Abstract
We provide data on the helminth fauna from the digestive tract of the lizard Mabuya arajara Rebouças-Spieker, 1981 from Chapada do Araripe, northeastern Brazil. Seventy one of the 127 lizards examined (56%) were infected with four nematode species: Physalopteroides venancioi and Physaloptera sp. (Physalopteridae), Strongyluris oscari (Heterakidae), and Parapharyngodon alvarengai (Pharyngodonidae), the latter being the component species (prevalence 53.5%; mean intensity of infection 3.37 ± 2.0; discrepancy index D = 0.69). The helminth P. alvarengai infected M. arajara throughout the year and showed increased infection rates in July, at the beginning of the dry season. In addition to the relationship with seasonality, lizards with greater body length and/or body mass were more infected. Relationships between number of parasites and body mass and with the sexes of lizards, on the other hand, were not found. Mabuya arajara represents a new host for these nematodes. This study contributes to the knowledge of the helminth fauna associated with the digestive tract of lizards from South America and the Caatinga domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cosmopolitan and known to encompass interactions between members of all animal classes, parasitism represents the most successful mode of life in terms of animal diversity (Aho 1990; Poulin 1999; Bush et al. 2001). Neglected throughout history, parasite organisms have a great relevance in population dynamics and act directly on several factors of community structure (Poulin and Morand 2000; Bush et al. 2001; Marcogliese 2004). Their actions are reflected in behavioral changes because of metabolic imbalance (Dare and Forbes 2008), or due to their effects on sexual selection and reproductive output in the host populations (Hudson and Greenman 1998; Nordling et al. 1998; Kose and Muller 1999; Marcogliese 2004).
The number of endoparasite species infecting reptiles has long been considered low compared to other vertebrates (Aho 1990; Bush et al. 1990; Rocha and Vrcibradic 2003). The last decade, however, saw the identification of considerable species richness coming from the increased number of studies involving lizards (Ávila and Silva 2010), although in the Brazilian Caatinga domain, the knowledge of helminth faunas of lizards is still limited (Ávila and Silva 2010; Ávila et al. 2012). Currently, this domain has 78 known species of lizards (Delfin 2012), of which only ~30% have undergone a parasitological survey (e.g., Anjos et al. 2011, 2012; Ávila et al. 2012; Ribeiro et al. 2012a; Brito et al. 2014).
Among the Mabuya Fitzinger 1826 (Mabuyidae) occurring in the Caatinga, the only records on parasite infection have been provided by Ribeiro et al. (2012a) for lung pentastomids in M. arajara and Brito et al. (2014) for intestinal helminths in M. heathi. Although lizards of this genus have a wide Neotropical distribution, in Brazil, most parasitological records are from the midwestern area, characterized by Amazonian, Cerrado, and Pantanal domains and from southeastern regions, characterized by Atlantic Forest domain (e.g., Vrcibradic et al. 2002a, b; Rocha et al. 2003; Rocha and Vrcibradic 2003; Ávila 2009; Ávila et al. 2011; Ávila and Silva 2011).
Mabuya arajara Rebouças-Spieker 1981 has a restricted geographical distribution, in enclaves of humid forest (locally known as “brejos de altitude”) in Ceará and Piauí States, Brazil (Roberto and Loebmann 2010; Roberto et al. 2012; Ribeiro et al. 2012b). This species is one of the largest genus in Brazil (SVL up to 114 mm) and the females grow larger than males (Ribeiro et al. 2015). Mabuya arajara feeds on a wide diversity of invertebrates, mainly arthropods, but termites tend to dominate the diet (Ribeiro et al. 2015), and has seasonal reproduction occurring in the dry season (Ribeiro et al. 2015). Therefore, this study aimed to provide information on the helminth fauna from the digestive tract of the lizard M. arajara and its correlation with climatic seasonality, time of the year, body size, and sex of the lizards.
Material and methods
This study was conducted in the Crato (07° 15′S, 39° 28′W) and Barbalha Municipalities (07° 21′S, 39° 17′W), in Chapada do Araripe, Ceará State, northeastern Brazil. The localities are within the Área de Proteção Ambiental do Araripe (APA Araripe) (600 to 800-m altitude), with seasonal tropical climate and mean annual temperature of 24 ± 2.1 °C. The rainy season occurs from January to June and the dry season from July to December, and annual average rainfall is 1100 mm3 (MMA 2000; IPECE 2010).
Specimens of M. arajara were collected bimonthly from September 2009 to July 2010. Immediately after collection, all animals were weighed with an analytical balance (precision 0.001 mg), killed with a lethal injection of 2% lidocaine, and their snout-vent length (SVL) was measured with a digital caliper (0.1-mm accuracy). The lizards were fixed in 10% formaldehyde solution, preserved in 70% ethanol, and housed in the collection of the Universidade Regional do Cariri (LZ-URCA 599–621, 677–738).
After dissection, the digestive tract (stomach and intestines) was removed for analysis under a stereomicroscope. The nematodes found were cleared using lactophenol, mounted on temporary slides, and analyzed with a light microscope (Carl Zeiss Microimaging GmbH, Gottingen, Germany). Voucher specimens were housed in the collection of the Universidade Regional do Cariri (URCA-P 1203, 1204, 1205, and 1026).
