further sequencing of the PCR products revealed one cyst of Taenia mustelae (field vole), two cysts of Taenia polyacantha (field vole and common vole), one cyst of Mesocestodied sp. (wood mouse) and one cyst (with clear protoscolices) of Cladotaenia sp. (field vole).
Discussion
In this study, E. multilocularis infection was investigated in 1345 Danish red foxes and raccoon dogs throughout 4 years (2012–2015), with special focus on Mid- and South Jutland (Fig. 1). Infections with E. multilocularis were demonstrated in 21 wild carnivores from only two localities in South Jutland, one of which (Højer) borders the endemic area of North Germany (Fig. 1). Infections were primarily identified in red foxes (n = 19), but for the first time in Denmark, we observed two E. multilocularis positive raccoon dogs. Likewise, none of the examined rodents or insectivores sampled in the high endemic area was positive for E. multilocularis.
In the present study, animals from the Copenhagen area (North-East Zealand) were all negative, confirming the low occurrence (0.7%) of E. multilocularis infection demonstrated in red foxes in 1999. From 2000 to 2010, no E. multilocularis positive animals were identified in Denmark, most likely due to the absence of an active surveillance program. However, in 2011, one E. multilocularis positive red fox was detected in Højer (Enemark et al. 2013; Al-Sabi et al. 2013), followed by consistent identification every year during 2012–2015. This may indicate recent invasion of infected red foxes from adjacent endemic areas or detection of established infections of low incidence in suspected areas.
Molecular analysis of the 12S rRNA gene confirmed the morphological diagnosis of the Danish isolates of E. multilocularis. However, the origin of the infections could not be established using the 12S rRNA gene, and further molecular analysis using for example microsatellite markers is advised for exploring the origin of the Danish infections (Knapp et al. 2007), which is beyond the scope of this study.
In Højer, 28.5% (CI 95% 11.7–45.3) red foxes were positive for E. multilocularis per year, and positive foxes were identified all 4 years. The consistent detection of the parasite in this area demonstrates that E. multilocularis is endemic in Højer with a high local prevalence, although infected intermediate host remains unidentified. The sample size of the rodents was probably too low to detect any positive animals, as seen in other studies. For example in an endemic area in Belgium with a fox prevalence of 24.55%, only 2 out of 1249 rodents were positive (Hanosset et al. 2008). Hence, future studies must include a larger sample size aiming at increasing the likelihood of detecting positive intermediate hosts and focus on the most important intermediate hosts in Europe like the common vole (M. arvalis), the water vole (Arvicola terrestris) (which was recently separated into Arvicola scherman and Arvicola amphibius (Wilson and Reeder 2005)) and the field vole (M. agrestis) (Stieger et al. 2002; Pleydell et al. 2004; Romig et al. 2006). In the present study, no Arvicola species were examined, and only eight M. arvalis and 15 M. agrestis were investigated. Despite infected intermediate hosts remain unidentified in Denmark, the E. multilocularis lifecycle can only be completed if infected intermediate hosts are present.
Højer is localised in South Jutland, adjacent to the German border and The Wadden Sea. The area consists of lakes, streams, reeds and marshlands; fields grazed by domestic animals and cultivated farmland. The Danish E. multilocularis infection highly likely originated from Germany, since the infection has been endemic in red foxes in North Germany for years (Berke et al. 2008; Staubach et al. 2011). However, dogs returning to Denmark after visiting other endemic European areas could have contributed to the introduction of the parasite, if they were not treated with prophylactic anthelmintics according to the guidelines of the Danish Veterinary and Food Administration and the European Food Safety Authority (No author, 2016).
