Abstract
We propose a model involving the oral inoculation of Echinococcus multilocularis eggs in a vole species and examine the infection dynamics in a dose-response experiment. Defined doses, 100 (n = 8), 500 (n = 5) and 1000 (n = 5) of E. multilocularis eggs were used to inoculate Microtus agrestis. Four female C57BL/6j mice were inoculated with 1000 eggs as positive controls. The groups inoculated with 100 and 500 eggs exhibited significantly higher lesion numbers, and relatively smaller lesion size was observed in the 1000 dose group. Undetectable abortive lesions may be responsible for some form of resource limitation early in the infection, resulting in lower lesion counts and size in the 1000 dose group. The C57BL/6j mice exhibited significantly fewer lesions than M. agrestis. The feasibility of measuring corticosterone (which has been shown to downregulate Th1 cytokines) in rodent hair and tumour necrosis factor (TNF) production in spleen cells was demonstrated by a positive correlation between corticosterone levels and higher lesion counts and TNF production in C57BL/6j, respectively. These results suggest that M. agrestis is more prone to a Th2 immune response than C57BL/6j, which is associated with E. multilocularis susceptibility and may explain why the parasite develops more slowly in murine models. This is the first data to suggest that M. agrestis is capable of supporting E. multilocularis transmission and thus may be suited as a model to describe the infection dynamics in an intermediate host that affects transmission under natural conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small fox tapeworm, Echinococcus multilocularis is a zoonotic parasite distributed within arctic to temperate climates in the Northern Hemisphere (Davidson et al. 2012). Foxes (Vulpes spp.) serve as the principal definitive host, with the adult worm residing within the small intestine shedding eggs with the faeces. Rodents that serve as intermediate host become infected after ingesting eggs on contaminated vegetation. Once ingested, the eggs hatch and the oncospheres migrate to the liver, developing into metacestodes that produce protoscolices. Fox predation on infected rodents completes the transmission cycle, with the protoscolices establishing in the intestine (Eckert et al. 2011).
Experimental E. multilocularis infection in rodents can be achieved via other routes than oral inoculation. Hence, the oral inoculation of E. multilocularis eggs is referred to as primary inoculation. Secondary inoculation involves the injection of metacestode homogenates into the animal and can be achieved intraperitoneally (IP), intrahepatically (IH) or intravenously (IV). Due to the unnatural route of inoculation, the parasite bypasses the early stages of oncosphere activation and development, providing a more narrow view of E. multilocularis infection dynamics. The majority of experimental studies have utilised these methods (Matsumoto and Yagi 2008). Primary inoculation is more similar to the natural route of exposure, as the inoculated oncospheres have to pass through the gastrointestinal passage prior to liver establishment; however, due to the extensive safety measures required and difficulties in obtaining eggs, few laboratories utilise this method (Romig and Bilger 1999).