The prevalence and mean intensity of infection were determined according to Bush et al. (1997). The discrepancy index was calculated overall and for each parasite species, as suggested by Poulin (1993):
The value of x is the number of parasites in host j and N is the total number of hosts. The index has a minimum value (0) when the parasites are uniformly distributed among the hosts and maximum value (1) when all parasites are found in a single host. Both evaluations were calculated using Quantitative Parasitology 3.0 software (Rózsa et al. 2000). Student’s t test was used to assess the difference in mean total parasite intensity between the dry season and the wet season. The relationship between parasite abundance and SVL, body mass, time of the year, and sex of M. arajara was calculated by the generalized linear model (GLM) of the Poisson model. After GLM, we performed an analysis of variance (ANOVA) comparing the total parasite intensity among the sampled months, followed by the Tukey test. To avoid the influence of ontogenetic factors, only adults were used for statistical analysis. The determination of sexual maturity and snout-vent length measurements were based on Ribeiro et al. (2015). The analysis was performed using the R Core Team (2017) platform and a significance level of p < 0.05.
Results
We analyzed a total of 127 specimens of Mabuya arajara, being 65 males, 54 females, and 8 juveniles (Table 1). Two-hundred and eighty helminths were obtained from 71 of the 127 lizards examined, representing an overall prevalence (P) of 56%; mean intensity of infection (I) was 3.5 ± 2.0 and discrepancy index (D) was 0.68. The helminth fauna of M. arajara was composed of four species of nematodes: Parapharyngodon alvarengai Freitas 1957 (Pharyngodonidae), infecting the intestines; Physalopteroides venancioi Lent et al. 1946 (Physalopteridae), infecting the stomach; Strongyluris oscari Travassos 1823 (Heterakidae) found in the intestine of a single individual; and Physaloptera sp. (Physalopteridae) found in the stomach of a single individual (Table 2).
The parasite abundance in M. arajara was influenced by seasonality and the highest infection rates were related to the dry season (Student t test = − 3.1994, gl = 111.81, p < 0.005).
The relationships between number of parasites × SVL (GLM; X(1,118) = 9.176, p = 0.005) and number of parasites and SVL × body mass of the lizards (GLM; X(1,110) = 6.051, p = 0.005) were positively and statistically significant. On the other hand, the relationship between number of parasites and body mass of the lizards was not significant (GLM; X(1,117) = 2.953, p = 0.085), nor was the relationship with sex (GLM; X(1,116) = 0.705, p = 0.401) (Table 3).
We observed a strong relationship between number of parasites and the month of collection for M. arajara (GLM; X(5,111) = 91.934, p < 0.001); (ANOVA; F(5,114) = 6.127, p < 0.001). While most months showed no differences, the Tukey test identified that July had the highest parasite load (p < 0.001) (Fig. 1). In that month, the recorded species were P. alvarengai, P. venancioi, and Physaloptera sp.
Discussion
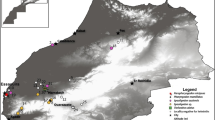
The helminth fauna described for M. arajara was composed of nematodes known to use many reptile hosts (Vicente et al. 1993; Anderson 2000; Ávila and Silva 2010). Our data showed a low helminth diversity and intensity of infection, but high prevalence, a common characteristic of South American Mabuya (Vrcibradic et al. 1999; Rocha and Vrcibradic 2003; Rocha et al. 2003). Parasitological studies with representatives of the genus are restricted to 41% of the species from South America (Fig. 2), but it is important to mention that some of these, such as M. agilis, M. macrorhyncha, and M. nigropunctata, were widely sampled (Van Sluys et al. 1997; Ribas et al. 1998b; Vrcibradic et al. 2000, 2002a, b; Rocha and Vrcibradic, 2003; McAllister et al. 2010a, b, c; Ávila and Silva 2011). The proportion of specimens infected can define the existence of component parasite species, that is, only those parasites found in ≥ 10% of the host population (Bush et al. 1990). In this study, besides being classified as a component species, since it is was recorded in two thirds of the population of M. arajara, the helminth P. alvarengai can also be considered a core species, since its infection prevalence was higher than 50% (Aho 1990).
Map of areas of South America represented by studies with lizards of the Mabuya genus parasitized by intestinal helminths. Country names are abbreviated: AR Argentina, BL Bolívia, BR Brazil, CH Chile, CO Colômbia, EC Ecuador, FG French Guiana, GY Guiana, PA Paraguay, PE Peru, NS Suriname, UY Uruguay, VE Venezuela
We provide the first infection record of P. alvarengai on M. arajara, and the second occurrence of this nematode among South American Mabuya; the other record is for M. heathi (infection rates P = 25%; I = 2.25) (Brito et al. 2014). Evolutionarily, Parapharyngodon spp. infected lizards and transferred to amphibians (Sousa et al. 2015; Montes-Oca et al. 2016). Among lizards, previous host records include the families Dactyloidae (Cabrera-Guzmán and Garrido-Olvera 2014), Gekkonidae (Anjos et al. 2011; Bezerra 2014; Brito et al. 2014), Iguanidae (Mayén-Peña and Salgado-Maldonado 1998), Scincidae (Freitas 1957; Brito et al. 2014), Teiidae (Padilha and Duarte 1979; Macedo et al. 2017), and Tropiduridae (Brito et al. 2014; Galdino et al. 2014; Bezerra et al. 2015; Araújo-Filho et al. 2016; Václav et al. 2017). Among amphibians, hosts include members of the Bufonidae (Luque et al. 2005) and Hylidae families (Montes-Oca et al. 2016).
