Abstract
The genus Anableps is composed of species Anableps anableps, Anableps dowi, and Anableps microlepis. These fishes are tropical and usually live on the surface of brackish water, being popularly known as four-eyed-fishes due to the presence of prominent eyes and a pupil split horizontally. A. anableps and A. microlepis are considered as sister species that live in sympatry in South America. A. dowi, however, is restricted to the Pacific Ocean (Central America) and is considered the most primitive species of this genus. The aims of this study were to investigate the presence of endoparasites in A. anableps from the Parnaíba’s Delta and characterize them morphologically. During the necropsy, larvae of Contracaecum sp. in the third larval stage (L3) were collected from the pancreas of A. anableps, but no endoparasites were observed in other organs. The worms had a cuticular tooth and excretory pore located at the anterior end, a thread like body, whitish color, and without distinction of sex. The length of the ventricular appendix of the larvae was much greater than in other studies. This is the first report of endoparasitism in A. anableps and the first report of nematodes in four-eyed-fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The largest viviparous fish of the order Cyprinodontiformes are represented by species of the genus Anableps. They are popularly known as four-eyed fish due to the presence of prominent eyes with pupils slipt horizontally. These features allow the monitoring of both water and air environments simultaneously and the efficient exploitation of shallow environments (Miller 1979; Santos 1987; Bone et al. 1995). Three species are allocated in the genus Anableps Scopoli, 1777: Anableps anableps (Linnaeus, 1758), Anableps microlepis Müller and Troschel, 1844, and Anableps dowi Gill, 1861. Among these, the first two are considered sister species and live in sympatry in northern part of South America. However, A. dowi occurs only in the Pacific Ocean (Central America), and it is considered the most primitive species of the genus (Ghedotti 2003). According to Cervigón et al. (1993), the distribution of A. anableps extends from the Gulf of Paria in Venezuela to the Amazon River Delta, Brazil. A. microlepis can be found in South America, on Trinidad island and coastal basins from Venezuela to the Delta of the Amazon River, Brazil (Reis et al. 2003). According to published reports, observations from fish collections revealed the presence of A. anableps and A. microlepis at the delta of the Parnaíba River, in the state of Piauí, Brazil. Anableps spp. are omnivorous fish with a diet consisting of macroalgae, insects, small crustaceans, and suspended particulate matter (Brenner and Krumme 2007).
Studies on parasitic fauna of four-eyed-fish are extremely scarce. Only one species of endoparasitic helminth has been reported to date, Amapacanthus amazonicus Salgado-Maldonado & Santos, 2000 (Acanthocephala), which parasitizes A. microlepis from the Maracá island in the state of Roraima, Brazil (Salgado-Maldonado and Santos 2000). In addition, there is only one report of ectoparasitism by Gnathiidae praniza larvae in A. anableps from the city of Bragança, state of Pará, Brazil (Diniz et al. 2008).
Materials and methods
Sampling and handling
Twenty-eight A. anableps were captured in the Parnaíba River delta, located in the city of Luis Correia, State of Piauí, Brazil, during the night, when the fish are less active. The fish were caught by local fishermen on the banks of the mangrove channels using circular fishing net (flue net type) with 20-mm mesh. Shortly after collection, the fish were packed in insulated boxes containing ice. Later, still in the collection site, the fish were fixed in 10% formalin and stored in plastic containers for subsequent analysis. At the laboratory, the fish were identified by morphological characters, biometric data were analyzed (weight, total length, and standard length), and the sex of each individual was determined. All data were recorded in autopsy forms.
Necropsy procedures, collection, fixation, conservation, and clarification of the parasites were performed according to Amato et al. (1991) and Eiras et al. (2000). Internal organs were placed in Petri dishes, separately, with 0.65% saline solution, dissected, and observed by stereomicroscopy. After removal of the viscera, the coelomic cavity was washed with distilled water. The wash water was passed through a 0.25-mm-mesh sieve. The retained material was collected and placed in a Petri dish containing 0.65% saline solution and examined by stereomicroscopy. Parasite indices (prevalence, mean intensity, and mean abundance) were calculated as described by Bush et al. (1997).
Light microscopy
A portion of the collected nematode larvae was preserved in 70% ethanol, and then clarified in lactophenol between a slide and coverslip.