In general, murine models are used in the study of E. multilocularis, thereby taking advantage of the easily obtainable standardised animals from commercial producers. These studies have provided useful insight into E. multilocularis antigen profiles (Wang et al. 2010), the importance of the laminated layer for metacestode survival (Gottstein et al. 2002) and host-parasite interplay, particularly in regard to immunological challenge to infection and its role in determining the outcome (Gottstein 2010). Severe combined immune-deficient mice lacking functional B and T lymphocytes have been shown to be highly susceptible to E. multilocularis (Playford et al. 1993), and resistance to metacestode development is associated with a strong delayed-type hypersensitivity cellular response (Emery et al. 1996; Liance et al. 1990; Playford and Kamiya 1992), a Th-1-type cytokine profile through IL-12 (Emery et al. 1998) and tumour necrosis factor (TNF) (Amiot et al. 1999). Given that the expression of the cellular response is regulated by glucocorticoids (GCs), it can be deduced that the level of stress would affect the outcome of E. multilocularis infection (Baschant and Tuckermann 2010; Busillo and Cidlowski 2013; Vegiopoulos and Herzig 2007; Webster Marketon and Glaser 2008). Specifically, the expression of GC has been demonstrated to inhibit the transcription of IL-12 (Blotta et al. 1997; DeKruyff et al. 1998), IL-6 (Tobler et al. 1992; Waage et al. 1990) and TNF (Swantek et al. 1997), resulting in the differentiation of tolerogenic dendritic cells (TDCs) (Piemonti et al. 1999; Rutella et al. 2006) which results in the suppression of the Th-1 cellular immune immunity and upregulation of Th-2 cytokine secretion (Chamorro et al. 2009). While there is no doubt that the commonly available murine models have provided insight into the immunology and physiology of E. multilocularis infection, it remains uncertain whether these mechanism also apply in the intermediate hosts that support the natural E. multilocularis transmission, since it is the species of the family Cricetidae that are considered to be the typical intermediate hosts for E. multilocularis (Eckert et al. 2011). Hence, experimental studies concerning the susceptibility of Cricetidae species coupled with information on the immunological and endocrinological aspects that could account for varying susceptibility would be useful from an ecological perspective.
The field vole (Microtus agrestis) has a broad geographical range and is closely related to the common vole (Microtus arvalis), which is considered to be a key species in the maintenance of the parasitic cycle in Europe (Pleydell et al. 2004). In neither species, or in fact any other Cricetidae intermediate host, has primary infection, followed by a morphological, immunological and endocrinological response to various defined doses of E. multilocularis eggs, been conducted.
This study aims to test the feasibility of the vole E. multilocularis parasite model by assessing the establishment of the metacestode at three different doses of E. multilocularis eggs. The long-term corticosterone (CORT) levels and TNF production in the field vole were examined. Differences and similarities between Cricetidae and Muridae models are discussed.
Materials and method
Animals and experimental inoculation
Female (n = 12) and male (n = 6) M. agrestis, born in the summer of 2012, were obtained from METLA (Finnish Forest Research Institute, Finland). The colony was reared on 25 × 25-m field plots until shipment. Female C57BL/6j mice (n = 4) were purchased from Charles River Laboratories (Germany). Animals were individually housed and fed ad libitum, within a safety facility (Biosafety Level 2++ approved by the Danish Working Environment Authority, journal no. 20120014119/21) at the Department of Plant and Environmental Sciences (University of Copenhagen, Denmark), under experimental licence no. 2012-15-2934-00150. Animals were imported under permission from the Danish AgriFish Agency (CVR 29979812, no. 1013624417).
The E. multilocularis eggs used for inoculation were collected from the intestines of naturally infected foxes from Zurich and the surrounding area, during the official Swiss hunting season. Eggs were tested for viability by the sodium hypochlorite (s-h)-resistant test (Deplazes et al. 2005). The amount of viable eggs was determined to be 23.5 %. On 3 May 2013, the animals were anesthetised with isoflurane and by gavage feeding of egg solution, inoculated with three doses of viable eggs: 100 (n = 8), 500 (n = 5) and 1000 (n = 5) eggs. As positive controls, four female C57BL/6j mice (age 66 days) were each inoculated with 1000 eggs. All animals were euthanised 6 weeks post inoculation (p.i.) with CO2.
Immediately following euthanasia, animals were weighed, and the liver subsequently removed and lesions counted and measured along the longest plane. PCR on the lesion material was performed on randomly selected animals (100 dose group n = 4, 500 dose group n = 2). PCR was performed according to Stieger et al. (2002). Protoscolex quantification was conducted as described by (Burlet et al. 2011).
Animals were housed for 2 months prior to inoculation to ensure full acclimatisation. A group of animals allocated as negative controls and immunised controls in addition to larger groups for each dose cohort was originally planned. Due to high mortality following transport, animal numbers were not sufficient to allow this. Lack of such controls precludes analysis of specific responses to antigen stimulation; however, within the framework of the three Rs (Russell et al. 1959) in order to maximise the information gained from experimental animal use, we measured TNF production in unstimulated and Con-A stimulated cells for species comparison.