In Grindsted, E. multilocularis was identified in a red fox in 2013, followed by additionally three cases in red foxes in 2014. Grindsted is located approx. 100 km north of Højer, and since red foxes can disperse over long distances (Meia and Weber 1995), it is likely that the transmission of parasites to Grindsted originate from Højer or other endemic areas, e.g. in North Germany. Alternatively, the parasite might have been introduced with infected dogs or the infection could have been present with low prevalence in the area or nearby areas all along, although undetected. Since E. multilocularis was identified in red foxes in Grindsted for two consecutive years, the environment has most likely been contaminated with infective eggs, increasing the risk of an establish parasite lifecycle in the area. However, parameters like presence of suitable intermediate hosts are also needed for the lifecycle to be fully established, and the density of these hosts play a crucial role in the transmission, establishment and maintenance of the life cycle in a given habitat. The continuous observation of positive animals in only two specific areas in Jutland could be linked to the distribution or absence of intermediate hosts in the remainder of Jutland. Giraudoux et al. (2013) have demonstrated that key rodent species can be used to describe the ranges of E. multilocularis over large areas. Moreover, in a study from southern Switzerland, an analysis of rodent communities revealed that M. arvalis was present in the E. multilocularis endemic area and completely absent from the non-endemic areas (Guerra et al. 2014). Hence, the endemic focus in Switzerland is believed to coincide with the availability of rodents. Unfortunately, our study is limited by the lack of sampling of rodents/voles from other areas than Højer, which prevents the analysis of a possible relationship between the distribution of potential intermediate hosts and the observed spread of E. multilocularis in wild animals in Denmark. According to the Danish mammal atlas (Baggøe and Jensen 2007), M. arvalis is most prevalent in the southern part of Jutland with a decreasing frequency northwards in Jutland and absents on the majority of the Danish Islands. Contrasting, M. agrestis and A. arvicola are widespread in Denmark, while A. schermann is absent in Denmark (Baggøe and Jensen 2007).
In total, 512 red foxes originating from the central and northern part of Jutland all tested negative for E. multilocularis, which may indicate either absence or very low incidence of infection in these areas. Nonetheless, the probability of detecting E. multilocularis-infected animals in low endemic areas depends on the sample size. For example, approx. 3000 red foxes were examined before the first positive red fox was identified in Sweden (Lind et al. 2011).
The finding of E. multilocularis positive raccoon dogs in Denmark is described in other epidemiological and experimental studies from Europe (Bagrade et al. 2016; Enemark et al. 2013; Kapel et al. 2006; Laurimaa et al. 2015; Schwarz et al. 2011). Together with the high biotic potential for E. multilocularis (Kapel et al. 2006), the raccoon dog can potentially contribute to the establishment and spread of E. multilocularis throughout Denmark.
In conclusion, the cluster of E. multilocularis positive red foxes in two distinct areas in South Jutland demonstrates high local prevalence, especially in Højer. This high local prevalence of E. multilocularis in red foxes in Højer suggests an established full lifecycle including both rodents and carnivores, entailing a high risk of spread to dogs and humans.
References
(2016) Reconsider timing of E. multilocularis treatment, suggests EFSA. Vet Rec 178:79
Al-Sabi MNS, Chriél M, Jensen TH, Enemark HL (2013) Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012—a comparative study. Int J Parasitol Parasites Wildl 2:144–151. https://doi.org/10.1016/j.ijppaw.2013.04.001
Al-Sabi MNS, Halasa T, Kapel CMO (2014) Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol 59:98–107. https://doi.org/10.2478/s11686-014-0214-6
Baggøe HJ, Jensen TS (2007) Dansk Pattedyratlas. Gyldendal