Measurements were taken to the nearest micrometer (range (mean ± S.D.)) of six third larval stage specimens isolated from different fishes. Measurements were performed with an Axioplan Zeiss light microscope (Carl Zeiss, Germany) equipped with a Canon Power-Shot A640 digital camera (Canon, China) and Zeiss AxionVision Sample Images Software (Carl Zeiss, Germany) for image analysis. Drawings of the parasites were performed with the aid of an Axioplan Zeiss light microscope (Carl Zeiss, Germany) equipped with a camera lucida and were digitized using Adobe Photoshop Elements 8.0 software with the aid of an Intuos4 Wacon® pen tablet (Wacon Co. Ltd., Japan).
Scanning electron microscopy
Nematode larvae were fixed in 2.5% glutaraldehyde, 4% freshly prepared paraformaldehyde, 5 mM calcium chloride in 0.1 M cacodylate buffer, pH 7.2 and postfixed in 2% osmium tetroxide in 0.1 M cacodylate buffer. The samples were dehydrated in an acetone series, critical point dried with CO2, sputter-coated with gold, and examined in a Zeiss EVO MA 10 scanning electron microscope (SEM) operating at 15 kV.
Representative specimens were deposited in the Helminthological Collection of the Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, RJ, under record number CHIOC no 38546.
Results
Third larval stage (L3) parasites were encysted in the pancreas of 17 fish, of which nine were females and eight were males. During the necropsies, a total of 52 larval specimens were collected, of which 36 were collected from females and 16 from males. A prevalence of 61% of parasitism was observed in the analyzed fishes, with a mean abundance of 3.06 and mean intensity of 1.86 parasites per fish.
More larvae were collected from female fish (average intensity of 4 ± 4.85, ranging from 1 to 16 larvae), than male fish (average intensity of 2 ± 0.93, ranging from 1 to 3 larvae per fish).
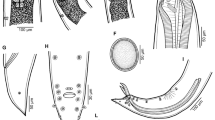
According to the morphological characters, the larvae were identified as Contracaecum sp. (Figs. 1, 2, and 3). They possessed an intestinal cecum directed to the anterior end, and excretory pore located laterally, next to the mouth opening, prior to the nerve ring (Figs. 1 b and 3b).
Scanning electron microscopy of anterior end of third stage larvae of Contracaecum sp. collected from four-eyed-fishes, A. anableps. a Anterior extremity showing lips (arrows) and oral aperture (OA). Bar 10 μm. b Ventral view of anterior region, showing lip (arrow) and aperture of excretory pore (arrow head). Bar 20 μm
The larvae were classified as third larval stage (L3) due the absence of a developed reproductive tract. Larvae present a filiform body, whitish in color, with tiny cuticular teeth at the anterior end, with a delicate contour and triangular shape (Figs. 1b, 2a, and 3). Total length ranged from 9496.6 to 12,717.6 (11,065.5 ± 1261.6); greater body width was observed at the posterior third ranging from 327.4 to 547.9 (425.2 ± 68.0); distance from the nerve ring to anterior end ranging from 310.0 to 328.9 (314.7 ± 11.0). Muscular esophagus measured 1079.7 to 1571.9 (1214.2 ± 190.9); intestinal caeca directed to the anterior end, measuring 586.5 to 849.6 (717.9 ± 109.5); presence of a long ventricular appendix measuring 3221.7 to 4001.2 (3116.1 ± 321.1). Intestinal caeca: ventricular appendix ratio about 1:5 (Fig. 1a). Tail with conical end without caudal spine (Fig. 1c) measuring 130.5 to 174.3 (158.1 ± 17.2). Body width at anus level ranging from 79.4 to 111.8 (96.9 ± 11.1).
Discussion
This study is the first report of endoparasites in A. anableps, and is also the first report of nematodes parasitizing fish of the genus Anableps. Previous studies only described ectoparasites isolated from this same fish species (Diniz et al. 2008; Martins et al. 2005) and a species of Acanthocephala in A. microlepis (Salgado-Maldonado & Santos, 2000). Thus far, there has been no report of parasitism in A. dowi.
The nematode larvae of Contracaecum are similar to those from Hysterothylacium by having the intestinal cecum directed to anterior end and a well-developed ventricular appendix. However, these two genera can be differentiated by the location of the excretory pore: in Contracaecum spp. it is located at the anterior end, near the oral aperture, following the cuticular tooth; in Hysterothylacium spp. the excretory pore is located in the esophagus region, at the level of nerve ring (Køie and Fagerholm 1995). According to these authors, it can be inferred that the larvae collected in this study belong to the genus Contracaecum. In addition to the morphological characteristics common to these two genera, the larvae observed in the present study have the excretory pore in the anterior region, which can be confirmed both by the scanning electron microscopy (Fig. 3b) and light microscopy (Fig. 1b).