TNF assay
In a sterile petri dish with 10 ml RPMI-1640, single splenocyte cell suspensions (SPC) were prepared by mashing the spleen through a 70-μm cell strainer. The SPCs were centrifuged at 400 g for 5 min, the supernatant discarded and pellet resuspended in 1 ml RPMI-1640 before adding 1 ml 1× red blood cell lysis buffer. Samples were kept on ice for 5 min before adding 20–30 ml of phosphate-buffered saline (PBS) as stop solution. After centrifugation at 500 g for 5 min, the supernatant was discarded, the cell pellet resuspended in 1 ml RPMI-1640 and live nucleated lymphocytes counted using 0.4 % Trypan Blue (1:50) in a hematocytometer. For concentration adjustment of SPCs to 4 × 106 cells/ml, RPMI-1640/10 was used. Cells were cultured in flat-bottomed 96-well microplates at 2 × 105 cells/well (50 μl/well) with additional 50 μl/well of either RPMI-1640/10 or 2 μg/ml Con-A. After 48 h of incubation, 50 μl cell culture supernatant was collected, pooled and frozen at −80 °C until analysis. The quantification of TNF in supernatant was determined using a monoclonal mouse TNF-α ELISA kit (Invitrogen™) and expressed as picograms per millilitre. All pooled samples were analysed in duplicates.
Corticosterone analysis
Glucocorticoids can be measured in a number of matrices (blood, saliva faeces, hair) which all provide indication for differences between animals. Assessing GC quantity in hair has become an increasingly recognised method of analysing chronic stress (Russell et al. 2012), and hair cortisol concentration has been used as a biological marker in a number of animals including rhesus macaques, dogs and cats with these studies concluding that salivary (Davenport et al. 2006), faecal (Accorsi et al. 2008) and hair cortisol measurements reflect (at least to a certain extent) the same adrenal activity. The present study assess CORT from hair extracts as stated by Davenport et al. (2006), because a single postmortem sample should reflect the average CORT over the entire period of infection. Hair samples were washed twice with 5 ml isopropanol and left to dry in a protected fume hood. The hair was pulverised and homogenised with a Retsch Ball (mixer mill MM 200; 10-ml stainless steel grinding jars; single 12-mm stainless steel grinding balls) for 5 min at 30 Hz. For the extraction of steroids, 10–45 mg of sample was incubated with 1 ml methanol in microtubes with slow rotation for 24 h. The samples were centrifuged for 30 s, and 600 μl supernatant of steroid extract was collected. The collected supernatant was evaporated under a stream of nitrogen gas at 38 °C before analysis with DetectX® multispecies Corticosterone EIA kit (Arbor Assays, MI, USA). Evaporated samples were resuspended with 400 μl assay buffer provided in the kit and analysed according to the manufacturer’s instructions. All samples were run in duplicates, to avoid intra-assay variation. The lower limit of detection was 16.9 pg/ml. A Four-parameter Logistic Calibration (4PLC) online data analysis tool (http://www.myassays.com/four-parameter-logistic-curve.assay) (MyAssays Ltd) was used for calculating the concentration of corticosterone in extract, reported as picograms per millilitre, which subsequently was transformed to picograms per milligram of hair.
Statistical methods
Total lesion counts per liver were analysed in a generalised linear model against sex, weight, dose, species and CORT (PROC GENMOD). Analyses of lesion size distribution was performed by analysing the proportion of large cysts, i.e. lesion size was analysed with lesion sizes pooled into small (<2 mm) and large (≥2 mm) as compared to the total lesion number (PROC GENMOD) using the same independent variables as for the lesion number followed by stepwise exclusion of insignificant variables (p > 0.05). Similarly, differences in the TNF response for Con-A was analysed by PROC GLM. Effects were considered significant if p < 0.05. All analysis was conducted using SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA).