Climatic seasonality, a fundamental factor responsible for the dry season having the highest parasite abundance in M. arajara, has a considerable influence on the ecology of species, such as habitat use and feeding (Grifftiths and Christian 1996; Vrcibradic and Rocha 1995, 2005; Brito 2003). These aspects, although determinants in the structuring and composition of the parasite assemblages Caatinga lizards (Brito et al. 2014), are apparently not observed in M. arajara, since the mode of life of this species varies little throughout the year (Ribeiro et al. 2015), and P. alvarengai has a monoxenous life cycle (Anderson 2000). A probable explanation for the increased infection rates in this period, therefore, is the consumption of contaminated particles of the substrate. On the other hand, during the rainy season, parasite eggs may be carried away by rain, providing less direct contact between hosts and parasites. This phenomenon is known to influence infection rates in Tropidurus torquatus (Pereira et al. 2012). The increased infection rates may also be related to physiological mechanisms in these animals, such as energy imbalance and hormonal changes (Aho 1990; Marcogliese 2004; Roberts et al. 2004; Martin et al. 2008). Although the low intensity of infection is a common characteristic among organisms infected by P. alvarengai (Pereira et al. 2012), there is evidence that susceptibility to infection by this parasite has an impact on the reproduction of host species (Bezerra 2014; Galdino et al. 2014).
The month of July, i.e., the beginning of the dry season, was highlighted by the highest parasite infection rates in M. arajara. This is a key period in the reproduction of this species (Ribeiro et al. 2015; Cabral 2017). Among males, seminiferous tubules go through a regenerative phase (Cabral A.N., unpublished data), peak hormonal production occurs, spermatogenesis begins, and the development of sexual structures is initiated (Fox 1977; Krohmer et al. 2004). It is known that testosterone is mostly used for reproductive functions, inducing decreased immunity in the organism, which increases susceptibility to opportunistic species (Nordling et al. 1998; Roberts et al. 2004; Martin et al. 2008). Among the females of M. arajara, there was a decrease in fat bodies with concomitant accelerated embryo development during that period (Ribeiro et al. 2015). Although this relationship is unclear, the investment of resources to the production of offspring leads to an energy imbalance in females, inducing a decrease in immunity similar to that observed in males (Nordling et al. 1998; Roberts et al. 2004). This harmful parasite-reproduction relationship has also been observed in amphibians (Dare and Forbes 2008), birds (Nordling et al. 1998; Kose and Mueller 1999), and mammals (Barger 1993; Zuk and Mckean 1996).
The low prevalence and intensity of infection may represent an evolutionary strategy of the parasite infecting a large number of hosts or may be due to casual infections (Poulin 2007). Thus, in both cases, the parasites are not considered component species (Bush et al. 1990). In this study, these parasite species were Physalopteroides venancioi, Physaloptera sp., and Strongyluris oscari. Similar records were provided by Ribeiro et al. (2012a) for the pentastomid Raillietiella mottae, a lung parasite recorded in M. arajara.
Physalopteroides venancioi is the only species of the genus reported for South America (Vrcibradic et al. 2000), and its low prevalence in M. arajara is common in lizards of the genus Mabuya (Van Sluys et al. 1997; Vrcibradic et al. 2000; Rocha and Vrcibradic 2003). According to Golberg et al. (1993), these characteristics occur due to the existence of physiological mechanisms and make the organism unfavorable to the development of Physalopteroides. The life cycle of P. venancioi is not completely known, but it is known that this nematode uses insects during the intermediate phase (Anderson 2000). In addition to lizards (e.g., Ávila and Silva 2010; Ávila et al. 2012), P. venancioi uses amphibians (Lent et al. 1946), and snakes as the final host (Al-Moussawi 2016).
We could not achieve a specific identification for only one individual of the family Physalopteridae due to immaturity. Widmer (1970), during analyses on the snake Crotalus viridis, found the presence of larvae of this family in the third stage of development, identified as Physaloptera sp. The author suggested the possibility of using this vertebrate as a paratenic host, which can be extended to M. arajara. Recently, Goldberg et al. (2014) indicated that Physaloptera larvae need insects as intermediate hosts and are commonly observed in the digestive tract of vertebrates after feeding. Physaloptera spp. affect a broad spectrum of hosts (Brandão et al. 2009; Santoro et al. 2010; Sianto et al. 2014), especially amphibians and reptiles in South America (Ávila and Silva 2010; Campião et al. 2014).
Only one individual of M. arajara was infected by S. oscari. Since it occurred in the same infection site of P. alvarengai, we believe that this may be related to an interspecific competition between the nematodes, where the low prevalence of one derives from the competitive superiority of the other. It is known that S. oscari needs insects as intermediate hosts and acquisition in lizards occurs after the ingestion of eggs in the infectious stage (Anderson 2000; Barreto-Lima and Anjos 2014). Among lizards of the Mabuya genus, this helminth has been previously described only for M. agilis (Ribas et al. 1998b) and M. guaporicola (Ávila 2009). With wide geographical distribution and registered in all Brazilian domains (Ribas et al. 1998a, b; Bursey et al. 2005; Ávila and Silva 2011, 2013; Ávila and Silva 2013; Barreto-Lima and Anjos 2014; Bezerra et al. 2015), high infection rates are found in lizards of humid regions (Vrcibradic et al. 2008; Barreto-Lima et al. 2012; Araújo-Filho et al. 2016) (Table 4).
