Abstract
This study was developed in order to describe the early morphological events observed during the invasion of two pathogenic strains of Acanthamoeba (genotype T4); A. castellanii and A. culbertsoni, at the olfactory meatus and cerebral, pulmonary, renal, hepatic and splenic tissues levels, an in vivo invasion study. Histological and immunohistochemical description of the events at 24, 48, 72, and 96 h postintranasal inoculations of BALB/c mice was performed. A. castellanii showed a higher invasion rate than A. culbertsoni, which was only able to reach lung and brain tissue in the in vivo model. The current study supports previous evidence of lack of inflammatory response during the early stages of infection. Acanthamoeba invasion of the CNS and other organs is a slow and contact-dependent process. The early morphological events during the invasion of amoebae include the penetration of trophozoites into different epithelia: olfactory, respiratory, alveolar space, and renal tubule, which resemble the process of amoebae invasion described in corneal tissue. The data suggest that after reaching the nasal epithelium, trophozoites continued invasion, separating and lifting the most superficial cells, then migrating and penetrating between the cell junctions without causing a cytolytic effect on adjacent cells. These results reaffirm the idea that contact-dependent mechanisms are relevant for amoebae of Acanthamoeba genus regardless of the invasion site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free-living amoebae (FLA) are protozoa commonly found worldwide in natural habitats such as soil, bodies of water, as well as man-made environments. Among FLA, some species such as Naegleria fowleri, Balamuthia mandrillaris, and the genus Acanthamoeba are known as etiological agents of often fatal CNS infections in humans and other animals known as Granulomatous Amoebic Encephalitis (GAE) and Primary Amoebic Encephalitis (PAM) in the case of N. fowleri infections (Kinde et al. 2007; Martinez 1982; Visvesvara 2013). Moreover, Acanthamoeba is also the causative agent of other serious human diseases, including skin lesions and a painful keratitis which if misdiagnosed and not treated in early stages often ends in blindness (Marciano-Cabral and Cabral 2003). Most of the reported cases of GAE due to Acanthamoeba are limited to individuals with a weakened immune system; particularly at risk are individuals with fungal, mycobacterial, and viral infections, with systemic lupus erythematosus (Grunnet et al. 1981; Koide et al. 1998; Thamtam et al. 2016), cancer (Memari et al. 2015), renal failure, patients on immunosuppressive therapy (Martinez 1991; Martinez and Janitschke 1985; Martinez and Visvesvara 1997), HIV seropositive (Martinez and Visvesvara 1997), diabetic (Blanco-Vidal et al. 2013; Petry et al. 2006), undernourished, alcoholics, and cirrhotic patients (Martinez and Visvesvara 1997), with only 2–3% survival rate (Kaushal et al. 2008). Immunosuppressed solid organ transplant recipients are at increased risk for acquisition of opportunistic pathogens such as Acanthamoeba species, with potentially fatal consequences (Abd et al. 2009; Doan et al. 2015; Fung et al. 2008; Salameh et al. 2015; Satlin et al. 2013; Young et al. 2010;). Surprisingly, in the recent years, several GAE cases have been related with healthy pediatric patients, in which the source of infection was not associated with contact with bodies of water; conversely, the patients had a history of geophagia and soil handling (Das et al. 2016; Shukla et al. 2016; Singhal et al. 2001). Immunocompetent patients develop a granulomatous reaction (Martinez 1982), while in immunodeficient individuals, granuloma formation is weak or lacking, especially in HIV/AIDS patients (Marciano-Cabral and Cabral 2003).
The clinical picture of GAE may resemble viral, bacterial, or tubercular meningitis, complicating the diagnosis of these pathogenic agents. Neurological symptoms develop insidiously, and most patients present focal deficits or alterations in mental status, involving headache, fever, behavioral changes, hemiparesis, lethargy, stiff neck, aphasia, ataxia, vomiting, nausea, cranial nerve palsies, increased intracranial pressure, and seizures. Over the course of weeks to several months, the infection progresses to coma leading to death as a consequence of increased intracranial pressure and brain herniation or from secondary infection and multi-organ failure. Postmortem examination often shows severe edema and hemorrhagic necrosis (Ma et al. 1990; Martinez 1991; Visvesvara et al. 2007).
GAE may occur during any season of the year. The portal of entry is not clearly known, since it becomes apparent only after several weeks or even months (Martinez and Janitschke 1985). Based on postmortem studies, it had been possible to elucidate the route of infection, which is thought to be through skin lesions or by inhalation of amoebae through the nasal passages and lungs (Marciano-Cabral and Cabral 2003).