Results
All animals, except three (one in 100 dose group, one in 1000 dose group and one C57BL/6j), had liver lesions consistent with metacestode development, and all livers examined by PCR were found to be positive. No metacestode metastasis or establishment was found in any other organs. In two heavily infected voles where the precise number of lesions could not be determined, the number of lesions was set at 50 for the statistical analysis. These animals were in the 100 and 500 dose groups. The average lesion counts for M. agrestis was 23.9 ± 15.3, whereas the C57BL/6j mice had an average of 14.0 ± 15.4 lesions. For the 100 dose group, 24.5 % of inoculated eggs developed into single metacestode lesions, whereas the 500 and 1000 dose groups had establishment rates of 5.3 and 2.0 %, respectively (Table 1). Comparison of lesion counts demonstrated significantly higher lesions in 100 and 500 dose groups than the 1000 with C57BL/6j exhibiting significantly fewer lesions (p = 0.003). No metacestodes had developed protoscoleces, and none were found calcified as morphologically observed and confirmed by the consistency of the lesions at necropsy.
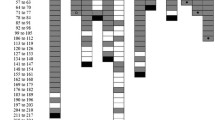
No C57BL/6j exhibited lesions >2 mm, in contrast with 71.43 % (10/14) of M. agrestis (with enumerable lesions) that did and 35.71 % had lesions >4 mm (5/14) (Fig. 1). In M. agrestis, there was a significant effect of dose with both 100 and 500 egg doses exhibiting a greater proportion of larger lesions than the 1000 dose group (p = 0.04 and p = 0.019 respectively) when adjusted for weight. With weight removed, the 500 dose group was significant (p = 0.027) while the 100 dose group demonstrated a strong tendency (p = 0.054).
The basic activity in TNF, as evidenced by the response to Con-A, differed between the two species (p = 0.002). No effect of sex or weight was noted (p’s > 0.5). There was no significant difference in CORT between species (p = 0.5). Rather, CORT was significantly correlated with lesion counts (p = 0.006), but not lesion size (p = 0.936).
Discussion
The establishment data demonstrate that M. agrestis is susceptible to E. multilocularis. This was not unexpected considering the proclivity for other species in the genus to demonstrate susceptibility (Ohbayashi et al. 1971).
Lesion counts were negatively correlated with egg dose, which could indicate an upper limit of parasite establishment in this species. However, livers were only observed macroscopically and very early abortive lesions may be have been undetectable but still responsible for some form of resource limitation early in the infection, resulting in lower lesion counts and size in the 1000 dose group. Immune protection mechanisms resulting in lower parasite survival as dose increases has been observed in a number of species including Taenia taeniaeformis in C57BL/6j mice (Mitchell et al. 1980), Haemonchus contortus in sheep (Coyne et al. 1991) and Oesophagostomum dentatum in pigs (Christensen et al. 1995). In this latter study, the proportion of immature parasites was greater in pigs receiving higher doses. Whether similar dynamics are occurring in this model will require alternative methods of detection of the parasite in the liver. No significant differences in lesion size were observed between M. agrestis and C57BL/6j. This is likely a consequence of small sample size of C57BL/6j (their primary function being positive controls for egg viability) and high S.E.M. However, none of the C57BL/6j was found to have lesions >2 mm compared to 10 of 14 M. agrestis with measurable lesions (71.43 %) that did. It is likely that a larger sample size of C57BL/6j would have demonstrated a significant difference.
No protoscolices were found in any of the animals at 6 weeks (42 days) p.i., which was expected. The cysts, however, did not appear calcified, and thus it would seem that they would have developed into larger cysts, with protoscolices at a later date. In a previous study with Microtus spp., brood capsule formation (the precursor to protoscolex development) occurred between 33–44 days post infection. In the same study, brood capsule formation in C57BL/6j mice was reported at 120–150 days p.i. (Ohbayashi 1960). Similar observations have been made in M. arvalis where protoscolices were absent in animals at 6 weeks p.i. but present at 10 weeks p.i. (Woolsey et al., unpublished data).
