Abstract
The effect of infection by Echinostoma paraensei on the activity of the enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the concentration of total proteins, uric acid and urea in the hemolymph of Biomphalaria glabrata were investigated after exposure to five or 50 miracidia. The biochemical concentrations were measured weekly until the end of the fourth week after exposure. There was a significant decrease in the concentrations of total proteins in the snails exposed both to five and 50 miracidia, as well as an increase in the nitrogenous products of excretion, ALT and AST activities. The higher ALT activity in the hemolymph of the snails after infection with 50 miracidia suggests highest energetic requirement in these snails in relation to snails exposed to five miracidia. The results also suggest an increase in the use of total proteins, since there was increased formation of nitrogenous catabolites, in conformity with an increase in the aminotransferase activities, frequently associated with tissue damages. This can be explained by damage due to penetration by the miracidia and subsequent development of intramolluscan sporocysts and rediae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinostoma paraensei is a trematode species that can infect snails of genera Biomphalaria, Physa, and Lymnaea (Maldonado et al. 2001a). It was first described in naturally infected specimens of Biomphalaria glabrata (Lie and Basch 1967). Subsequently this trematode was isolated from its natural host, the wild rodent Nectomys squamipes (Maldonado et al. 2001b). Both B. glabrata and N. squamipes are natural hosts of Schistosoma mansoni (Maldonado et al. 2001c, 2006). During its intramolluscan development, E. paraensei presents both sporocyst and redia stages that migrate through the host snail’s tissues, causing tissue destruction, and impairing its metabolic processes (Fried et al. 2004; Pinheiro et al. 2004a).
Infection by trematodes can change the biochemical characteristics of the hemolymph of snails (Perez et al. 1994; Bandstra et al. 2006). The higher energy demand resulting from infection by trematodes results in redirection of the metabolic routes, such as that of the proteins. When infected by parasites, snails drastically increase their mobilization of carbohydrates. The glucose concentration in the hemolymph is severely reduced and the snails resort to their reserves from the digestive gland and cephalopedal mass. This has been shown to be related to the intoxication caused by the increase in nitrogenous products of excretion (Pinheiro and Amato 1994, 1995). This mobilization depends on various factors, such as number of parasites and age of the snail, and is followed by compensatory phenomena until the individual’s death after exhausting the energy reserves or intoxication by excess nitrogenous catabolites (de Souza et al. 2000).
Several studies have shown significant changes on the biological patterns of infected snails by different miracidial doses (Massoud 1974; Sluiters et al. 1980; Vasquez and Sullivan 2001). Théron et al. (1992) studying the functional relationship of B. glabrata/S. mansoni with different miracidial doses observed that snails subjected to plurimiracidial infections showed smaller digestive gland relative to infected snails with a low parasitic load, indicating a balance of space and energy between the development of the parasite/host. In this context, our group observed that, although changes in the reproductive profile of B.glabrata infected with E. paraensei occur, this was independent of the miracidial dose used (Tunholi et al. 2010).
In this paper, we revisit the idea of the possible interference of miracidial dose in relation to biochemical changes as additional tools for comparative studies using systemic parameters that allow a more specific way to provide many of the changes observed. For this, we analyzed the concentrations of total proteins, uric acid and urea, along with the activity of l-aspartate/2 oxoglutarate aminotransferase (E.C.2.6.1.1; AST) and l-alanine/2 oxoglutarate aminotransferase (E.C.2.6.1.2; ALT), in B. glabrata resulting from experimental infection by different miracidial doses (five or 50 miracidia) of E. paraensei as parameters of metabolic changes and tissue injury (Blasco and Puppo 1999; Pinheiro et al. 2001).
