Abstract
Despite many efforts, the currently available treatments for leishmaniasis are not fully effective. To discover new medications, drug repurposing arises as a promising strategy. We present data that supports the use of the antidepressant clomipramine against Leishmania amazonensis. The drug presented selective activity at micromolar range against both the parasite forms and stimulated nitric oxide production in host macrophages. Regarding the mechanism of action, clomipramine led parasites do mitochondrial depolarization, which coupled with the inhibition of trypanothione reductase induced strong oxidative stress in the parasites. The effects observed in promastigotes included lipoperoxidation, plasma membrane permeabilization, and apoptosis hallmarks (i.e., DNA fragmentation, phosphatidylserine exposure, and cell shrinkage). The mechanism of action in both parasitic forms was quite similar, but amastigotes also exhibited energetic stress, reflected by a reduction of adenosine triphosphate levels. Such differential effects might be attributable to the metabolic particularities of each form of the parasitic. Ultrastructural alterations of the endomembrane system and autophagy were also observed, possibly indicating an adaptive response to oxidative stress. Our results suggest that clomipramine interferes with the redox metabolism of L. amazonensis. In spite of the cellular responses to recover the cellular homeostasis, parasites underwent programmed cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmania is a diverse genus of pathogenic protozoa that can penetrate and replicate inside macrophages, the front-line cells of the immune system (Berman et al. 1979; Moradin et al. 2012). These intracellular protozoa present a complex digenetic life cycle, requiring a susceptible vertebrate host and a permissive insect vector, generally phlebotomines. Leishmaniasis comprises a group of diseases that are caused by these parasites, presented as a severe public health problem in many countries worldwide, affecting mainly regions that are socially and economically vulnerable (WHO 2018; Pace 2014).
The chemotherapeutics that are currently available for the treatment of leishmaniasis are not completely effective. Drugs that have proven leishmanicidal activity have severe toxic effects. Treatment can result in the emergence of resistant strains and involves a complex protocol of administration. The liposomal formulation of amphotericin B (L-AMB) is the best option that is currently available for the treatment of leishmaniasis. L-AMB presents the highest therapeutic efficacy and best safety, but its use is limited by its high cost (Georgiadou et al. 2015). Considering these limitations, several drug discovery approaches have been employed to find more effective and less toxic medications for the treatment of leishmaniasis, including the isolation of natural products and screening of large libraries of synthetic compounds. Nevertheless, despite increasing investments in drug discovery, these traditional approaches have not yet successfully resulted in safer or more effective therapeutic alternatives (Munos 2009). The repurposing of already approved drugs may be a less expensive and faster approach to discover new treatments for these parasitic diseases (Andrews et al. 2014; Kaiser et al. 2015).
Clomipramine is a tricyclic antidepressant that is currently used for the treatment of psychiatric disorders, including obsessive-compulsive disorder. It presents a mechanism of action that is based on the inhibition of serotonin reuptake (El Mansari and Blier 2006). By employing a repurposing approach, clomipramine was shown to have promising efficacy against different trypanosomatids, including Trypanosoma brucei (Rodrigues et al., 2016), Trypanosoma cruzi (Hammond et al., 1984; Rivarola et al., 2005), and Leishmania donovani (Zilberstein et al., 1984). In addition, imipramine another tricyclic antidepressant has shown to be active against Leishmania donovani (Mukherjee et al., 2012). Initial studies suggested that clomipramine acts through the competitive inhibition of trypanothione reductase, an enzyme that plays a key role in the unique redox metabolism of these parasites (Benson et al. 1992).
Still unknown are the effects of clomipramine against the causative agents of cutaneous leishmaniasis in the New World. The present study evaluated the activity of clomipramine against Leishmania amazonensis. This parasite has a wide spectrum of clinical manifestations, including cutaneous, mucocutaneous, and diffuse cutaneous forms (Barral et al. 1991). We investigated biochemical and ultrastructural alterations that may be involved in the mechanism of action of clomipramine in L. amazonensis.
Materials and methods
Chemicals
Dimethyl sulfoxide (DMSO), thiazolyl blue tetrazolium bromide (MTT), actinomycin D, antimycin A, carbonyl cyanide m-chlorophenylhydrazone (CCCP), camptothecin, potassium cyanide (KCN), Nile red, N-acetyl-L-cysteine (NAC), digitonin, lipopolysaccharides (LPS), and monodansylcadaverine (MDC) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Brain heart infusion (BHI) was acquired from Beckton Dickinson (Sparks, MD, USA). Fetal bovine serum (FBS), RPMI-1640 medium, and Giemsa were obtained from Invitrogen (Grand Island, NY, USA). Annexin V fluorescein isothiocyanate (FITC) conjugate, the APO bromodeoxyuridine (BrdU) terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit, 2′,7′-dichlorofluorescin diacetate (H2DCFDA), 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM), Amplex red hydrogen peroxide/peroxidase assay kit, and tetramethylrhodamine (TMRE) were obtained from Invitrogen (Eugene, OR, USA). Cell titer-glo luminescent cell viability assay was obtained from Promega ® (Madison, USA). All of the other reagents were of analytical grade.
Compound
Clomipramine hydrochloride (Clomi) was purchased from Sigma-Aldrich (St. Louis, MI, USA). The drug was diluted in DMSO before each experiment and the final concentration of diluent in all assays never exceeded 0.5%. Equimolar concentrations of DMSO were applied in all the treatments and controls, and had no influence on parasites and cells.
Parasites and mammalian cells
The experiments were performed with L. amazonensis (WHOM/BR/75/JOSEFA strain) that was originally isolated by C.A. Cuba-Cuba (Universidade de Brasília, Brazil) from a patient with diffuse cutaneous leishmaniasis. Promastigotes were cultivated in Warren medium (brain heart infusion, hemin, and folic acid; pH 7.0) supplemented with 10% FBS and maintained at 25 °C. J774.A1 macrophages (Banco de Células do Rio de Janeiro, Rio de Janeiro, Brazil) were maintained in RPMI-1640 medium (pH 7.2), supplemented with sodium bicarbonate, L-glutamine, and 10% FBS, maintained at 37 °C in a 5% CO2 atmosphere. Intracellular amastigotes were obtained from macrophages that were infected with promastigotes and cultivated under the same conditions that were established for macrophages but at 34 °C.