Bagrade G, Deksne G, Ozoliņa Z, Howlett SJ, Interisano M, Casulli A, Pozio E (2016) Echinococcus multilocularis in foxes and raccoon dogs: an increasing concern for Baltic countries. Parasit Vectors 9:615. https://doi.org/10.1186/s13071-016-1891-9
Beerli O, Guerra D, Baltrunaite L, Deplazes P, Hegglin D (2017) Microtus arvalis and Arvicola scherman: key players in the Echinococcus multilocularis life cycle. Front Vet Sci 4:216. https://doi.org/10.3389/fvets.2017.00216
Berke O, Romig T, von Keyserlingk M (2008) Emergence of Echinococcus multilocularis among red foxes in northern Germany, 1991-2005. Vet Parasitol 155:319–322. https://doi.org/10.1016/j.vetpar.2008.05.017
Bouwknegt M, Devleesschauwe B, Graham H et al (2018) Prioritisation of food-borne parasites in Europe, 2016. Euro Surveill 23:1–11. https://doi.org/10.2807/1560-7917.ES.2018.23.9.17-00161
Bružinskaitė-Schmidhalter R, Šarkūnas M, Malakauskas A et al (2012) Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 139:120–127. https://doi.org/10.1017/S0031182011001715
Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ (2012) The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol 28:239–247. https://doi.org/10.1016/j.pt.2012.03.004
Denzin N, Schliephake A, Froehlich A et al (2014) On the move? Echinococcus multilocularis in red foxes of Saxony-Anhalt (Germany). Transbound Emerg Dis 61:239–246. https://doi.org/10.1111/tbed.12026
Eckert J, Gemmell MA, Meslin F, Pawlowski ZS (2001) WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. OIE, France
Enemark HL, Al-Sabi MN, Knapp J et al (2013) Detection of a high-endemic focus of Echinococcus multilocularis in red foxes in southern Denmark, January 2013. Euro Surveill 18:2–5
European Food Safety Authority EFS (2015) EFSA journal. European Food Safety Authority
Giraudoux P, Raoul F, Afonso E et al (2013) Transmission ecosystems of Echinococcus multilocularis in China and Central Asia. Parasitology 140:1655–1666. https://doi.org/10.1017/S0031182013000644
Guerra D, Hegglin D, Bacciarini L et al (2014) Stability of the southern European border of Echinococcus multilocularis in the Alps: evidence that Microtus arvalis is a limiting factor. Parasitology 141:1593–1602. https://doi.org/10.1017/S0031182014000730
Hanosset R, Saegerman C, Adant S, Massart L, Losson B (2008) Echinococcus multilocularis in Belgium: prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet Parasitol 151:212–217. https://doi.org/10.1016/j.vetpar.2007.09.024
Hofer S, Gloor S, Müller U et al (2000) High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology 120:135–142
Isaksson M, Hagstrom A, Armua-Fernandez MT et al (2014) A semi-automated magnetic capture probe based DNA extraction and real-time PCR method applied in the Swedish surveillance of Echinococcus multilocularis in red fox (Vulpes vulpes) faecal samples. Parasit Vectors 7:583. https://doi.org/10.1186/s13071-014-0583-6
Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P (2006) Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol 36:79–86. https://doi.org/10.1016/j.ijpara.2005.08.012
Knapp J, Bart JM, Glowatzki ML, Ito A, Gerard S, Maillard S, Piarroux R, Gottstein B (2007) Assessment of use of microsatellite polymorphism analysis for improving spatial distribution tracking of Echinococcus multilocularis. J Clin Microbiol 45:2943–2950. https://doi.org/10.1128/JCM.02107-06
Laurimaa L, Süld K, Moks E, Valdmann H, Umhang G, Knapp J, Saarma U (2015) First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet Parasitol 212:200–205. https://doi.org/10.1016/j.vetpar.2015.06.004
Liccioli S, Giraudoux P, Deplazes P, Massolo A (2015) Wilderness in the “city” revisited: different urbes shape transmission of Echinococcus multilocularis by altering predator and prey communities. Trends Parasitol 31:297–305. https://doi.org/10.1016/j.pt.2015.04.007
Lind EO, Juremalm M, Christensson D et al (2011) First detection of Echinococcus multilocularis in Sweden, February to March 2011. Euro Surveill 16:4–6
Meia J-S, Weber J-M (1995) Home ranges and movements of red foxes in central Europe: stability despite environmental changes. Can J Zool 73:1960–1966. https://doi.org/10.1139/z95-230
Oksanen A, Siles-Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, la Torre G, Boufana B, Casulli A (2016) The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasit Vectors 9:519. https://doi.org/10.1186/s13071-016-1746-4