Material and methods
Obtaining the E. paraensei miracidia
Four-week-old hamsters were gastrically infected with 50 metacercariae and then euthanized and necropsied after 4 weeks of infection to obtain adult E. paraensei worms. The uterus of each helminth was dissected to release the eggs, which were incubated in dechlorinated tap water and kept in a BOD chamber at 26°C for 14 days. After this period, the eggs were exposed to light (100 W) to stimulate miracidia hatching (Pinheiro et al. 2004a, b, 2005).
Experimental infection of B. glabrata (Sumidouro, RJ isolate)
The snail specimens were obtained from a breeding stock maintained in the Laboratório de Esquistossomose Experimental of Instituto Oswaldo Cruz, Rio de Janeiro, Brazil. They were then kept in 2-L glass aquariums (ten snails/aquarium) and fed ad libitum every other day with lettuce (Lactuca sativa L.) for 1 week, to allow them to adapt to the experimental conditions. Three groups of 40 snails were formed: a control, not exposed to miracidia, and two infected. The infected groups were individually exposed to either five or 50 miracidia for 24 h (Sluiters et al. 1980; Vasquez and Sullivan 2001). These doses were used to characterize low and high parasitemia, since in nature there is no way to control these parameters and the sucess of infection is related to the presence and number of snails and miracidia. Then these snails were transferred to new aquariums and maintained as described previously. The tests were performed in duplicate.
Dissection and collection of the hemolymph
Each week after infection ten specimens from each group were randomly chosen, dissected and the hemolymph was collected by heart puncture and maintained at −10°C until utilization for the biochemical analyses. The haemocytes were removed after a centrifugation step (5 min–103 rpm).
Aminotransferases activities (AST and ALT)
To test for aminotransferases activities, 0.5 ml of substrate for ALT or AST (solution containing 0.2 M l-alanine or 0.2 M l-aspartate; 0.002 M α-cetoglutarate and 0.1 M sodium phosphate buffer, pH 7.4) was incubated at 37°C for 2 min. Then, 100 or 200 μl of hemolymph (for ALT and AST, respectively), were added, homogenized and again incubated at 37°C for 30 min. After this, 0.5 mL of 0.001 M 2,4 dinitrophenylhydrazine was added and the solution was kept at 25°C for 20 min. The reactions were interrupted by adding 5 mL of 0.4 M NaOH. The readings were taken in a spectrophotometer at 505 nm (Kaplan and Pesce 1996) and the results were expressed as URF per milliliter.
Determination of total proteins
This assay was performed according to the biuret technique (Weichselbaum 1946). A mixture of 50 μl of hemolymph and 2.5 ml of the biuret reagent (0.114 M trisodium citrate, 0.21 M sodium carbonate and 0.01 M copper sulfate) was homogenized and left at 22°C for 5 min, after which the readings were taken in a spectrophotometer at 550 nm. The results were expressed as grams per dilliliter.
Determination of the concentrations of uric acid and urea
To measure the uric acid level, 50 μl of hemolymph was mixed with 2 ml of the dye reagent (100 mmol/L of sodium phosphate buffer [pH 7.8] containing 4 mmol/L of dichlorophenol-sulfonate, 0.5 mmol/L of 4-aminoantipirina, 120U ≤ uricase, 4.980U ≤ ascorbate oxidase, 1.080U ≤ peroxidase). The mixture was homogenized and incubated at 37°C for 5 min. The readings were taken in a spectrophotometer at 520 nm (Bishop et al. 1996), and the results were expressed as milligrams per dilliliter.
The urea concentration was measured by adding 2 ml of a solution containing 60 mmol of sodium salicylate, 3.4 mmol of sodium nitroprusside and 1.35 mmol of disodium EDTA. Then 2 μl of urease and 20 μl of hemolymph were added. This mixture was homogenized and incubated at 37°C for 5 min. The readings were taken in a spectrophotometer at 600 nm, and the results were expressed as milligrams per dilliliter (Connerty et al. 1955).