The positive correlation between parasite load/SVL and parasite load /SVL/body mass observed in M. arajara was similar to what has been observed in its congeners M. agilis, M. macrorhyncha (Vrcibradic et al. 2000), and M. frenata (Vrcibradic et al. 1999). It is known that older individuals present greater SVL/body mass when compared to younger animals, thus increasing the chances of acquiring parasites due to greater time for contact with the environment throughout life (Ribas et al. 1998a; Pereira et al. 2012). In addition, a large body volume provides the parasite an abundance of resources (space to feed) available for colonization (Aho 1990; Van Sluys et al. 1994; Poulin 1997), even more so if we consider that M. arajara has a large body size among lizards of the genus Mabuya (Ribeiro et al. 2015).
In terms of overall infection and as the component species, P. alvarengai showed a tendency toward an intermediate distribution between aggregation and uniformity. Although parasite aggregation is the distribution between individuals (Poulin 1993; Bush et al. 2001; Begon et al. 2006; Simões et al. 2010), it is known that as long as the proportion of infected hosts increases, the parasite tends to exploit a growing number of individuals in the population. Thus, it is expected that the parasites are distributed uniformly, resulting in a high number of infected hosts (Poulin 1993). Similar trends were observed in Enyalius perditus (Barreto-Lima et al. 2012), Tropidurus semitaeniatus (Bezerra et al. 2015), and T. torquatus (Pereira et al. 2012). However, low prevalence of secondary helminths coincides with the maximum aggregation of these parasites, reflected in the underutilization of their respective hosts, as described by Poulin (1993).
This study contributes to the knowledge of the helminth fauna associated with the digestive tract of South American lizards inhabiting the Caatinga domain. Mabuya arajara is a new recorded host for all four nematodes recorded herein. Our data also show that the helminth P. alvarengai infects M. arajara throughout the year. However, we believe that more research should be undertaken in order to fill knowledge gaps in this parasite-host relationship.
References
Albuquerque S, Ávila RW, Bernarde PS (2012) Occurrence of helminths in lizards (Reptilia: Squamata) at lower Moa river forest, Cruzeiro do Sul, Acre, Brazil. Comp Parasitol 79(1):64–67. https://doi.org/10.1654/4539.1
Aho JM (1990) Helminth communities of amphibians and reptiles: comparative approaches to understanding pattern and process. In: Esch GW, Bush AO, Aho JM (eds) Parasite communities: patterns and processes. Chapman and Hall, London, pp 157–195. https://doi.org/10.1007/978-94-009-0837-6_7
Al-Moussawi AA (2016) The parasitic nematode Physalopteroides venancioi in the snake Platyceps ventromaculatus (Gray, 1834) in Baghdad city, Central Iraq. Int J Curr Microbiol App Sci 5(5):350–357. https://doi.org/10.20546/ijcmas.2016.505.036
Anderson RC (2000) Nematodes parasites of vertebrates. Their development and transmission. CAB International, Wallingford, p 650. https://doi.org/10.1079/9780851994215.0000
Anjos LA, Ávila R, Ribeiro SC, Almeida WO, Silva RJ (2012) Gastrointestinal nematodes of the lizard Tropidurus hispidus (Squamata: Tropiduridae) from a semi-arid region north-eastern Brazil. J Helminthol 87:1–7
Anjos LA, Bezerra CH, Passos DC, Zanchi D, Barbosa CA (2011) Helminth fauna of two gecko lizards, Hemidactylus agrius and Lygodactylus klugei (Gekkonidade), from Caatinga biome, northeastern Brazil. Neotrop Helminthol 5:285–289
Araújo-Filho JA, Brito SV, Lima VF, Pereira AMA, Mesquita DO, Albuquerque RL, Almeida WO (2016) Influence of temporal variation and host condition on helminth abundance in the lizard Tropidurus hispidus from north-eastern Brazil. J Helminthol 4:1–8
Ávila RW (2009) Padrões de infecção por helmintos em comunidades de lagartos do Brasil Central. Universidade Estadual Paulista, Doctoral thesis
Ávila RW, Anjos LA, Ribeiro SC, Morais DH, Silva RJ, Almeida WO (2012) Nematodes of lizards (Reptilia: Squamata) from Caatinga biome, northeastern Brazil. Comp Parasitol 79(1):56–63. https://doi.org/10.1654/4518.1
Ávila RW, Cardoso MW, Oda FH, Silva RJ (2011) Helminths from lizards (Reptilia: Squamata) at the Cerrado of Goiás state, Brazil. Comp Parasitol 78(1):120–128. https://doi.org/10.1654/4472.1
Ávila RW, Silva RJ (2013) Helminths of lizards from the municipality of Aripuanã in the southern Amazon region of Brazil. J Helminthol 87(01):12–16. https://doi.org/10.1017/S0022149X11000769
Ávila RW, Silva RJ (2010) Checklist of helminths from lizards and amphisbaenians (Reptilia, Squamata) of South America. J Venom Anim Toxins incl Trop Dis 16:1–30