Although no difference was observed between TNF production in unstimulated cells in this study, the significantly higher TNF production in response to Con-A in C57BL/6j is indicative of a fundamental difference between the two species and could represent immune dynamics responsible for the significantly higher lesion counts and the presence of larger lesions in M. agrestis. Cellular (Th1) immunity is considered to be responsible for the host’s resistance to metacestode growth (Vuitton and Gottstein 2010) with such a profile characterised by the secretion of IL-2, TNF and IFN-γ (Romani et al. 1997; Vuitton 2003). Consistent with this, transgenic TNF-deficient mice were found to display higher burdens of the parasite in the liver compared to wild-type controls (Amiot et al. 1999). In canine definitive hosts, E. multilocularis antigens also suppressed Con-A-induced proliferative responses (Kato et al. 2005). It would be interesting to see if there is reduced suppression in a less susceptible definitive host such as cats. If so, it would suggest that aspects of E. multilocularis immune modulation strategy are maintained throughout the life cycle.
No differences were found in CORT between species. CORT levels were significantly correlated with higher lesion counts although there was no effect of CORT on lesion growth/size. GCs have been shown to downregulate Th1 cytokines, e.g. (Swantek et al. 1997) with murine hosts of E. multilocularis demonstrating increased establishment of the parasite when treated with synthetic GCs (Hildreth and Granholm 2003). Evidence exists that suggests E. multilocularis shifts the immune profile of the host towards Th2 (Aumüller et al. 2004; Issaadi et al. 2006). Whether there is an association between CORT and the metacestode’s induction of Th2 immunity warrants further study. Although hair CORT concentration analysis was not accompanied by additional faecal, serum or saliva CORT concentrations and there is some discussion regarding whether GC levels in hair represent systemic levels, most authors assume they do (Russell et al. 2012) and it has been shown in rhesus macaques that prolonged stressful experience produces increased levels of hair cortisol (Davenport et al. 2006). Here, the combined TNF and CORT observations would inspire the idea that M. agrestis is more prone to a Th2 response as compared to C57BL/6j.
The observations clearly suggest that M. agrestis and C57BL/6j differ in respect to establishment and early growth of E. multilocularis. Further investigations utilising M. agrestis should be conducted to determine minimum infectious dose and whether this perceived upper limit of oncosphere establishment is caused by host physiology, immune response, intraspecific competition of the parasite or a combination of these factors. Flow cytometry analysis of CD4+ and CD8+ lymphocyte populations in immunised and infected BALB/c mice demonstrated a predominance of CD4+ lymphocytes, potentially inducing the humoral response associated with parasite growth (Issaadi et al. 2006). Determining the differences in these lymphocyte profiles could shed further light on inherent susceptibility between Cricetidae species.
The TNF results provide a promising foundation for the future use of immunological assays in this model. This coupled with the proof of parasite establishment in M. agrestis, and the demonstration of the feasibility of CORT quantification in the hair of this species points to the viability of this model for testing dose levels, hormones, immunology and stress in response to E. multilocularis and provides a promising framework for comparative studies utilising a range of Cricetidae species. An in-depth understanding of the this particular model would better allow for assessing the potential spread of E. multilocularis in northern Europe and in particular Scandinavia and Great Britain (the latter ostensibly free of E. multilocularis), were M. arvalis is absent. To this, we may add that our data does certainly not preclude M. agrestis from playing a role in parasite transmission, if the suspected northern shift in transmission of this parasite occurs (Atkinson et al. 2013).
To conclude, E. multilocularis lesion counts in M. agrestis in this study was negatively correlated with egg dose. This is thought to be a result of an upper limit of oncospheres that can establish in this species, which also is associated with different metacestode growth patterns between M. agrestis and C57BL/6j. This study thus demonstrates the feasibility of utilising voles to assess metacestode establishment and development in species more ecologically relevant to parasite maintenance and the long-term stress and immunological profiles associated with infection. Crucially, it provides a background to which other Cricetidae species can be comparatively assessed for E. multilocularis susceptibility.