Morphological analysis of larvae collected in this study did not present a developed reproductive system. For this reason, these parasites can be considered as third larval stage (L3). Moreover, according to Huizinga (1967), the second larval stage (L2) are thin, elongated, possess a cuticular tooth present on the ventral side of the buccal opening, have an undeveloped intestinal cecum, and esophagus, ventricle, and nerve ring with incomplete development, being difficult to be visualized with an magnification of ×400. In addition, L2 possess a thin intestine containing granular material inside.
The identification of the collected larvae at the species level was not possible, since the specific identification of nematodes is performed based on a set of morphological characters of the reproductive system, together with the morphometry of male and female specimens (Barson 2004). Moreover, there is a lack of studies describing the morphology and ultrastructure of nematode larvae. To identify L3 larvae, molecular biological studies are required, but in this case were not available.
The length of the ventricular appendix of the larvae of Contracaecum that were collected in this study was much greater than that of other parasites of the same genus isolated from different fish species (Yamaguti 1954; Martins et al. 2005; Pardo et al. 2009;. Shamsi and Aghazadeh-Meshgi 2011). The only exception to this are larvae collected from Otolithes sp. (Bloch & Schneider, 1801) which presented a length ranging from 3000 to 4600 μm (Yamaguti 1954) very close to that found in our study (3221.7 to 4001.2 μm) (Table 1).
The ratio between the total length of the larvae of Contracaecum found in the present study and the length of their ventricular appendix is greater than that described in other parasites of the same genus isolated from other fish species (Table 1). The larvae studied by Yamaguti (1954) in Otolithes sp. displayed a lower ratio between body length and the ventricular appendix than that observed in our study (1:4 and 1:3, respectively). This ratio is the closest to that of the larvae collected from A. anableps. Martins et al. (2005) and Pardo et al. (2009) found much lower ratios between the total body length and the length of the ventricular appendix in Contracaecum larvae collected from Hopliuserythrinus unitaeniatus (Spix & Agassiz, 1829) (1:42) and Soribim cuspicaudus Littmann, Burr & Nass (2000) (1:53). Medians proportions of the characteristics described above were reported in larvae collected from Synagris taeniopterus (Yamaguti, 1953) (1:17), Saurida gracilis (Quoy & Gaimard, 1824) (1:11), Chorinemus moadetta Cuvier, 1832 (1:11) (Yamaguti 1954), Barbus spp. (Cuvier & Cloquet, 1816) (1:26) (Shamsi and Aghazadeh-Meshgi 2011) when compared to the present study (Table 1). Variability in the length of the ventricular appendix in L3 between species of Contracaecum is observable. This characteristic can be used to help identify species of infective larvae. Klöser et al. (1992) distinguished two types of larvae according to total body length and the ratio of the length of the esophagus and intestinal cecum, although no other morphological differences between the two types of larvae were identified.
The body length of L3 of Contracaecum sp. isolated from A. anableps presented a total length longer than that of parasites of the same genus isolated from other fish species, with exception of larvae collected from H. unitaeniatus (Table 1). The total length of the larvae is not a reliable characteristic for distinguish species, since it continues its growth inside the fish, and where it can pass its whole life cycle (Køie and Fagerholm 1995; Garbin et al. 2013).
Worms collected from A. anableps presented cuticular teeth, but caudal spines were absent. Yamaguti (1954) and Shamsi and Aghazadeh-Meshgi (2011) described larvae of Contracaecum moadettae Yamaguti, 1954, collected from fish of the genus Barbus without cuticular tooth (Table 1), but with a visible caudal spine.
The current study has revealed that A. anableps participate in the epidemiological chain of at least one species of Contracaecum, since this parasite has a heteroxenous life cycle. Further studies are necessary to identify the Contracaecum larvae observed in A. anableps at the species level.