Statistical analyses
The results obtained were expressed as mean±standard deviation, and the Tukey test and ANOVA were used to compare the means. A polynomial regression was calculated to analyze the relation between the values obtained and the infection time, and for the parasite load to which the snails were exposed (α = 5%; InStat, GraphPad, v.4.00, Prism, GraphPad, v.3.02, Prism, Inc.).
Results
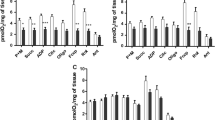
There was a significant increase in the AST and ALT activities in the snails infected with five and 50 miracidia (Table 1). There was a direct relation between the infection period and the enzyme activities, indicated by the high r 2 values (r 2 = 0.95 and 0.94 for ALT and r 2 = 0.99 and 0.99 for AST, for the snails infected with five and 50 miracidia, respectively) (Figs. 1, 2).
Relationship between the ALT activity, expressed in URF per milliliter, in the hemolymph of Biomphalaria glabrata infected by five (a) and 50 (b) Echinostoma paraensei miracidia and the time, in weeks. Zero (0) represents the mean values of the snails in the control group obtained at the end of each week (1, 2, 3, and 4 weeks). a–c Means differ significantly (mean±SD)
Relationship between the AST activity, expressed in URF per milliliter, in the hemolymph of Biomphalaria glabrata infected by five (a) and 50 (b) Echinostoma paraensei miracidia and the time, in weeks. Zero (0) represents the mean of the snails in the control group obtained at the end of each week (1, 2, 3, and 4 weeks). a–c Means differ significantly (mean±SD)
The ALT activity increased by 329% at the end of the fourth week of infection by five miracidia (123.30 ± 2.65 URF/ml) relative to the control group (28.74 ± 3.11 URF/ml; Fig. 1a). Likewise, infection with 50 miracidia caused an increase of 326% in ALT activity 4 weeks after exposure (122.67 ± 3.14 URF/ml), and this increase was even higher 3 weeks after infection (126.11 ± 4.39 URF/ml), although the difference between this result and that of the fourth week was not significant (Fig. 1b).
The AST activity also increased significantly with time of infection, with the highest values occurring 4 weeks post-infection for snails infected both with five and 50 miracidia (39.41 ± 3.44 URF/ml and 134.61 ± 1.97 URF/ml, respectively). The increase in AST activity was 3.42 times greater in the snails infected with 50 miracidia than in those infected with five miracidia (Fig. 2a, b).
The total protein levels in the hemolymph of B. glabrata infected with E. paraensei were significantly different from the uninfected specimens (Table 2). There was a strong negative relation between the time of infection and the total protein content in the hemolymph of the snails infected with five miracidia (r 2 = 0.98) (Fig. 3a) and for those infected with 50 miracidia (r 2 = 0.98) (Fig. 3b). There was a 41.46% decline in the total protein levels in the snails infected with five miracidia four weeks after infection (4.73 ± 0.51 g/dl) in comparison with the control group (8.08 ± 0.39 g/dl). This reduction was even more pronounced in the snails infected with 50 miracidia 4 weeks after infection (2.97 ± 0.29 g/dl).
Relationship between the concentration of total proteins, expressed in grams per dilliliter, in the hemolymph of Biomphalaria glabrata infected by five (a) and 50 (b) Echinostoma paraensei miracidia and the time, in weeks. Zero (0) represents the mean of the snails in the control group obtained at the end of each week (1, 2, 3, and 4 weeks). a–c Means differ significantly (mean±SD)
The infection of B. glabrata with both five and 50 E. paraensei miracidia caused changes in the metabolism for nitrogenous products of excretion, with increases in the concentrations of uric acid and urea in the hemolymph of the snails at the end of the test period (Table 2). Nevertheless, there were weak relations (r 2 = 0.53 and r 2 = 0.84) for the urea content in the snails infected with five and 50 miracidia, respectively, and weak relations as well (r 2 = 0.74 and r 2 = 0.66) for the uric acid content in the snails infected with five and 50 miracidia, respectively. Furthermore, despite the overall increase at the end of 4 weeks of infection, there were decreases of 47.64% (0.060 ± 0.008 mg/dl) and 77.37% (2.273 ± 0.293 mg/dl) in the concentrations of urea and uric acid, respectively, in the hemolymph of B. glabrata infected with five miracidia when compared with the control group (0.114 ± 0.011 mg/dl and 10.048 ± 0.201 mg/dl, respectively) one week after infection (Figs. 4a and 5a). Similar results were observed for the snails infected with 50 miracidia (Figs. 4b and 5b). The results of the control group (0 weeks of infection) did not change during the 4 weeks analyzed, for this reason they were grouped and expressed as a mean (Figs. 1, 2, 3, 4, and 5).