Antiproliferative activity against promastigotes of L. amazonensis
Parasites were harvested in the exponential growth phase (1 × 106 promastigotes/ml) and incubated in the presence or absence of different concentrations of clomipramine for 72 h. Parasite growth was estimated by counting the number of parasites in a Neubauer hemocytometer. The results were expressed as a percentage of growth inhibition relative to control cultures. The inhibitory concentrations for 50% (IC50) and 90% (IC90) of parasite growth were determined by non-linear regression using the sigmoidal inhibition curve of better adjustment. The amount of drugs (8 and 22 μM) applied to further experiments with promastigotes was based on the IC50 and IC90 values.
Antiproliferative activity against intracellular amastigotes of L. amazonensis
To assess the activity of clomipramine against intracellular parasites, JJ74A.1 macrophages were infected with promastigotes (10 parasites per host cell) and incubated for 24 h at 34 °C in a 5% CO2 atmosphere. The infected macrophages were then treated with different concentrations of clomipramine and incubated for 48 h, followed by fixation in methanol and Giemsa staining. The number of infected cells and amastigotes was counted in 200 cells. The infection indices (number of amastigotes per cell × percentage of cells infected / total number of cells) were determined, and IC50 and IC90 values were calculated as described for promastigotes. The amount of drugs (15 and 30 μM) applied of further experiments with amastigotes was based on the IC50 and IC90 values.
Cytotoxicity in J774A.1 macrophages
Macrophages were cultured (5 × 105 cells/ml) in RPMI-1640 medium supplemented with 10% FBS in 96-well microplates at 37 °C in a 5% CO2 atmosphere. After 24 h, the cells were treated with different concentrations of clomipramine and incubated for 48 h. The microplates were then washed with phosphate-buffered saline (PBS), and 50 μl of MTT solution (2 mg/ml) was added to each well. The plate was incubated for 4 h at 37 °C while protected from light. After incubation, 150 μl of DMSO was added to each well, and absorbance was read in a Bio-Tek Power Wave XS spectrophotometer at 570 nm. The MTT assay is based on the conversion of water-soluble MTT to an insoluble formazan precipitate by viable mitochondria. The cytotoxic concentration for 50% of the cells (CC50) was determined by non-linear regression using the sigmoidal inhibition curve of better adjustment. The selectivity index (SI) was calculated as CC50/IC50.
Isolation of intracellular amastigote forms
To study the mechanism of action of clomipramine in intracellular amastigotes, we established a protocol for isolating these parasites. J774.A1 macrophages were infected with promastigotes (10 parasites per host cell) and incubated for 48 h at 34 °C in a 5% CO2 atmosphere. The infected macrophages were treated with clomipramine (15 and 30 μM) for additional 24 h. After treatment, the infected macrophages were removed using a cell scraper and lysed by aspiration and extrusion with a syringe and needle (30 gauge, 0.5 in., BD PrecisionGlide, Canada). Amastigotes were separated from unlysed macrophages and debris by differential centrifugation at 1000 rpm for 5 min, and the supernatant was collected. The number of amastigotes was counted in a Neubauer hemocytometer and adjusted to 1 × 106 parasites/ml.
Nitric oxide production in macrophages
Macrophages were plated in black 96-well plates and incubated at 37 °C for 48 h in a 5% CO2 atmosphere. The cells were then treated with clomipramine (15 and 30 μM) and incubated for 24 h. After treatment, the cells were loaded with DAF-FM (1 μM), incubated for 30 min at room temperature, washed with PBS, and incubated for an additional 15 min. Fluorescence was measured in a spectrofluorometer (Victor X3; PerkinElmer) at λex = 495 nm and λem = 515 nm. H2O2 (4 mM) and LPS (1 μg/ml) were used as a positive control. In parallel, we pretreated the same groups with N-acetyl cysteine (NAC; 5 mM) for 3 h.
Morphological and ultrastructural analyses
To assess morphological and ultrastructural alterations that were induced by clomipramine, parasites were analyzed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Promastigotes were treated with clomipramine (8 and 22 μM) for 72 h and prepared for SEM. To visualize alterations of the intracellular form of L. amazonensis, macrophages were infected with promastigotes for 48 h, treated for 24 h with clomipramine at 15 and 30 μM, and then processed for TEM. After treatment, both forms of the parasite were washed with PBS and fixed in 2.5% glutaraldehyde and 0.1 M sodium cacodylate buffer at 4 °C. For TEM, samples of infected macrophages were postfixed in 1% osmium tetroxide (OsO4), 0.8% potassium ferrocyanide, and 10 mM CaCl2 in 0.1 M cacodylate buffer, dehydrated in an increasing acetone gradient, and embedded in Epon resin for 72 h at 60 °C. Ultrathin sections were obtained, stained with uranyl acetate and lead citrate, and examined using a JEOL JM 1400 transmission electron microscope. For SEM, promastigotes were placed on a glass specimen support with poly-L-lysine, dehydrated in a graded series of ethanol, critical-point dried in CO2, coated with gold, and examined using a FEI Quanta 250 FEG scanning electron microscope.
Evaluation of the mechanism of action of clomipramine in L. amazonensis promastigotes and amastigotes
To elucidate the mechanism of action of clomipramine in L. amazonensis, we performed a series of spectrometric experiments. For that promastigotes (1 × 106 cells/ml) were treated with clomipramine (8 and 22 μM) for 24 h. Intracellular amastigotes (1 × 106 cells/ml) were isolated from infected macrophages previously incubated with clomipramine (15 and 30 μM) for 24 h. In parallel, for assays that involved promastigotes, before drug treatment, half of the parasites were pre-incubated with the antioxidant NAC (200 μM) for 3 h to investigate the possible involvement of reactive oxygen species (ROS) in some of the parameters. For all the fluorimetric assays, parasites were distributed in black 96-well plates before reading, and measurements were performed at a plate multireader (Victor X3; PerkinElmer).