References
Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E (2008) Cortisol determination in hair and faeces from domestic cats and dogs. Gen Comp Endocrinol 155:398–402
Amiot F, Voung P, Defontaines M, Pater C, Dautry F, Liance M (1999) Secondary alveolar echinococcosis in lymphotoxi-α and tumor necrosis factor-α deficient mice: exacerbation of Echinococcus multilocularis larval growh is associated with cellular changes in the periparasitic granuloma. Parasite Immunol 21:475–483
Atkinson JA, Gray DJ, Clements AC, Barnes TS, McManus DP, Yang YR (2013) Environmental changes impacting Echinococcus transmission: research to support predictive surveillance and control. Glob Chang Biol 19:677–688
Aumüller E, Schramm G, Gronow A, Brehm K, Gibbs BF, Doenhoff MJ, Haas H (2004) Echinococcus multilocularis metacestode extract triggers human basophils to release interleukin-4. Parasite Immunol 26:387–395
Baschant U, Tuckermann J (2010) The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol 120:69–75
Blotta MH, DeKruyff RH, Umetsu DT (1997) Cortiscosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce il-4 synthesis in CD4+ lymphocytes. J Immunol 158:5589–5595
Burlet P, Deplazes P, Hegglin D (2011) Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit Vectors 4:6
Busillo JM, Cidlowski JA (2013) The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab 24:109–119
Chamorro S et al (2009) TLR triggering on tolerogenic dendritic cells results in TLR2 up-regulation and a reduced proinflammatory immune program. J Immunol 183:2984–2994
Christensen CM, Barnes EH, Nansen P, Roepstorff A, Slotved HC (1995) Experimental Oesphagostomum dentatum infection in the pig: worm populations resulting from single infections with three doses of larvae. Int J Parasitol 25:1491–1498
Coyne MJ, Smith G, Johnstone C (1991) A study of the mortality and fecundity of Haemonchus contortus in sheep following experimental infections. Int J Parasitol 21:847–853
Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS (2006) Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261
Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ (2012) The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol 28:239–247
DeKruyff RH, Fang Y, Umetsu DT (1998) Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 160:2231–2237
Deplazes P, Grimm F, Sydler T, Tanner I, Kapel CM (2005) Experimental alveolar echinococcosis in pigs, lesion development and serological follow up. Vet Parasitol 130:213–222
Eckert J, Deplazes P, Kern P (2011) Alveolar echinococcosis (Echinococcus multilocularis).
Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M (1998) In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol 20:81–91
Emery I, Liance M, Deriaud E, Vuitton DA, Houin R, Leclerc C (1996) Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol 18:463
Gottstein B (2010) Echinococcus spp. and echinococcosis. Acta Vet Scand 52(Suppl 1):S5
Gottstein B, Dai WJ, Walker M, Stettler M, Muller N, Hemphill A (2002) An intact laminated layer is important for the establishment of secondary Echinococcus multilocularis infection. Parasitol Res 88:822–828
Hildreth MB, Granholm NH (2003) Effect of mouse strain variations and cortisone treatment on the establishment and growth of primary Echinococcus multilocularis hydatid cysts. J Parasitol 89:493–495
Issaadi N, Fraize M, Azzouz S, Petavy AF, Sarciron ME (2006) Echinococcus multilocularis: immunity response to purified alkaline phosphatase in BALB/c mice. Parasitol Res 98:218–226
Kato N, Nonaka N, Oku Y, Kamiya M (2005) Modified cellular immune responses in dogs infected with Echinococcus multilocularis. Parasitol Res 95:339–345
Liance M, Bresson-Hadni S, Meyer JP, Houin R, Vuitton DA (1990) Cellular immunity in experimental Echinococcus multilocularis infection I. Sequential and comparative study of specific in vivo delayed-type hypersensitivity against E. multilocularis antigens in resistant and sensitive mice. Clin Exp Immunol 82:373–377