Relationship between the concentration of uric acid, expressed in mg/dl, in the hemolymph of Biomphalaria glabrata infected by five (a) and 50 (b) Echinostoma paraensei miracidia and the time, in weeks. Zero (0) represents the mean of the snails in the control group obtained at the end of each week (1, 2, 3, and 4 weeks). a–c Means differ significantly (mean±SD)
Relationship between the concentration of urea, expressed in mg/dl, in the hemolymph of Biomphalaria glabrata infected by five (a) and 50 (b) Echinostoma paraensei miracidia and the time, in weeks. Zero (0) represents the mean of the snails in the control group obtained at the end of each week (1, 2, 3, and 4 weeks). a–c Means differ significantly (mean±SD)
Discussion
One of the most important features of the present study is the time course assessment of infection with different miracidial doses of E. paraensei on the metabolic patterns of the snail host, B. glabrata. Ultimately, this continuation of earlier studies developed by our group yields insights into some mechanisms associated with response of B. glabrata to different miracidial doses, and it provides the first description of metabolic changes in snails infected by different doses of E. paraensei miracidia.
Many studies have shown changes in the metabolism of snails infected by larval trematodes, particularly in the concentrations of carbohydrates. The interaction between the intermediate host and the helminth parasite causes hypometabolism of snail hosts, which begin to use their metabolic reserves to maintain their vital functions, as well as to supply the energetic requirements of the larvae during their development stages. Hence, these changes lead to a negative energy balance, characterized by consumption of glycogen from digestive gland and cephalopedal mass, and galactogen deposits in the albumen gland (Pinheiro and Amato 1994). In this fashion, infected snails begin to use non-carbohydrate sources to survive and reproduce, resulting in many physiological alterations (Pinheiro et al. 2009) and a process of compensatory fecundity as observed in the E. paraensei–B. glabrata model (Tunholi et al. 2010).
The decrease in the total protein contents in the hemolymph of parasitized snails suggests the use the carbon structures obtained from gluconeogenic amino acids, because as mentioned earlier, changes in the metabolism of carbohydrates have been observed in snails under physiological stress, especially caused by infection by trematode larvae (Pinheiro and Amato 1994; Lustrino et al. 2008; Oliveira et al. 2008). In addition, the decrease in the total proteins contents in the hemolymph of snails likely would be related to the amino acid absorption by larval trematodes during development stages in the snail, because a relationship between the number of larvae to which snails were experimentally exposed and the total protein levels in the hemolymph was observed, with a reduction of 63.25% in the snails infected with 50 miracidia compared with those infected with five miracidia 4 weeks after infection. A similar result was observed by Pinheiro and Amato (1994), studying Bradybaena similaris infected with larval Eurytrema coelomaticum. These authors suggested that the sporocysts attach themselves to the outside surface of the host’s digestive gland, where they are bathed by the hemolymph and can remove glucose monomers to maintain their intense metabolic processes during larval development. Therefore, it is possible that a similar situation occurs, characterized by absorption of amino acids from proteins by the larval stages of E. paraensei.