Determination of mitochondrial membrane potential
To investigate the influence of clomipramine on the mitochondrial membrane potential (ΔΨm), the probe Tetramethylrhodamine Ethyl Ester (TMRE) was applied with the modified protocol previously described by Miranda et al. (2017). Treated promastigotes were incubated with 25 nM TMRE for 30 min. Fluorescence was read at λex = 540 nm and λem = 595 nm. CCCP (100 μM) was used as a positive control.
Measurement of intracellular adenosine triphosphate levels
The cellular ATP levels in parasites were determined using the Cell Titer-Glo Luminescent Cell Viability Assay, according to the manufacturer instruction. After treatment, parasites were washed, resuspended in PBS, and incubated, in a white 96-well plate, with an equal volume of CellTiter-Glo reagent for 10 min. Luminescence was measured in a microplate reader (VICTOR X3, PerkinElmer). KCN (500 μM) was used as a positive control.
Evaluation of oxidative stress
To determine the influence of clomipramine on redox metabolism in Leishmania, we evaluated the total ROS, the H2O2 levels, and the amount of nitric oxide. For that, after treatment parasites were stained, respectively, with the fluorescent markers H2DCFDA, Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit, and DAF-FM diacetate according to the manufacturer’s instructions in protocols previously modified (Miranda et al. 2017; Lazarin-Bidóia et al. 2013). Fluorescence values were measured at λex/em = 488/530 nm (ROS), λex/em = 571/585 nm and λex/em = 495/515 nm. Cells treated with H2O2 (4 mM) were used as positive control.
Measurement of mitochondrial O2 ·− levels
The generation of mitochondrial O2·− was detected using the MitoSOX Red mitochondrial superoxide assay kit. The experiments were performed according to the manufacturer instructions, with minor modifications (Lazarin-Bidóia et al. 2013). Treated promastigotes were resuspended in Krebs-Henseleit (KH) buffer (15 mM NaHCO3, 5 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4, and 1.5 mM NaH2PO4, pH 7.3), and incubated with MitoSOX reagent (5 μM) for 20 min in the dark at 25 °C. Readings were performed at λex = 510/580 nm. Antimycin A (10 μM) was used as a positive control.
Measurement of reduced thiol levels
Considering that the antioxidant defense of kinetoplastids mainly relies on trypanothione, a thiol-based molecule, we quantified the levels of reduced thiols using the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) as previously described (Lazarin-Bidóia et al. 2013). Clomipramine-treated promastigotes (1 × 107 cells/ml) were collected, resuspended in Tris-HCl buffer (100 mM, pH 2.5), and sonicated. The supernatant was collected and incubated with DTNB (1 mM) in PBS. Absorbance was read in a microplate reader at 412 nm (BIO-TEK Power Wave XS spectrophotometer). H2O2 (4 mM) was used as a positive control.
Evaluation of lipid peroxidation and lipid droplets accumulation
In addition, we measured the accumulation of lipid droplets and the lipid peroxidation using, respectively, the probes Nile red and DPPP (1,3-bis (diphenylphosphino) propane (Takahashi et al. 2001). Treated promastigotes were washed in PBS buffer and incubated with DPPP (5 μM) or Nile red (50 μM) in the dark at room temperature for 15 or 30 min, respectively. Fluorometric readings were performed at λex/em = 355/460 nm for DPPP and λex/em = 385/535 nm for Nile red. In both assays, H2O2 (4 mM) was used as a positive control.
Analysis of cell membrane integrity
The integrity of cell membrane was assessed by propidium iodide (PI), is modified protocol as previously described (Miranda et al. 2017). After treatment with clomipramine, the parasites were stained with PI (0.2 μg/ml) for 5 min at room temperature while protected from light. Samples were read at λex = 535 nm and λem = 617 nm. Digitonin (40 μM) was used as a positive control.
Detection of DNA fragmentation by the TUNEL assay
To determine DNA fragmentation, clomipramine-treated parasites were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 for 10 min, and stained according to the manufacturer’s instructions, with minor modifications (APO-BrdU TUNEL Assay Kit with Alexa Fluor 488 Anti-BrdU). Fluorescence was quantified in a spectrofluorometer at λex/em = 485/520 nm. Camptothecin (10 μM) was used as a positive control.
Detection of phosphatidylserine exposure
Phosphatidylserine exposure was detected using the probe annexin V/FITC as previously described (Miranda et al. 2017). After treatment, promastigotes were washed and resuspended in binding buffer (140 mM NaCl, 5 mM CaCl2, and 10 mM HEPES-Na, pH 7.4) with annexin V/FITC for 15 min. Readings were performed at λex/em = 494/518 nm. Camptothecin (10 μM) was used as a positive control.
Determination of caspase 3/7-like activity
To analyze the occurrence of apoptosis, caspase-like activity was determined using the EnzChek Caspase-3 Assay Kit #1 Z-DEVD-AMC Substrate. Clomipramine-treated promastigotes were washed in PBS buffer, and stained according to the manufacturer’s instructions. Readings were performed in a spectrofluorometer at λex/em = 342/441 nm. Camptothecin (10 μM) was used as a positive control. Similar groups were also pre-incubated with the caspase inhibitor (Ac-DEVD-CHO) available on the kit.
Detection of autophagic vacuoles
Autophagic vacuoles were quantified using MDC. After treatment with clomipramine, promastigotes and amastigotes were resuspended in PBS buffer, and incubated with MDC (0.05 mM) for 1 h. As a positive control for autophagy, we used 7-day-old cultures of promastigotes. The fluorescence was read at λex/em = 335/518 nm. For promastigotes, in parallel, we pretreated a group with wortmannin (0.5 μM), a potent inhibitor of PI3 kinase and consequently autophagic vacuole formation (Blommaart et al. 1997).
Statistical analysis
All of the quantitative experiments were conducted in at least three independent experiments that were performed in duplicate. The data are expressed as mean and standard error of the mean. The data were analyzed using one-way or two-way analysis of variance (ANOVA) when appropriate, followed by the Bonferroni post hoc test using Prism 5.0 software (GraphPad, San Diego, CA, USA). Values of p ≤ 0.05 were considered statistically significant.