Ávila RW, Silva RJ (2011) Helminths of lizards (Reptilia: Squamata) from Mato Grosso state, Brazil. Comp Parasitol 78(1):129–139. https://doi.org/10.1654/4473.1
Bain O (1974) Description de nouvelles filaires Oswaldofilariinae de lézards sud-américains; Hyphthése sur l’evolution des filaires de Reptiles. Bull Mus Nat Hist Paris 138:169–200
Bain O, Sulahian E (1974) Trois nouvelles Filaires du genre Oswaldofilaria chez des lézards sud-américains; essain de classification des Oswaldofilariinae. Bull Mus Nat Hist Paris 156:827–841
Barger IA (1993) Influence of sex and reproductive status on susceptibility of ruminants to nematode parasitism. Int J Parasitol 23(4):463–469. https://doi.org/10.1016/0020-7519(93)90034-V
Barreto-Lima AF, Anjos LA (2014) Occurrence of Strongyluris oscari (Nematoda; Heterakidae) in Enyalius bilineatus from the Brazilian Atlantic Forest. Herpetol. Notes 7:455–456
Barreto-Lima AF, Toledo GM, Anjos LA (2012) The nematode community in the Atlantic rainforest lizard Enyalius perditus Jackson, from southeastern Brazil. J Helminthol 86(04):395–400. https://doi.org/10.1017/S0022149X11000599
Begon M, Harper JL, Townsend CR (2006) Ecology: individuals, populations and communities. (3rd Ed) Cambridge, Blackwell Science, pp.1068
Bezerra CH (2014) Parasitas do lagarto exótico Hemidactylus mabouia (Moreau de Jonnes, 1818) (Squamata, Gekkonidae): padrões de infecção e efeitos da distância geográfica na similaridade das comunidades. Master’s dissertation, Universidade Federal do Ceará, Brasil
Bezerra CH, Ávila RW, Passos DC, Zanchi-Silva D, Galdino CAB (2015) Levels of helminth infection in the flat lizard Tropidurus semitaeniatus from northeastern Brazil. J Helminthol 2:1–5
Brandão ML, Chame M, Cordeiro JLP, Chaves SAM (2009) Diversidade de helmintos intestinais em mamíferos silvestres e domésticos na Caatinga do Parque Nacional Serra da Capivara, Sudeste do Piauí, Brasil. Rev Bras Parasitol V 18(e1):19–28. https://doi.org/10.4322/rbpv.018e1004
Brito JC (2003) Seasonal variation in movements, home range and habitat use by male Vipera latastei in northern Portugal. J Herpetol 37(1):155–160. https://doi.org/10.1670/0022-1511(2003)037[0155:SVIMHR]2.0.CO;2
Brito SV, Corso G, Almeida AM, Ferreira FS, Almeida WO, Anjos LA, Mesquita DO (2014) Phylogeny and micro-habitats utilized by lizards determine the composition of their endoparasites in the semiarid Caatinga of northeast Brazil. Parasitol Res 113(11):3963–3972. https://doi.org/10.1007/s00436-014-4061-z
Bursey CR, Goldberg SR, Parmelee JR (2005) Gastrointestinal helminths from 13 species of lizards from Reserva Cuzco Amazónico, Peru. Comp Parasitol 72(1):50–68. https://doi.org/10.1654/4132
Bush AO, AHO JM, Kennedy CR (1990) Ecological versus phylogenetic determinants of helminth parasite community richness. Evol Ecol 4(1):1–20. https://doi.org/10.1007/BF02270711
Bush AO, Fernández JC, Esch GW, Seed JR (2001) Parasitism: the diversity and ecology of animal parasites (Ed). Cambridge University Press, Cambridge, p 567
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583. https://doi.org/10.2307/3284227
Cabral AN (2017) The relationship between morphology and functional renal sexual segment and seminiferous tubules of Mabuya arajara Rebouças-Spieker, 1981 (Squamata: Mabuyidae). Universidade Federal de Pernambuco, Brasil, Master’s dissertation
Cabreba-Guzmán E, Garrido-Olvera L (2014) Helminth parasites of the lesser scaly anole, Anolis uniformis (Squamata: Dactyloidae), from Los Tuxtlas, southern Mexico: evidence of diet and habitat use. South Am J Herpetol 9(3):183–189. https://doi.org/10.2994/SAJH-D-14-00035.1
Campião KM, Morais DH, Dias OT, Aguiar A, Toledo G, Tavares LER (2014) Checklist of helminth parasites of amphibians from South America. Zootaxa 3843(1):1–93. https://doi.org/10.11646/zootaxa.3843.1.1
Dare OK, Forbes MR (2008) Rates of development in male and female wood frogs and patterns of parasitism by lung nematodes. Parasitology 135(3):385–393. https://doi.org/10.1017/S0031182007003836
Delfin FR (2012) Riqueza e padrões de distribuição dos lagartos do Domínio morfoclimático da Caatinga. Doctoral thesis, Universidade Federal da Paraíba, Brasil
Fitzinger LJ (1826) Neue Classification der Reptilien nach ihren natürlichen Verwandtschaften. Vienna 66
Fox W (1977) A comparison of the male urogenital systems of blind snakes, Leptotyphlopidae and Typhlopidae. Herpetologica 21:241–256
Freitas JFT (1957) Sobre os gêneros Thelandros Wedl, 1962 e Parapharyngodon Chatteuji, 1933, com descrição de Parapharyngodon alvarengai sp. n. (Nematoda, Oxyuroidea). Mem Inst Oswaldo Cruz 55(1):21–45. https://doi.org/10.1590/S0074-02761957000100003