Freshwater snails typically have a uricotelic excretion pattern because the availability of water allows uric acid to be easily removed (Becker 1980). However, changes are observed in the excretion pattern of snails exposed to adverse situations, such as infection by trematode larvae (Becker and Schmale 1975; de Souza et al. 2000).
Significant increases in the urea concentrations in the hemolymph of B. glabrata infected with both five and 50 E. paraensei miracidia were observed, with the highest concentrations at 4 weeks after infection. A similar relation occurred in the content of uric acid. This suggests that the parasites are able to stimulate the snail host to change the routes for formation of nitrogenous products of excretion during the course of the infection. Probably the increased concentration of nitrogenous products results from the increased catabolism of proteins to meet the infected snail’s energetic requirements. As a consequence of this, the amino acids are deaminated and directed to glucose synthesis, with accumulation of detoxified nitrogenous products through the formation of uric acid and urea, whose concentrations increase in the hemolymph of infected snails (Becker and Schmale 1975; de Souza et al. 2000).
After the first week of infection the concentrations of urea and uric acid decreased in the snails infected with five and 50 miracidia, although at the end of the experiment the levels of urea and uric acid increased in the infected snails. Thompson et al. (1991) also observed lower levels of urea in B. glabrata infected with S. mansoni, corroborating the results here. Similarly, de Souza et al. (2000), studying B. similaris infected by E. coelomaticum, observed a marked reduction in the levels of urea in the hemolymph of the snails from 30 until 40 days after infection, although this species is a land snail. This fact suggests that in the beginning of the infection, the snails use compensatory physiological mechanisms in the process of excretion of nitrogenous products, exhibiting another predominant mechanism of excretion, which is already postulated for the species Achatina fulica Bowdich, 1822 (Hiong et al. 2005).
The steady increases in the levels of both aminotransferases analyzed are in line with the reduction in protein levels and the increased concentrations of urea and uric acid. This is probably related to the greater gluconeogenesis activity, with higher consumption of carbon structures from the amino acids, reduction of the total protein concentrations and accumulation of nitrogenous products of excretion. AST and ALT are key enzymes related to amino acids metabolism, because they relate the amino acids to the metabolic routes involved in generating energy, particularly the Krebs cycle. The increase in the activity of these enzymes can be a sign of heightened energy demand, resulting in the mobilization of potential energy sources, including amino acids (Masola et al. 2008; Pinheiro et al. 2001).
The increases of AST and ALT were more pronounced towards the end of the pre-patent period when the formation of rediae occurs. Larvae at this stage are capable of causing damage to the host’s tissues because they have an oral aperture, by which the parasites ingest tissue fragments (Pinheiro et al. 2004a). The aminotransferases have been suggested as valid markers of tissue injury, especially in the digestive gland, an organ similar to the liver, where carbohydrates are stored, proteins are recycled and nitrogenous products of excretion are formed (Blasco and Puppo 1999; Pinheiro et al. 2001).
The results observed in this study indicate that infection with five or 50 E. paraensei miracidia causes considerable metabolic changes in the B. glabrata, mainly in digestive gland because this site is the site of various metabolic routes and by the physical contact of some larval stages that are hosted in this organ, as shown by Pinheiro et al. (2004a). Ultimately, the infection with 50 miracidia caused greater damages to snail host, as shown through increases in the AST activity and protein consumption, when compared with those snails infected with five miracidia of E. paraensei.