Results
Clomipramine selectively inhibits the proliferation of L. amazonensis
The antidepressant clomipramine was a selective inhibitor of both extracellular and intracellular forms of the parasite. Against promastigotes, clomipramine had an IC50 of 8.31 ± 3.29 μM and IC90 of 21.58 ± 3.44 μM. Clomipramine also inhibited proliferation of the intracellular form of the parasite, with an IC50 of 15.45 ± 4.92 μM and IC90 of 31.38 ± 3.27 μM. The cytotoxicity testing of the drug revealed a good safety profile, inhibiting 50% of macrophage cell growth at 181.22 ± 8.04 μM. These results indicate that clomipramine is more selective against the parasites than host cells (Selective Indexes of 11.72 in amastigotes and 21.81 in promastigotes).
Nitric oxide production by macrophages is stimulated by clomipramine
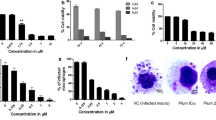
To investigate the effect of clomipramine directly on macrophages, we quantified NO production in these cells using the probe DAF-FM diacetate. Clomipramine dose-dependently augmented intracellular NO levels in macrophages, increasing fluorescence by more than 50% after 30 μM treatment (Fig. 1). The well-known macrophage activator LPS also significantly increased NO production.
Production of nitric oxide (NO) in macrophages (J774A.1) after 24 h treatment with clomipramine. Nitric oxide levels were measured in a spectrofluorimeter using the fluorescent probe DAF-FM. The data are expressed as the mean ± SEM of at least three independent experiments. Lipopolysaccharide (LPS) was used as an activator of macrophages. *p ≤ 0.05, compared with untreated control
Clomipramine induces morphological and ultrastructural alterations in L. amazonensis
After determining the inhibitory concentrations of clomipramine, we performed SEM of promastigotes to determine the morphological alterations that are caused by clomipramine. Untreated cells exhibited a normal elongated body with a prominent flagellum and smooth and intact cell surface (Fig. 2a). Parasites that were treated with clomipramine exhibited severe alterations of cell shape (Fig. 2b), with a wrinkled cell surface, a smaller cell body size, and distortions of the flagellum but with the maintenance of cell membrane integrity. Interestingly, we also observed the accumulation of vesicles on the surface of the flagellar pocket (Fig. 2c) in treated promastigotes. Clomipramine also caused ultrastructural alterations in L. amazonensis. We focused on large parasitophorous vacuoles in infected macrophages. One notable alteration that we observed in treated parasites was flagellar pockets that were full of vesicles, possibly indicating intense exocytic activity (Fig. 3b, d, f). Another prominent consequence of clomipramine treatment was large multivesicular vacuoles that were spread around the cytosol, filled with electron-dense cargo, and full of membranous arrangements (Fig. 3c–f). An increase in lipid inclusion bodies was also observed (Fig. 3c, e, f). These alterations were already evident at the IC50 of clomipramine but became more pronounced at the IC90. In amastigotes, we also observed mitochondrial swelling (Fig. 3e), an increase in endoplasmic reticulum profiles, and an increase in Golgi complex vesiculation.
Morphological analysis (scanning electron microscopy) of L. amazonensis promastigotes that were treated with clomipramine for 72 h. a Control parasites had a normal elongated body, with a smooth and intact cell surface. b Promastigotes that were treated with 8 μM clomipramine had a wrinkled cell surface, altered flagellum, and smaller cell size. c, d Treatment with 22 μM clomipramine caused the same alterations as in b and the additional accumulation of vesicles in the flagellar pocket region (detail in c). Scale bar = 2 μm
Ultrastructural analysis (transmission electron microscopy) of L. amazonensis amastigotes, detail on J774A.1 macrophage vacuoles after 48 h treatment with clomipramine. a Control parasites exhibited a normal ultrastructure with preserved organelles. Parasites that were treated with b, c 15 μM and d–f 30 μM exhibited alterations of the Golgi complex with an enlarged cisternae, disassembled endoplasmic reticulum (white arrows), intense vesicle traffic (black arrows), and a flagellar pocket full of cargo. Numerous lipid inclusions (+) were also observed throughout the cytoplasm with all treatments. Autophagy-related structures, such as double-membrane autophagosomes, large autolysosomes (#), and myelin figures, were often observed. Plasma membrane shedding (black arrowhead) was seen in the most affected cells. N, nucleus; K, kinetoplast; F, flagellum; M, mitochondrion; G, Golgi complex; fp, flagellar pocket. Scale bar = 0.5 μm
Clomipramine induces oxidative stress in L. amazonensis
Treatment with 8 μM clomipramine induced several alterations of redox metabolism in promastigotes, which was clearly reflected by a significant increase in total ROS levels (Fig. 4a). We assessed which ROS were more abundant during this state of oxidative stress. The fluorescent values of H2O2 almost doubled after treatment with 8 μM clomipramine (Fig. 4b). Different responses were observed for mitochondrial superoxide (Fig. 4c) and NO (Fig. 4d), in which no statistically significant alterations were detected at the lower concentration of clomipramine. Only the 22 μM concentration led to a significant response. Pretreatment with the antioxidant NAC prevented oxidative stress that was induced by clomipramine in most cases.
Levels of reactive oxygen/nitrogen species in promastigotes of L. amazonensis that were treated for 24 h with clomipramine (8 and 22 μM). a H2DCFDA as an indicator of total ROS. b Amplex Red as an indicator of H2O2 production. c MitoSOX Red as an indicator of mitochondrial O2·−. d DAF-FM as an indicator of intracellular NO levels. Gray bars, treatment with only clomipramine. Black bars, parasites pre-incubated with 200 μM NAC for 3 h before the addition of clomipramine. Antimycin A (2 μM) and H2O2 (0.25 mM) were used as inducers of oxidative stress. *p ≤ 0.05, compared with untreated control; αp ≤ 0.05, comparison between pretreatment with NAC and no pretreatment
Related to redox metabolism, the levels of reduced thiols were also affected by clomipramine treatment (Fig. 5a), indicating inhibition of the enzyme trypanothione reductase. When intracellular amastigotes were treated, we observed a clear increase in the amount of total ROS in the parasites (Fig. 6d), an increase in H2O2 levels (Fig. 6c), and a dose-dependent increase in DCF and Amplex Red fluorescence in isolated amastigotes that were treated with clomipramine (15 and 30 μM).