Galdino CAB, Ávila RW, Bezerra CH, Passos DC, Melo GC, Zanchi-Silva D (2014) Helminths infection patterns in a lizard (Tropidurus hispidus) population from a semiarid Neotropical area: associations between female reproductive allocation and parasite loads. J Parasitol 100(6):864–867. https://doi.org/10.1645/13-264.1
Goldberg SR, Bursey CR, Arreola J (2014) Gastrointestinal helminths of the Santa Cruz Island sator, Sceloporus angustus (Squamata: Phrynosomatidae), from Isla Santa Cruz, Baja California Sur, Mexico. Comp Parasitol 81(2):276–277. https://doi.org/10.1654/4676.1
Golberg SR, Bursey CR, Tawil R (1993) Gastrointestinal helminths of the western brush lizard, Urosaurus graciosus graciosus (Phrynosomatidae). Bull S calif. Acad Sci 92:43–51
Griffiths AD, Christian KA (1996) Diet and habitat use of frillneck lizards in a seasonal tropical environment. Oecologia 106(1):39–48. https://doi.org/10.1007/BF00334405
Hudson P, Greenman J (1998) Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390
IPECE- Instituto de Pesquisa e Estatística Econômica do Ceará (2010) Perfil básico municipal: Crato. Governo do Estado do Ceará, Secretaria do Planejamento e Coordenação
Kose M, Mueller AP (1999) Sexual selection feather breakage and parasites: the importance of white spots in the tail of the born swallow (Hirundo rustica). Behav Ecol Sociobiol 45(6):430–436. https://doi.org/10.1007/s002650050581
Krohmer RW, Martinez D, Mason RT (2004) Development of the renal sexual segment in immature snakes: effect of sex steroid hormones. Comp Biochem Physiol 139(1):55–64. https://doi.org/10.1016/j.cbpb.2004.06.015
Lent H, Freitas JFT, Proença MC (1946) Alguns helmintos batráquios colecionados no Paraguai. Mem Inst Oswaldo Cruz 44(1):195–214. https://doi.org/10.1590/S0074-02761946000100007
Luque JL, Martins AL, Tavares LER (2005) Community structure of metazoan parasites of the yellow cururu toad, Bufo ictericus (Anura, Bufonidae) from Rio de Janeiro, Brazil. Acta Parasitol 50:215–220
Macedo LC, Gardner SL, Melo STV, Giese EG, Santos JN (2017) Nematodes parasites of Teiidae lizards from the Brazilian Amazon rainforest. J Parasitol 103(2):176–182. https://doi.org/10.1645/16-69
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theater. Eco Health 1:151–164
Martin LB, Weil ZM, Nelson RJ (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos T R Soc B 363(1490):321–339. https://doi.org/10.1098/rstb.2007.2142
Mayén-Peña E, Salgado-Maldonado G (1998) Helminths of four lizards from Nayarit, México: Anolis nebulosus (Polychrotidae), Ctenosaura pectinata (Iguanidae), Phyllodactylus lanei (Gekkonidae), and Sceloporus nelsoni (Phrynosomatidae). J Helminthol Soc W 65:108–111
McAllister CT, Bursey CR, Fred PS (2010a) Helminth parasites (Cestoidea: Nematoda) of select herpetofauna from Paraguay. J Parasitol 96(1):222–224. https://doi.org/10.1645/GE-2191.1
McAllister CT, Bursey CR, Fred PS (2010b) Helminth parasites of amphibians and reptiles from the Ucayali region, Peru. J Parasitol 96(2):444–447. https://doi.org/10.1645/GE-2206.1
McAllister CT, Bursey CR, Fred PS (2010c) Helminth parasites of herpetofauna from the Rupunini District, southwestern Guyana. Comp Parasitol 77:185–201
Ministério do Meio Ambiente (2000). Avaliação e ações prioritárias para a conservação da biodiversidade da Mata Atlântica e Campos Sulinos. Fundação SOS Mata Atlântica, Fundação Biodiversitas, Instituto de Pesquisas Ecológicas, Secretaria do Meio Ambiente do Estado de São Paulo, SEMAD/Instituto Estadual de Florestas-MG. Brasília: MMA/ SBF. http://www.mma.gov.br/
Montes-Oca EUG, Mata-López R, León-Règagnon V (2016) Two species of Parapharyngodon parasites of Sceloporus pyrocephalus, with a key to the species found in Mexico (Nematoda, Pharyngodonidae). Zookeys 559:1–16
Nordling D, Anderson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond 265(1403):1291–1298. https://doi.org/10.1098/rspb.1998.0432
Padilha TN, Duarte MJF (1979) Ocorrência de Parapharyngodon alvarengai Freitas, 1957, em Ameiva ameiva no Estado do Rio de Janeiro (Nematoda, Oxyuroidea). Atlas Soc Biol Rio de Janeiro 20:21–22
Pereira FB, Sousa BM, Lima SS (2012) Helminth community structure of Tropidurus torquatus (Squamata: Tropiduridae) in a rocky outcrop area of Minas Gerais state, southeastern Brazil. J Parasitol 98(1):6–10. https://doi.org/10.1645/GE-2689.1
Poulin R (1993) The disparity between observed and uniform distributions: a new look at parasite aggregation. Int J Parasitol 23(7):937–944. https://doi.org/10.1016/0020-7519(93)90060-C
Poulin R (1999) The functional importance of parasites in animal communities: many roles at many levels? Int J Parasitol 29(6):903–914. https://doi.org/10.1016/S0020-7519(99)00045-4