Matsumoto J, Yagi K (2008) Experimental studies on Echinococcus multilocularis in Japan, focusing on biohazardous stages of the parasite. Exp Parasitol 119:534–541
Mitchell GF, Rajasekariah GR, Rickard D (1980) A mechanism to account for mouse strain variation in resistance to the larval cestode. Immunology 39:481–489
Ohbayashi M (1960) Studies on echinococcosis X: histological observations on experimental cases of multilocular echinococcosis. Jpn J Vet Res 8:134–160
Ohbayashi M, Rausch RL, Fay FH (1971) On the ecology and distribution of Echinococcus spp. (Cestoda: Taeniidae), and characteristics of their development in the intermediate host: II. Comparative studies on the development of larval E. multilocularis. Leukart, 1863 in the intermediate host. Japanese Journal of Veterinary Research 19 (supplement):1–53
Piemonti L et al (1999) Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol 162:6473–6481
Playford MC, Kamiya M (1992) Immune response to Echinococcus multilocularis infection in the mouse model: a review. Jpn J Vet Res 40:113–130
Playford MC, Ooi H, Ito M, Kamiya M (1993) Lymphocyte engraftment conveys immunity and alters parasite development in scid mice infected with Echinococcus multilocularis. Parasitol Res 79:261–268
Pleydell DR, Raoul F, Tourneux F, Danson FM, Graham AJ, Craig PS, Giraudoux P (2004) Modelling the spatial distribution of Echinococcus multilocularis infection in foxes. Acta Trop 91:253–265
Romani L, Puccetti P, Bistoni F (1997) Interleukin-12 in infectious diseases. Clin Microbiol Rev 10:611–636
Romig T, Bilger B (1999) Animal models of echinococcosis. In: Zak O, Sande MA (eds) Animal models of infection. Academic, San Diego, pp 877–884
Russell E, Koren G, Rieder M, Van Uum S (2012) Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601
Russell WMW, Burch RL, Hume CW (1959) The principles of humane experimental technique. Methuen, United Kingdom
Rutella S, Danese S, Leone G (2006) Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108:1435–1440
Stieger C, Hegglin D, Schwarzenback G, Mathis A, Deplazes P (2002) Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitilogy 124:631–640
Swantek JL, Cobb MH, Geppert TD (1997) Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol 17:6247–6282
Tobler A, Meier R, Seitz M, Dewald B, Baggiolini M, Fey MF (1992) Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8 and IL-6, but not M-CSF in human fibroblasts. Blood 79:45–51
Vegiopoulos A, Herzig S (2007) Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275:43–61
Vuitton DA (2003) The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop 85:119–132
Vuitton DA, Gottstein B (2010) Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. J Biomed Biotechnol 2010:923193
Waage A, Slupphaug G, Shalaby R (1990) Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol 20:2439–2443
Wang X et al (2010) Production and immunoanalytical application of 32 monoclonal antibodies against metacestode somatic antigens of Echinococcus multilocularis. Parasitol Res 107:177–185
Webster Marketon JI, Glaser R (2008) Stress hormones and immune function. Cell Immunol 252:16–26
Acknowledgments
The authors would like to thank Otso Huitu and Heikki Henttonen at METLA (Finnish Forest Research Institute, Finland) for providing the voles for this study. This study was conducted under the framework of the project “Echinococcus multilocularis in Rodents (EMIRO)” funded by EMIDA, Era-Net under the EU-FP7.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woolsey, I.D., Bune, N.E.T., Jensen, P.M. et al. Echinococcus multilocularis infection in the field vole (Microtus agrestis): an ecological model for studies on transmission dynamics. Parasitol Res 114, 1703–1709 (2015). https://doi.org/10.1007/s00436-015-4355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4355-9