Levels of a reduced thiols, b lipoperoxidation, c lipid droplet accumulation, and d permeability of the plasma membrane in promastigotes of L. amazonensis that were treated for 24 h with clomipramine (8 and 22 μM). Gray bars, treatment with only clomipramine. Black bars, parasites pre-incubated with 200 μM NAC for 3 h before the addition of clomipramine. H2O2 (0.25 mM) was used as an inducer of oxidative stress. The detergent digitonin (40 μM) was used as a disruptor of the membrane. *p ≤ 0.05, compared with untreated control; αp ≤ 0.05, comparison between pretreatment with NAC and no pretreatment
Evaluation of the effects of clomipramine (15 and 30 μM) on the physiology and metabolism of intracellular amastigotes of L. amazonensis. a Mitochondrial membrane potential, b ATP levels, c H2O2 production, d total ROS levels, e permeability of the plasma membrane, and f lipid droplet accumulation were measured in intracellular amastigotes that were isolated from macrophages after 24 h treatment. H2O2 (4 mM) was used as an inducer of oxidative stress. CCCP (100 μM) was used as a mitochondrial uncoupler. KCN (500 μM) was used as a cytochrome c oxidase inhibitor. Camptothecin (10 μM) was used as an inducer of apoptosis. *p ≤ 0.05, compared with untreated control
Oxidative stress affects the cell membrane of promastigotes
The intense oxidative stress that was caused by clomipramine treatment compromised the cell membrane integrity of the parasites. Although no significant effect was observed at the IC50 of clomipramine, treatment with 22 μM increased lipid peroxidation in promastigotes (Fig. 5b). Similarly, clomipramine caused the accumulation of lipid bodies (Fig. 5c) and alterations of cell membrane permeability (Fig. 5d) at the higher concentration tested. These effects were at least partially inhibited when the parasites were pretreated with NAC. Intracellular amastigotes presented a different profile. Despite high ROS levels that were induced by clomipramine, intracellular parasites did not suffer lipoperoxidation (data not shown) or the loss of cell membrane integrity (Fig. 6e). Intracellular amastigotes still presented an increase in the accumulation of lipid bodies at 30 μM clomipramine (Fig. 6f).
Clomipramine disrupts mitochondrial membrane potential in L. amazonensis
The effect of clomipramine on mitochondrial physiology in L. amazonensis varied in the two different forms of the parasite. In promastigotes, the uncoupler CCCP inhibited ∆Ψ by 35.3% when compared with untreated controls. Clomipramine also induced an inhibition in ∆Ψ by 32.3 and 49.2% at 8 and 22 μM, respectively (Fig. 7a). Despite the alterations of mitochondrial physiology, cellular ATP levels were unaffected in promastigotes, even at the higher concentration tested (Fig. 7b). Intriguingly, we observed an analogous effect on ∆Ψ in amastigotes (Fig. 6a) but also an additional reduction of intracellular ATP levels at the IC50 and IC90 of clomipramine, similar to the effects of the uncoupler CCCP (Fig. 6b).
Evaluation of mitochondrial metabolism and cell death parameters in promastigotes of L. amazonensis that were treated for 24 h with clomipramine (8 and 22 μM). a Mitochondrial membrane potential and b ATP levels were measured as indicators of energetic metabolism. c DNA fragmentation, d phosphatidylserine exposure, and e caspase 3–7 activity were evaluated as hallmarks of apoptosis. Gray bars, treatment with only clomipramine. Black bars, parasites pre-incubated with 200 μM NAC for 3 h before the addition of clomipramine. White dotted black bars, cells incubated with the apoptosis inhibitor Ac-DEVD-CHO. H2O2 (0.25 mM) was used as an inducer of oxidative stress. CCCP (100 μM) was used as a mitochondrial uncoupler. KCN (200 μM) was used as a cytochrome c oxidase inhibitor. Camptothecin (10 μM) was used as an inducer of apoptosis. *p ≤ 0.05, compared with untreated control; αp ≤ 0.05, comparison between pretreatment with NAC and no pretreatment
Clomipramine-treated promastigotes exhibit hallmarks of apoptosis
The mechanism of cell death that was induced in promastigotes by clomipramine was assessed by fluorimetry. After 24 h treatment with the antidepressant, promastigotes exhibited strong staining of annexin V/FITC, an indicator of phosphatidylserine (PS) and related compounds exposure, with 80.2 and 101.7% increases in total fluorescence at 8 and 22 μM, respectively (Fig. 7d). We also evaluated caspase 3–7 activity as another marker of apoptosis, which was unaffected at the lower concentration of clomipramine. Only slight activation was observed at 22 μM (Fig. 7e). Clomipramine dose-dependently induced DNA fragmentation, with a 42.7% increase at the lower concentration and a 98.7% increase at the higher concentration (Fig. 7c). Pretreatment with NAC prevented phosphatidylserine exposure that was caused by clomipramine but did not significantly affect DNA fragmentation.
Clomipramine induces autophagy in parasites
We performed MDC staining to determine whether autophagy is related to the mechanism of action of clomipramine. Clomipramine-treated promastigotes exhibited 20.9 and 53.9% increases in MDC fluorescence at the IC50 and IC90, indicating autophagic vacuole accumulation. As expected, 7-day-old cultures of promastigotes exhibited strong autophagy, with double the level of MDC fluorescence compared with untreated controls (Fig. 8a). In intracellular amastigotes, we observed the induction of autophagy, in which the IC50 of clomipramine increased MDC fluorescence by 55.8%, and the IC90 increased autophagic vacuole formation by 64.8% (Fig. 8b).