References
Amato JFR, Boeger WA, Amato SB (1991) Protocolos para laboratório—coleta e processamento de parasitos de pescado. Imprensa Universitária, Universidade Federal Rural do Rio de Janeiro, Seropédica
Barson M (2004) The occurrence of Contracaecum sp. larvae (Nematoda: Anisakidae) in the catfish Clarias gariepinus (Burchell) from Lake Chivero, Zimbabwe. Onderstepoort J Vet Res 71:35–39
Bone Q, Marshall NB, Blaxter JHS (1995) Biology of fishes. Chapman and Hall, London
Brenner M, Krumme U (2007) Tidal migration and patterns in feeding of the four-eyed fish Anableps anableps in a north Brazilian mangrove. J Fish Biol 70:406–427
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Cervigón F, Cipriani R, Fischer W, Garibaldi L, Hendrickx M, Lemus AJ, Márquez R, Poutiers JM, Robaina G, Rodriguez B (1993) FAO species identification sheets for fishery purposes: field guide to the commercial marine and brackish-water resources of the northern coast of South America. FAO, Rome
Diniz DG, Varella JEA, Guimarães MDF, Santos AFL, Fujimoto RY, Monfort KCF, Pires MAB, Martins ML, Eiras JC (2008) A note on the occurrence of praniza larvae of Gnathiidae (Crustacea, isopoda) on fishes from Northeast of Pará, Brazil. An Acad Bras Ciênc 80:657–664
Eiras JC, Takemoto RM, Pavanelli GC (2000) Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. EDUEM, Maringá
Garbin LE, Mattiucci S, Paoletti M, Diaz JI, Nascetti G, Navone GT (2013) Molecular identification and larval morphological description of Contracaecum pelagicum (Nematoda: Anisakidae) from the anchovy Engraulis anchoita (Engraulidae) and fish-eating birds from the Argentine North Patagonian Sea. Parasitol Int 62:309–319
Ghedotti MJ (2003) Family Anablepidae (four-eyed fishes, onesided livebears and the white eye). In: Reis RE, Kullander SO, Ferraris C (eds) Checklist of the freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre, pp 582–585
Huizinga HW (1967) The life cycle of Contracaecum multipatillatum (von Drasche, 1882) Lucker, 1941 (Nematoda: Heterocheilidae). J Parasitol 53:368–375
Klöser H, Plötz J, Palm H, Bartsch A, Hubold G (1992) Adjustment of anisakid nematode life cycles to the high Antarctic food web as shown by Contracaecum radiatum and C. osculatum in the Weddell Sea. Antarct Sci 4:171–178
Køie M, Fagerholm H (1995) The life cycle of Contracaecum osculatum (Rudolphi, 1802) sensustricto (Nematoda, Ascaridoida, Anisakidae) in view of experimental infections. Parasitol Res 81:481–489
Martins ML, Onaka EM, Fenerick J Jr (2005) Larval Contracaecum sp. (Nematoda: Anisakidae) in Hoplias malabaricus and Hoplerythrinus unitaeniatusv (Osteichthyes: Erythrinidae) of economic importance in occidenta marshlands of Maranhão, Brazil. Vet Parasitol 127:51–59
Miller RR (1979) Ecology, habitats and relationships of the middle American Cuatro Ojos, Anableps dowi (Pisces: Anablepidae). Copeia 1:82–91
Pardo S, Núnes D, Barrios R, Prieto M, Atencio V (2009) Índices parasitarios y descripcíon morfológica de Contracaecum sp. (Nematoda: Anisakidae) enblanquillo Sorubim cuspicaudus (Pimelodidae) del rio Sinú. Rev Med Vet Zootec Cóerdoba 14:1712–1722
Reis RE, Kullander SO, Ferraris CJ Jr (2003) Check list of freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre
Salgado-Maldonado G, Santos CP (2000) Amapacanthus amazonicus n.g., n.sp. (Acanthocephala: Diplosentidae: Allorhadinorhynchinae) from Arius passany and Anableps microleps (Pisces) at Maraca Island off northern Brazil. Syst Parasitol 46:111–116
Santos E (1987) Peixes de água doce. Editora Itatiaia, Belo Horizonte
Shamsi S, Aghazadeh-Meshgi M (2011) Morphological and genetic characterisation of selected Contracaecum (Nematoda: Anisakidae) larvae in Iran. Iran J Fish Sci 10:356–361
Yamaguti S (1954) Parasitic worms mainly from Celebes: nematodes of fishes. Part 9 Acta Med Okayama 9:122–135
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Conselho Nacional de Pesquisa (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Rights and permissions
About this article
Cite this article
Ribeiro, J.S., de Oliveira, F.C.R. & Ederli, N.B. Short communication: first report of nematodes parasitizing the four-eyed-fish, Anableps anableps (Pisces, Cyprinodontiformes). Parasitol Res 116, 2249–2254 (2017). https://doi.org/10.1007/s00436-017-5528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5528-5