Pleydell DRJ, Raoul F, Tourneux F, Danson FM, Graham AJ, Craig PS, Giraudoux P (2004) Modelling the spatial distribution of Echinococcus multilocularis infection in foxes. Acta Trop 91:253–265. https://doi.org/10.1016/j.actatropica.2004.05.004
Romig T, Thoma D, Weible A-K (2006) Echinococcus multilocularis—a zoonosis of anthropogenic environments? J Helminthol 80:207–212. https://doi.org/10.1079/JOH2006347
Romig T, Deplazes P, Jenkins D et al (2017) Ecology and life cycle patterns of Echinococcus species. Adv Parasitol 95:213–314. https://doi.org/10.1016/bs.apar.2016.11.002
Saeed I, Maddox-Hyttel C, Monrad J, Kapel CMO (2006) Helminths of red foxes (Vulpes vulpes) in Denmark. Vet Parasitol 139:168–179. https://doi.org/10.1016/j.vetpar.2006.02.015
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schwarz S, Sutor A, Staubach C, Mattis R, Tackmann K, Conraths FJ (2011) Estimated prevalence of Echinococcus multilocularis in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr Zool 57:655–661. https://doi.org/10.1093/czoolo/57.5.655
Statens Serum Institute (2017) Bændelorm påvist på Statens Serum Institut i perioden 2005–2015. In: EPI-NYT. http://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge 4–2017.aspx. Accessed 12 Jun 2017
Staubach C, Hoffmann L, Schmid VJ, Ziller M, Tackmann K, Conraths FJ (2011) Bayesian space-time analysis of Echinococcus multilocularis-infections in foxes. Vet Parasitol 179:77–83. https://doi.org/10.1016/j.vetpar.2011.01.065
Stefanic S, Shaikenov BS, Deplazes P et al (2004) Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol Res 92:347–351. https://doi.org/10.1007/s00436-003-1043-y
Stieger C, Hegglin D, Schwarzenbach G et al (2002) Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology 124:631–640. https://doi.org/10.1017/S0031182002001749
Takeuchi-Storm N, Woolsey ID, Jensen PM, Fredensborg BL, Pipper CB, Kapel CMO (2015) Predictors of Echinococcus multilocularis prevalence in definitive and intermediate hosts: a meta-analysis approach. J Parasitol 101:297–303. https://doi.org/10.1645/14-645.1
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, Tarr PE, Halkik N, Müllhaupt B (2008) Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol 49:72–77. https://doi.org/10.1016/j.jhep.2008.03.023
Trachsel D, Deplazes P, Mathis A (2007) Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 134:911. https://doi.org/10.1017/S0031182007002235
Wilson DE, Reeder DM (2005) Mammal species of the world: a taxonomic and geographic reference. Online database from Smithson. Natl. Museum Nat. Hist. http//vertebrates.si.edu/msw/mswCFApp/msw/index.cfm 2142
Winge H (1908) Pattedyr - Bind 5 i Danmarks fauna. G.E.C. Gads Forlag, Copenhagen, Denmark
Woolsey ID, Bune NET, Jensen PM, Deplazes P, Kapel CMO (2015) Echinococcus multilocularis infection in the field vole (Microtus agrestis): an ecological model for studies on transmission dynamics. Parasitol Res 114:1703–1709. https://doi.org/10.1007/s00436-015-4355-9
Woolsey ID, Jensen PM, Deplazes P, Kapel CMO (2016) Peroral Echinococcus multilocularis egg inoculation in Myodes glareolus, Mesocricetus auratus and Mus musculus (CD-1 IGS and C57BL/6j). Int J Parasitol Parasites Wildl 5:158–163. https://doi.org/10.1016/j.ijppaw.2016.05.004
Acknowledgements
Danish hunters and the Danish Nature Agency are thanked for supplying carnivores for the surveillance study. Cynthia D Juel, Boi-Tien T Pham, Leif B Eiersted and Aleksandra Tofteby are acknowledged for skilled technical assistance. The Institute of Parasitology, Zurich University (Prof. Deplazes) and The Danish Serum Institut (Dr. Steensvold) are thanked for assistance with the analysis of cysts isolated from rodents. The study was financed by the Danish Veterinary and Food Administration, The Danish Environmental Protection Agency (Grant nr. 410239) and The Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Petersen, H.H., Al-Sabi, M.N.S., Enemark, H.L. et al. Echinococcus multilocularis in Denmark 2012–2015: high local prevalence in red foxes. Parasitol Res 117, 2577–2584 (2018). https://doi.org/10.1007/s00436-018-5947-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5947-y