Detection of autophagy in a promastigotes and b isolated intracellular amastigotes of L. amazonensis that were treated for 24 h with clomipramine (8 and 22 μM). Monodansylcadaverine (MDC) is a marker that accumulates in autophagic vacuoles. Seven-day-old promastigotes were analyzed as a positive control for the occurrence of autophagy. *p ≤ 0.05, compared with untreated control
Discussion
The repurposing of drugs is often successfully applied for the treatment of several diseases. Two of the main drugs that are currently used for the treatment of leishmaniasis, miltefosine and amphotericin B, were originally developed as antineoplasic and antifungal agents, respectively (Padhy and Gupta 2011). In the present study, we evaluated the antidepressant clomipramine as a potential drug candidate for the treatment of leishmaniasis. This research was motivated by previous data that showed that clomipramine was a potent inhibitor of tripanosomatids (Rivarola et al. 2005; Kaiser et al. 2015; Rodrigues et al. 2016). We observed the inhibitory activity of clomipramine at micromolar concentrations against both the extracellular and intracellular forms of L. amazonensis. The safety of clomipramine was reflected by the good SIs against both forms of the parasite when activity was compared with toxicity against mammalian kidney cells. Its safety is further assured by the fact that it has long been used by general population.
The elimination of an intracellular parasite by an antimicrobial agent occurs mainly through two mechanisms: direct killing of the parasite by the compound or stimulation of the host cell to combat the microbial intruder. By analyzing aspects of the host, we observed marked stimulation of NO production in macrophages that were incubated with clomipramine. Together with the antiproliferative activity of the compound, such a response may contribute to the complete elimination of L. amazonensis amastigotes, in which NO plays a major role in intracellular parasite killing (Holzmuller et al. 2006).
Trypanosomes are microorganisms with a peculiar ultrastructure and unique features that differentiate them from host cells. Among these characteristics are single and ramified mitochondria that are attractive cellular targets for drug development (Menna-Barreto et al. 2009). To assess whether this organelle may be a target of the activity of clomipramine against L. amazonensis, we performed ultrastructural and biochemical analyses of treated parasites. Both forms of the parasite had mitochondria that were strongly affected by clomipramine, expressing intense mitochondrial membrane depolarization. Interestingly, pretreatment with the antioxidant NAC did not affect the response that was induced by the drug in promastigotes. Our findings clearly showed mitochondrial depolarization as an initial mechanism of action of clomipramine, rather than the induction of pronounced oxidative stress. The TEM analysis revealed mitochondrial swelling in treated parasites, and these alterations may be a physiological consequence of the ionic imbalance that is created by depolarization (Safiulina et al. 2006). A comparable effect was previously observed when T. brucei was treated with clomipramine (Rodrigues et al. 2016).
The tricyclic antidepressant imipramine was found to be more active than miltefosine in vivo against L. donovani (Mukherjee et al., 2012). These authors found that imipramine strongly reduced ΔΨm in L. donovani, similar to our previous observations in L. amazonensis, in both amastigotes and promastigotes. Higgins and Pilkington (2010) investigated the effects of tricyclic antidepressants on glioma cells and found that clomipramine is a strong inhibitor of ΔΨm and consequently a potent inducer of apoptosis, thus corroborating our hypothesis that mitochondria are a cellular target of this drug.
After finding that clomipramine disrupted ∆Ψm in L. amazonensis and knowing that ∆Ψm is coupled with the mitochondrial synthesis of ATP (Okuno et al. 2011), we further investigated the intracellular amounts of this energetic molecule. Intracellular amastigotes exhibited changes in ATP levels that were proportional to the disruption of ∆Ψm. In contrast, promastigotes exhibited severe disruption of ∆Ψ, with no changes in ATP levels. Such discrepancies may occur through metabolic specificities of the two distinct forms of the parasite.
Trypanosomatids pass through different environments and suffer numerous metabolic adaptations during their life cycle (Bringaud et al. 2006). A proteomic study of L. donovani showed that extracellular promastigotes utilize glucose as a main energy source, whereas amastigotes utilize fatty acids and amino acids as its main energy matrix (Rosenzweig et al. 2007). Considering the metabolic differences between the forms of the parasite and our observations of constant levels of ATP in clomipramine-treated promastigotes even after ∆Ψ disruption, substrate-level phosphorylation may be mainly responsible for maintaining ATP levels in these extracellular parasites, similar to previous observations in procyclic T. brucei (Bringaud et al. 2006).
To obtain additional information about mitochondrial physiology in clomipramine-treated L. amazonensis, we investigated redox metabolism. Both promastigotes and amastigotes exhibited intense oxidative stress after clomipramine treatment, with initially higher levels of total ROS and significant increases in H2O2. Consistent with a previous study (Benson et al. 1992), the present results suggest that clomipramine impairs the antioxidant enzyme trypanothione reductase and thus prevents ROS detoxification, clearly contributing to oxidative stress. Mitochondrial O2·− increased in promastigotes only at the higher concentrations of clomipramine, suggesting that nascent superoxide in the electron transport chain is not the main mechanism by which clomipramine acts initially.
The phenomena that occurred in the parasite when the lower dose (i.e., IC50) of clomipramine was used may reveal the initial steps of its complex mechanism of action. Higher concentrations (i.e., the IC90) of clomipramine resulted in more alterations, possibly because of the widespread cellular demise that occurred through several secondary effects.
As a consequence of extensive oxidative stress in the parasites, promastigotes exhibited high levels of lipoperoxidation already at 8 μM clomipramine, with membrane permeabilization and lipid body accumulation at the higher dose. The partial inhibition of these effects by the antioxidant NAC demonstrates the causal relationship between oxidative stress and alterations of cell membrane permeability in promastigotes. Intriguingly, the same effect was not observed in intracellular amastigotes. Although they presented high amounts of ROS even at the lower dose of clomipramine, they did not suffer lipoperoxidation or the loss of cell membrane integrity. This resilience of amastigotes to multiple stresses may reflect their slower growth and stringent metabolic response that were evolutionarily acquired as an adaptation to the harsh conditions of parasitophorous vacuoles (McConville et al. 2015).
Multiple alterations were observed at the mitochondrial level, including swelling, ROS generation, and membrane depolarization. This organelle is one of the sites of the initiation of apoptosis (Menna-Barreto and de Castro 2014). We investigated the occurrence of programmed cell death in clomipramine-treated promastigotes. Apoptosis, which may be the subtype of programmed cell death that is better described in higher eukaryotes, is characterized by cell shrinkage, chromatin condensation, chromosomal DNA fragmentation, a decrease in ΔΨm, phosphatidylserine exposure, and caspase activation (Smirlis et al. 2010; Galluzzi et al. 2015). Our results strongly support the occurrence of apoptosis-like programmed cell death.