Poulin R (1997) Species richness of parasite assemblages: evolution and patterns. Annu Rev Ecol Syst 28(1):341–358. https://doi.org/10.1146/annurev.ecolsys.28.1.341
Poulin R (2007) Evolutionary ecology of parasites. 2rd end. 332 pp. Princeton, Princeton University Press
Poulin R, Morand S (2000) The diversity of parasites. Q Rev Biol 75(3):277–293. https://doi.org/10.1086/393500
Core Team R (2017) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna URL https://www.R-project.org/
Rebouças-Spieker R (1981) Sobre uma nova espécie de Mabuya do Nordeste do Brasil (Sauria, Scincidae). Pap Avulsos Zool 34:121–123
Ribas SC, Rocha CFD, Teixeira-Filho PF, Vicente JJ (1998a) Nematode infection in two sympatric lizards (Tropidurus torquatus and Ameiva ameiva) with different foraging tactics. Amphibia-Reptilia 19(3):323–330. https://doi.org/10.1163/156853898X00232
Ribas SC, Teixeira-Filho PF, Rocha CFD, Vicente JJ (1998b) Parasitismo por nematoides em duas espécies simpátricas de Mabuya (Lacertilia: Scincidae) na restinga da Barra de Maricá, RJ. Anais do VIII Seminário Regional de Ecologia 8:883–894
Ribeiro SC, Ferreira FS, Brito SV, Teles DA, Ávila RW, Almeida WO, Anjos LA, Guarnieri MC (2012a) Pulmonary infection in two sympatric lizards, Mabuya arajara (Scincidae) and Anolis brasiliensis (Polychrotidae) from a cloud forest in Chapada do Araripe, Ceará, northeastern Brazil. Braz J Biol 72(4):929–933. https://doi.org/10.1590/S1519-69842012000500021
Ribeiro SC, Roberto IJ, Sales DL, Ávila RW, Almeida WO (2012b) Amphibians and reptiles from Araripe bioregion, northeastern Brazil. Salamandra 48:133–146
Ribeiro SC, Teles DA, Mesquita DO, Almeida WO, Anjos LA, Guarnieri MC (2015) Ecology of skink, Mabuya arajara Rebouças-Spieker 1981, in Araripe plateau, northeast Brazil. J Herpetol 49(2):237–244. https://doi.org/10.1670/13-018
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68(2):227–239. https://doi.org/10.1016/j.anbehav.2004.05.001
Roberto IJ, Brito PTP, Menezes BF, Bezerra LMB, Ribeiro SC (2012) Mabuya arajara geographical distribution. Herpetol Rev 43:655
Roberto IJ, Loebmann D (2010) Geographic distribution and parturition of Mabuya arajara Rebouças-Spieker, 1981 (Squamata, Sauria, Scincidae) from Ceará, northeastern Brazil. Herpetol Bull 13:4–10
Rocha CFD, Vrcibradic D (2003) Nematode assemblages of some insular and continental lizard hosts of the genus Mabuya Fitzinger (Reptilia, Scincidae) along the eastern Brazilian coast. Rev Bras Zool 20(4):755–759. https://doi.org/10.1590/S0101-81752003000400031
Rocha CFD, Vrcibradic D, Vicente JJ, Cunha-Barros M (2003) Helminths infecting Mabuya dorsivittata (Lacertilia, Scincidae) from a high-altitude habitat in Itatiaia National Park, Rio de Janeiro state, southeastern Brazil. Braz J Biol 63(1):129–132. https://doi.org/10.1590/S1519-69842003000100017
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86(2):228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Santoro M, Tripepi M, Kinsella JM, Panebianco A, Mattiucci S (2010) Helminth infestation in birds of prey (Accipitriformes and Falconiformes) in southern Italy. Vet J 186(1):119–122. https://doi.org/10.1016/j.tvjl.2009.07.001
Sianto L, Souza MV, Chame M, Luz MF, Guidon N, Pessis AM, Araújo A (2014) Helminths in feline coprolites up to 9000 years in the Brazilian northeast. Parasitol Int 63(6):851–857. https://doi.org/10.1016/j.parint.2014.08.002
Silva CC (2014a) Efeito do extrato renal de cascaveis (Crotalus durissus cascavella) como diluidor de sêmen de touro (Bos taurus) e de peixes (Colossoma macropomum). Universidade Federal de Pernambuco, Brasil, Master’s dissertation
Silva LAF (2014b) Helmintofauna associada a répteis provenientes da Reserva Particular do Patrimônio Natural Foz do Rio Aguapeí. Universidade Estadual Paulista, Brasil, Estado de São Paulo. Master’s dissertation
Simões RO, Souza JGR, Maldonado A, Luque JL (2010) Variation in the helminth community structure of three sympatric sigmodontine rodents from the coastal Atlantic Forest of Rio de Janeiro, Brazil. J Helminthol 85:171–178
Sousa AMV, Harris DJ, Leg, AP (2015) Assessment of cophylogenetic patterns between the nematode genus Parapharyngodon spp. and their reptile hosts in the Canary Islands. Master’s dissertation, Faculty of Sciences of University of Porto
Travassos LP (1823) Informações sobre a fauna helminthologica de Mato Grosso. Folha Medicinal 4:58–60
Václav ABHP, Anjos LA, Queiróz MS, Nascimento LB, Galdino CAB (2017) Nematode infection patterns in a Neotropical lizard species from a insular mountain habitat in Brazil. J Helminthol 91(05):578–582. https://doi.org/10.1017/S0022149X16000754