References
Bandstra SR, Fried B, Sherma J (2006) High-performance thin-layer chromatographic analysis of neutral lipids and phospholipids in Biomphalaria glabrata patently infected with Echinostoma caproni. Parasitol Res 99:414–418
Becker W (1980) Metabolic interrelationship of parasitic trematodes and molluscs, especially Schistosoma mansoni in Biomphalaria glabrata. Z Parasitenkd 63:101–111
Becker W, Schmale H (1975) The nitrogenous products of degradation—ammonia, urea and uric acid—in the hemolymph of the snail Biomphalaria glabrata. Z Parasitenkd 51A:407–411
Bishop ML, Duben-Engelkirk JL, Fody EP (1996) Clinical chemistry. Principles, procedures, correlations, thirdth edn. Lippincott, Filadélfia
Blasco J, Puppo J (1999) Effect of heavy metals (Cu, Cd and Pb) on aspartate and alanine aminotransferase in Ruditapes philippinarum (Mollusca: Bivalvia). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 122:253–263
Connerty HV, Briggs AR, Eaten EH (1955) Determination of blood urea nitrogen using a simple stabilizing reagent. Am J Clin Pathol 25:1321
de Souza RM, Gomes EM, Chagas GM, Pinheiro J (2000) The Influence of starvation and Eurytrema coelomaticum infection on the nitrogenads produts of degradation in the hemolymph of Bradybaena similaris. Braz Arch Biol Technol 43:365–371
Fried B, Graczyk TK, Tamang L (2004) Food-borne intestinal trematodiases in humans. Parasitol Res 93:159–170
Hiong KC, Loong AM, Chew SF, Ip YK (2005) Increases in urea synthesis and the ornithine-urea cycle capacity in the giant African snail, Achatina fulica, during fasting or aestivation, or after the injection with ammonium chloride. J Exp Zool A Comp Exp Biol 303:1040–1053
Kaplan LA, Pesce AJ (1996) Clinical chemistry, Thirdth edn. Mosby, St. Louis
Lie KJ, Basch PF (1967) The life history of Echinostoma paraensei sp. n. (Trematoda: Echinostomatidae). J Parasitol 53:1192–1199
Lustrino D, Tunholi-Alves VM, Tunholi VM, Bessa ECA, Pinheiro J (2008) Allamanda cathartica (Apocynaceae) seeds induces changes on carbohydrates deposits of Bradybaena similaris (Mollusca, Bradybaenidae). Rev Bras Zooc 10:23–27
Maldonado A Jr, Vieira GO, Garcia JS, Rey L, Lanfredi RM (2001a) Biological aspects of a new isolate of Echinostoma paraensei (Trematoda: Echinostomatidae): susceptibility of sympatric snails and the natural vertebrate host. Parasitol Res 87:853–859
Maldonado A Jr, Loker ES, Morgan JAT, Rey L, Lanfredi RM (2001b) Description of the adult worms of a new Brazilian isolate of Echinostoma paraensei (Platyhelminthes: Digenea) from its natural vertebrate host Nectomys squamipes by light and scanning electron microscopy and molecular analysis. Parasitol Res 88:40–848
Maldonado A Jr, Coura R, Garcia JG, Lanfredi RM, Rey (2001c) Changes on Schistosoma mansoni (Digenea: Schistosomatidae) worm load in Nectomys squamipes (Rodentia: Sigmodontidae) concurrently infected with Echinostoma paraensei (Digenea: Echinostomatidae). Mem Inst Oswaldo Cruz 96:193–198
Maldonado A Jr, Gentile R, Fernandes CM, D’andrea PS, Lanfredi RM, Rey L (2006) Helminth communities of Nectomys squamipes (Rodentia: Sigmodontinae) naturally infected by the exotic trematode Schistosoma mansoni in southeastern Brazil. J Helminthol 80:369–375
Masola B, Chibi M, Kandare E, Naik YS, Zaranyika MF (2008) Potential marker enzymes and metal-metal interactions in Helisoma duryi and Lymnaea natalensis exposed to cadmium. Ecotoxicol Environ Saf 70:79–87
Massoud J (1974) The effect of variation in miracidial exposure dose on laboratory infections of Ornithobilharzia turkestanicum in Lymnaea gedrosiana. J Helminthol 48:139–144