A similar occurrence of programmed cell death was observed in protozoan parasites (Menna-Barreto et al. 2009; Desoti et al. 2015; Rodrigues et al. 2016). Although the parasites presented morphological features of apoptotic cell death, they lacked crucial molecular events, such as caspase activation, which is necessary for apoptosis in higher eukaryotes (Menna-Barreto and de Castro 2014). Despite the presence of orthologues of caspases (i.e., metacaspases) in pathogenic trypanosomatids, their involvement in cell death is doubtful (Proto et al. 2012). This might explain the minimal increase in caspase 3–7-like activity that we observed in clomipramine-treated promastigotes, whereas our observations of a reduction of cell size, membrane shrinkage, DNA fragmentation, and phosphatidylserine exposure clearly indicate the occurrence of apoptosis even at the lower dose of clomipramine.
Trypanosomatids might exhibit a rather primitive type of apoptosis that does not primarily depend on caspases but rather relies on ROS formation (Duszenko et al. 2006). Our findings corroborate this possibility. Antioxidant pretreatment prevented PS exposure in clomipramine-treated promastigotes.
Regarding the PS exposure, even though our results shown strong annexin V staining after clomipramine treatment in promastigotes, it is important to consider that this marker can also bind at a lesser extent to other membrane phospholipids, including phosphatidylglycerol and phosphatidyl inositol-4,5 bisphosphate (Yeung et al. 2009). Even the existence of PS in leishmania parasites is still a matter of debate, though some authors reported the lack of PS in L. donovani membranes (Weingärtner et al. 2012), others reported the presence of PS and its relationship to apoptosis in Leishmania sp. (Shadab et al., 2017; Wanderley et al., 2013; Imbert et al., 2012), similarly to what we observed.
Together with mitochondrial injury, clomipramine-treated parasites exhibited disturbances of the endomembrane system, with alterations of the Golgi complex and a flagellar pocket that was full of vesicles, as detected by TEM. The relationship between apoptosis and the secretory pathway is not entirely clear, but some evidence suggests that the endoplasmic reticulum and Golgi complex can act as sensors of cellular stress and are involved in the so-called unfolded protein response (Walter and Ron 2011). During the unfolded protein response, these organelles might first trigger recovery mechanisms through widespread trafficking between cell compartments. In the case of extensive damage, they might participate in the initiation of apoptosis. As the cell is dismantled during apoptosis, the endoplasmic reticulum vesiculates, and the Golgi complex disassembles, resulting in the loss of their cisterna arrangement and turning them into vesicles and tubular clusters (Maag et al. 2003; Hicks and Machamer 2005).
The ultrastructural analysis of clomipramine-treated intracellular amastigotes also revealed the appearance of peculiar compartments, characterized by multivesicular vacuoles that were surrounded by a single membrane and filled with electron-dense cargo. According to the most recent guidelines for monitoring and interpreting autophagy (Klionsky et al. 2016), such observations are close to the definition of autolysomes, recognized as autophagy-related structural products of the fusion of double-membrane autophagosomes with lysosomes. Interestingly, similar structures were observed in clomipramine-treated T. brucei (Rodrigues et al. 2016). Together with the unfolded protein response, autophagy might be understood as a set of adaptive responses that are initially aimed at recovering an injured cell. Because of extensive damage and compromised cell function, autophagy may contribute to cell death (Galluzzi et al. 2015).
Additional ultrastructural findings showed that clomipramine caused intense exocytic activity in L. amazonensis, which was clearly demonstrated by the numerous vesicles inside the flagellar pocket in treated parasites. The trypanosomal flagellar pocket is the region where major exocytosis and endocytosis occur in the parasite (Field and Carrington 2009). The accumulation of vesicles may indicate the intense exocytosis of abnormal macromolecules, as remnants of autophagy or misfolded proteins and lipids (Lorente et al. 2004).
The present study showed that the antidepressant clomipramine has potential as a repurposed drug for the treatment of L. amazonensis infection. The demonstrated safety of the drug, its activity against both forms of the parasite, and the activation of macrophages support further studies of novel therapeutics for cutaneous leishmaniasis. Aware about the complexity and extensive cross talk between the cell death mechanisms in trypanosomatids, we tried to connect our results in order to shed some light on the mechanism of action of clomipramine. Our results support the suggestion that the antidepressant acts through a mitochondrial pathway in L. amazonensis (Fig. 9). By inhibiting trypanothione reductase activity and directly interfering with ΔΨm, clomipramine caused severe oxidative stress in the parasite. Autophagy and remodeling of the endomembrane system may reflect adaptive responses that unsuccessfully restored cellular homeostasis in the parasite, culminating in the appearance of programmed cell death hallmarks, characteristically apoptosis-like. Interestingly, differences between the two forms of the parasite were observed. Promastigotes were generally more susceptible to clomipramine-induced oxidative stress. Amastigotes, although resilient to most of the effects of ROS accumulation, exhibited mitochondrial depolarization and the consequent decline of ATP levels, with greater dependence on mitochondrial energetic metabolism.