Van Sluys M, Rocha CFD, Ribas SC (1994) Nematodes infecting the lizard Tropidurus itambere in southeastern Brazil. Amphibia-Reptilia 15(4):405–408. https://doi.org/10.1163/156853894X00443
Van Sluys M, Rocha CFD, Bergallo HG, Vrcibradic D, Ribas SC (1997) Nematode infection in three sympatric lizards in an isolated fragment of restinga habitat in southeastern Brazil. Amphibia-Reptilia 18(4):442–446. https://doi.org/10.1163/156853897X00503
Vicente JJ, Rodrigues HO, Gomes DC, Pinto RM (1993) Nematoides do Brasil. Parte III: nematoides de répteis. Rev Bras Zool 10(1):19–168. https://doi.org/10.1590/S0101-81751993000100003
Vicente JJ, Vrcibradic D, Muniz-Pereira LC, Pinto RM (2000) Skrjabinodon heliocostai sp. n. (Nematoda, Pharyngodonidae) parasitizing Mabuya frenata (Cope) (Lacertilia, Scincidae) in Brazil and the reallocation of Skrjabinodon capacyupanquii (Freitas, Vicente and Ibñez) in the genus Thelandros Wedl. Rev Bras Zool 17(2):361–367. https://doi.org/10.1590/S0101-81752000000200006
Vicente JJ, Vrcibradic D, Rocha CFD, Pinto RM (2002) Description of Skrjabinodon spinosulus sp. n. (Nematoda, Oxyuroidea, Pharyngodonidae) from the Brazilian lizard Mabuya dorsivittata Cope, 1862 (Scincidae). Rev Bras Zool 19(1):157–162. https://doi.org/10.1590/S0101-81752002000100014
Vrcibradic D, Anjos LA, Vicente JJ, Bursey CR (2008) Helminth parasites of two sympatric lizards, Enyalius iheringii and E. perditus (Leiosauridae), from Atlantic rainforest area of southeastern Brazil. Acta Parasitol 53:222–225
Vrcibradic D, Rocha CFD (1995) Variação sazonal na dieta de Mabuya macrorhyncha (Sauria, Scincidae) na restinga de Barra de Maricá, RJ. Oecologia Bras 1(01):143–153. https://doi.org/10.4257/oeco.1995.0101.05
Vrcibradic D, Rocha CFD (2005) Observations on the natural history of the lizard Mabuya macrorhyncha Hoge (Scincidae) in Queimada Grande island, São Paulo. Rev Bras Zool 4:1185–1190
Vrcibradic D, Rocha CFD, Bursey CR, Vicente JJ (2002a) Helminth communities of two sympatric skinks (Mabuya agilis and Mabuya macrorhyncha) from two ‘restinga’ habitats in southeastern Brazil. 76:355-361. J Helminthol 76(4):355–361. https://doi.org/10.1079/JOH2002134
Vrcibradic D, Rocha CFD, Bursey CR, Vicente JJ (2002b) Helminths infecting Mabuya agilis (Lacertilia, Scincidae) in a restinga habitat (Grumari) of Rio de Janeiro, Brazil. Amphibia-Reptilia 23:109–114
Vrcibradic D, Rocha CFD, Ribas SC, Vicente JJ (1999) Nematodes infecting the skink Mabuya frenata in Valinhos, São Paulo state, southeastern Brazil. Amphibia-Reptilia 20(3):333–339. https://doi.org/10.1163/156853899X00367
Vrcibradic D, Rocha CFD, Van Sluys M, Bursey CR (2001) Natural history notes. Mabuya macrorhyncha. Endoparasites. Herpetol Rev 32:256–262
Vrcibradic D, Vicente JJ, Bursey R (2000) Nematode infection patterns in four sympatric lizards from a restinga habitat (Jurubatiba) in Rio de Janeiro state, southeastern Brazil. Amphibia-Reptilia 21(3):307–316. https://doi.org/10.1163/156853800507507
Widmer EA (1970) Development of third-stage Physaloptera larvae from Crotalus viridis Rafinesque, 1818 in cats with notes on pathology of the larvae in the reptile (Nematoda, Spiruoidea). J Wildlife Dis 6(2):89–93. https://doi.org/10.7589/0090-3558-6.2.89
Zuk M, Mckean K (1996) Sex differences in parasite infections: patterns and processes. Int J Parasitol 26(10):1009–1024. https://doi.org/10.1016/S0020-7519(96)80001-4
Acknowledgements
We thank the owners of private areas in which fieldwork was conducted: Raimundo Marques (Nascente, Delvechia Farm) and Ivan de Araújo B. Filho (São Joaquim Farm, Itapuí-S.A). We also thank Guilherme Sousa, Ivonildo Dias, Rafael Brandão and Jonathan Ramos for the field assistance.We are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (PQ-302429/2015-8) for the research grant awarded to W.O.Almeida. We thank to referees who carefully reviewed the manuscript and offered great contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical standards
The collecting methods were defined and authorized by the regulatory agency in Brazil (ICMBio/SISBio 20388-1 and 23544-1). All processes followed the ethical guidelines provided by the American Society of Ichthyologists and Herpetologists (ASIH), the Herpetologists’ League (HL), the Society for the Study of Amphibians and Reptiles, and Conselho Brasileiro de Biologia (CBO).
Rights and permissions
About this article
Cite this article
Cabral, A.d., Teles, D.A., Brito, S.V. et al. Helminth parasites of Mabuya arajara Rebouças-Spieker, 1981 (Lacertilia: Mabuyidae) from Chapada do Araripe, northeastern Brazil. Parasitol Res 117, 1185–1193 (2018). https://doi.org/10.1007/s00436-018-5797-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5797-7