Oliveira CS, Vasconcellos MC, Pinheiro J (2008) The population density effects on the reproductive biology of the snail Bradybaena similaris (Mollusca, Gastropoda). Braz J Biol 68:631–637
Perez MK, Fried B, Sherma J (1994) High performance thin-layer chromatographic analysis of sugars in Biomphalaria glabrata (Gastropoda) infected with Echinostoma caproni (Trematoda). J Parasitol 80:336–338
Pinheiro J, Amato SB (1994) Eurytrema coelomaticum (Digenea, Dicroceliidae): the effect of infection on carbohydrate contents of its intermediate snail host, Bradybaena similaris (Gastropoda, Xanthnychidae). Mem Inst Oswaldo Cruz 89:407–410
Pinheiro J, Amato SB (1995) Eurytrema coelomaticum: influence of the infection on the reproduction and nucleic acids contents in the albumen gland and ovotestis of Bradybaena similaris. Mem Inst Oswaldo Cruz 90:635–638
Pinheiro J, Gomes EM, Chagas GM (2001) Aminotranferases activity in the hemolymph of Bradybaena similaris under starvation. Mem Inst Oswaldo Cruz 96:1161–1164
Pinheiro J, Maldonado A Jr, Attias M, Lanfredi RM (2004a) Morphology of the rediae of Echinostoma paraensei (Trematoda: Echinostomatidae) from its intermediate host Lymnaea columella (Mollusca, Gastropoda). Parasitol Res 93:171–177
Pinheiro J, Maldonado A Jr, Lanfredi RM (2004b) Light and scanning electron microscopy of the miracidium of Echinostoma paraensei (Trematoda: Echinostomatidae). Vet Parasitol 121:265–275
Pinheiro J, Maldonado A Jr, Attias M, Lanfredi RM (2005) Ultrastructure of the Miracidium of Echinostoma paraensei Lie and Basch, 1967 (Trematoda, Echinostomatidae). Parasitol Res 97:367–372
Pinheiro J, Maldonado A Jr, Lanfredi RM (2009) Physiological changes in Lymnaea columella (Say, 1818) (Mollusca, Gastropoda) in response to Echinostoma paraensei Lie and Basch, 1967 (Trematoda, Echinostomatidae) infection. Parasitol Res 106:55–59
Sluiters JF, Brussaard-Wust CCM, Meuleman EA (1980) The relationship between miracidial dose, production of cercariae, and reproductive activity of the host in the combination Trichobilharzia ocellata and Lymnaea stagnalis. Z Parasitenkd 63:13–26
Théron A, Moné H, Gérard C (1992) Spatial and energy compromise between host and parasite: the Biomphalaria glabrata-Schistosoma mansoni system. Int J Parasitol 22:91–94
Thompson SN, Mejia-Scales V, Borchardt DB (1991) Physiologic studies of snail-schistosome interactions and potential for improvement of in vitro culture of schistosomes. In Vitro Cell Dev Biol 27A:497–504
Tunholi VM, Lustrino D, Tunholi-Alves VM, Mello-Silva CC, Maldonado A Jr, Rodrigues MD, Pinheiro J (2010) Changes in the reproductive biology of Biomphalara glabrata infected with different doses of Echinostoma paraensei miracidia. J Invertebr Pathol. doi:10.1016/j.jip.2010.09.006
Vasquez RE, Sullivan JT (2001) Effect of miracidial dose on adoptively transferred resistance to Schistosoma mansoni in the snail intermediate host, Biomphalaria glabrata. J Parasitol 87:460–462
Weichselbaum CTE (1946) An accurate and rapid method for determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol 16:40–49
Acknowledgments
This study was supported in part by Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The authors thank M.Sc. Juberlan Silva Garcia for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunholi, V.M., Lustrino, D., Tunholi-Alves, V.M. et al. Biochemical profile of Biomphalaria glabrata (Mollusca: Gastropoda) after infection by Echinostoma paraensei (Trematoda: Echinostomatidae). Parasitol Res 109, 885–891 (2011). https://doi.org/10.1007/s00436-011-2330-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2330-7