Suggested mechanism of action of clomipramine in L. amazonensis. The drug acts first by inhibiting trypanothione reductase (TR) and impairing the mitochondrial membrane potential (ΔΨ). Parasites are taken to intense accumulation of reactive oxygen species (ROS), and consequently display autophagy and augmented exocytosis. Parasites end up dying by apoptosis-like programmed cell death, exhibiting several hallmarks
References
Andrews KT, Fisher G, Skinner-Adams TS (2014) Drug repurposing and human parasitic protozoan diseases. Int J Parasitol Drugs Drug Resist 4:95–111
Barral A, Pedral-Sampaio D, Grimaldi-Júnior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, Almeida R, Badaro R, Barral-Netto M, Carvalho EM (1991) Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg 44:536–546
Benson TJ, McKie JH, Garforth J, Borges A, Fairlamb A, Douglas KT (1992) Rationally designed selective inhibitors of trypanothione reductase. Phenothiazines and related tricyclics as lead structures. Biochem J 286:9–11
Berman JD, Dwyer DM, Wyler DJ (1979) Multiplication of Leishmania in human macrophages in vitro. Infect Immun 26:375–379
Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243:240–246
Bringaud F, Rivière L, Coustou V (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol 149:1–9
Desoti VC, Lazarin-Bidóia D, Martins-Ribeiro F, Martins SC, Rodrigues JHS, Ueda-Nakamura T et al (2015) The combination of vitamin K3 and vitamin C has synergic activity against forms of Trypanosoma cruzi through a redox imbalance process. PLoS One 10:1–23
Duszenko M, Figarella K, Macleod ET, Welburn SC (2006) Death of a trypanosome: a selfish altruism. Trends Parasitol 22:536–542
El Mansari M, Blier P (2006) Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 30:362–373
Field MC, Carrington M (2009) The trypanosome flagellar pocket. Nat Rev Microbiol 7:775–786
Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson S, Abrams JM, Adam D et al (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 22:58–73
Georgiadou SP, Makaritsis KP, Dalekos GN (2015) Leishmaniasis revisited: current aspects on epidemiology, diagnosis and treatment. J Transl Int Med 3:43–50
Hammond DJ, Cover B, Gutteridge WE (1984) A novel series of chemical structures active in vitro against trypomastigote form of Trypanosoma cruzi. Trans R Soc Trop Med Hyg 78:91–95
Hicks SW, Machamer CE (2005) Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta 1744:406–414
Higgins SC, Pilkington GJ (2010) The in vitro effects of tricyclic drugs and dexamethasone on cellular respiration of malignant glioma. Anticancer Res 398:391–397
Holzmuller P, Bras-Gonçalves R, Lemesre J (2006) Phenotypical characteristics, biochemical pathways, molecular targets and putative role of nitric oxide-mediated programmed cell death in Leishmania. Parasitology 132:19–32
Kaiser M, Mäser P, Tadoori LP, Loset JR, Brun R (2015) Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PLoS One 10:1–16
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G et al (2016) Guidelines for use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222
Lazarin-Bidóia D, Desoti VC, Ueda-Nakamura T, Dias Filho BP, Nakamura CV, Silva SO (2013) Further evidence of the trypanocidal action of eupomatenoid-5: confirmation of involvement of reactive oxygen species and mitochondria owing to a reduction in trypanothione reductase activity. Free Radic Biol Med 60:17–28
Lorente SO, Rodrigues JCF, Jime C, Joyce-menekse M, Rodrigues C, Croft SL et al (2004) Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob Agents Chemother 48:2937–2950
Maag RS, Hicks SW, Machamer CE (2003) Death from within: apoptosis and the secretory pathway. Curr Opin Cell Biol 15:456–461
McConville MJ, Saunders EC, Kloehn J, Dagley MJ (2015) Leishmania carbon metabolism in the macrophage phagolysosome-feast or famine? F1000Research 4:1–11
Menna-Barreto RFS, de Castro SL (2014) The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed Res Int 2014:1–14
Menna-Barreto RFS, Corrêa JR, Cascabulho CM, Fernandes MC, Pinto V, Soares MJ et al (2009) Naphthoimidazoles promote different death phenotypes in Trypanosoma cruzi. Parasitology 136:499–510
Moradin N, Descoteaux A, Beverley SM (2012) Leishmania promastigotes: building a safe niche within macrophages. Front Cell Infect Microbiol 2:1–7
Mukherjee S, Mukherjee B, Mukhopadhyay R, Naskar K, Sundar S, Dujardin JC et al (2012) Imipramine is an orally active drug against both antimony sensitive and resistant Leishmania donovani clinical isolates in experimental infection. PLoS Negl Trop Dis 6:1–15
Munos B (2009) Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 8:959–968
Okuno D, Lino R, Noji H (2011) Rotation and structure of FoF1-ATP synthase. J Biochem 149:655–664
Pace D (2014) Leishmaniasis. J Inf Secur 69:10–18
Padhy B, Gupta Y (2011) Drug repositioning: re-investigating existing drugs for new therapeutic indications. J Postgrad Med 57:153–160
Proto WR, Coombs GH, Mottram JC (2012) Cell death in parasitic protozoa: regulated or incidental? Nat Rev Microbiol 11:58–66
Rivarola HW, Bustamante JM, Presti SL, Fernández AR, Enders JE, Gea S et al (2005) Trypanosoma cruzi: chemotherapeutic effects of clomipramine in mice infected with an isolate obtained from an endemic area. Exp Parasitol 111:80–86
Rodrigues JHS, Stein J, Strauss M, Rivarola HW, Ueda-Nakamura T, Nakamura CV, Duszenko M (2016) Clomipramine kills Trypanosoma brucei by apoptosis. Int J Med Microbiol 306:196–205
Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, Zilberstein D (2007) Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J 22:590–602
`Safiulina D, Veksler V, Zharkovsky A, Kaasik A (2006) Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurones. J Cell Physiol 206:347–353
Smirlis D, Duszenko M, Ruiz A, Scoulica E, Bastien P, Fasel N, Soteriadou K (2010) Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit Vectors 3:107–132
Takahashi M, Shibata M, Niki E (2001) Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radic Biol Med 31(2):164–174
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
WHO (2018) Leishmaniasis. World Health Organization. http://www.who.int/mediacentre/factsheets/fs375/en/. Accessed 06 July 2018
Zilberstein D, Dwyer DM (1984) Antidepressants cause lethal disruption of membrane function in the human protozoan parasite Leishmania. Science 226:977–979
Acknowledgments
We thank all the staffs of the “Laboratório de Inovação Tecnológica no Desenvolvimento de Fármacos e Cosméticos” and the “Complexo de Centrais de Apoio à Pesquisa (COMCAP-UEM)”.
Funding
This study was supported by grants of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Financiadora de Estudos e Projetos (FINEP) and Programa de Núcleos de Excelência (PRONEX/Fundação Araucária).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Sarah Hendrickx
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Rodrigues, J.H., Miranda, N., Volpato, H. et al. The antidepressant clomipramine induces programmed cell death in Leishmania amazonensis through a mitochondrial pathway. Parasitol Res 118, 977–989 (2019). https://doi.org/10.1007/s00436-018-06200-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-06200-x