Furthermore, and supported by clinical findings, it has been suggested that the invasion of Acanthamoeba trophozoites of the central nervous system (CNS) occurs by hematogenous dissemination from a primary site of infection or directly through the olfactory neuroepithelium, leading to interactions with the blood-brain barrier and finally with the amoebae invading the CNS (Khan and Siddiqui 2009). The biopsy and/or autopsy findings reported so far in GAE patients have shown moderate to severe edema in cerebral hemispheres; the brain stem, midbrain, corpus callosum, and cerebellum may show multifocal lesions and areas of hemorrhagic infarcts. Over the cortex, a chronic inflammatory exudate has been observed. The microscopic findings revealed edema, severe hemorrhagic necrosis, encephalomalacia, and multinucleated giant cells. Occasionally, severe angiitis may be seen with perivascular cuffing by lymphocytes. Blood vessels are thrombotic with fibrinoid necrosis and cuffed by trophozoites, cysts, and polymorphonuclear leukocytes (Marciano-Cabral and Cabral 2003; Visvesvara et al. 2007; Visvesvara 2013). Trophozoites and cysts have been observed in CNS tissues; they can be distinguished from host cells by their prominent central nucleolus and vacuoles (Visvesvara et al. 2007).
During the infection, amoebic invasion depends on diverse factors including the ability to tolerate physiological conditions such as temperature, osmotic shock, and pH variations (Walochnik et al. 2000). Additionally, the trophozoites are able to induce contact-dependent mechanisms of damage such as adhesion, migration, penetration, and phagocytosis of target organs together with contact independent mechanisms, i.e., enzymatic amoebic processes (Omaña-Molina et al. 2013).
In vitro and in vivo studies have been undertaken to assess the pathogenic mechanisms of Acanthamoeba clinical and environmental isolates to elucidate the capacity of free-living organisms to establish and cause disease in hosts. Most in vivo studies of GAE infections have been performed using the rat and mouse as experimental animals (Alves et al. 2016; Culbertson et al. 1966). Many reports have described some features of the Acanthamoeba infection in mice, such as clinical presentation, CNS dissemination, affected organs, and host response, (Martinez and Visvesvara 1997; Massilamany et al. 2014; Siddiqui et al. 2011). Nevertheless, at present, the complete understanding of the pathogenesis and pathophysiology of Acanthamoeba encephalitis is still an urgent need, especially when relating to events occurring in the initial stages of infection.
In this study, in order to describe the early morphological events observed during the invasion of two pathogenic strains of Acanthamoeba (A. castellanii and A. culbertsoni, genotype T4) at the olfactory meatus and cerebral, pulmonary, renal, hepatic, and splenic tissues levels, an in vivo invasion study was developed. Moreover, a histological and immune-histochemical description of the events that took place at 24, 48, 72, and 96 h postintranasal inoculations of BALB/c mice with the strains mentioned above was also performed.
Material and methods
Amoebae
This study was undertaken with two strains of Acanthamoeba belonging to genotype T4: A. culbertsoni isolated from a clinical case of amoebic keratitis involving extra-corneal invasion where the amoeba reached the aqueous humor (Arnalich-Montiel et al. 2012), and A. castellanii, originally isolated in the Association to Prevent Blindness in Mexico, Luis Sánchez Bulnes Hospital, from a contact lens of an amoebic keratitis (AK) patient. Both isolates were identified using the morphological taxonomic criteria of Page (1988). Molecular identification of the amoebae strain was performed by genotyping through DNA sequencing of the DF3 region of 18S rRNA genes (Booton et al. 2002; Lorenzo-Morales et al. 2006). Both species were previously classified as members of the T4 genotype (Chávez-Munguía et al. 2016; Omaña-Molina et al. 2016) which is the most common genotype of Acanthamoeba related to GAE and AK cases (Alves et al. 2016).
Trophozoites of both strains were grown at 30 °C (optimal temperature of growth of both amoebae) in axenic culture in 2% bactocasitone (DIFCO, Spaks, MD) supplemented with 10% fetal bovine serum (Equitech-bio, Kerville, TX) and 1% (w/v) antibiotics (10,000 U/ml penicillin and 10 mg/ml streptomycin). Assays were performed with trophozoites harvested at the end of the logarithmic growth phase (72 h) by chilling at 4 °C and concentrated by centrifugation for 5 min at 300×g. It is also important to highlight that A. castellanii trophozoites described in this study were used as well to describe the early morphological events during the in vitro corneal invasion (Omaña-Molina et al. 2013).
Reactivation of Acanthamoeba strains virulence
Amoebae virulence was reactivated and maintained by the intranasal instillation of trophozoites of each strain in a mouse model of CNS. Briefly, trophozoites of both species were chilled at 4 °C and concentrated by centrifugation for 5 min at 300 × g; the pellets were counted and adjusted to obtain 1 × 106 trophozoites, then resuspended in 20 μl of fresh culture medium (without fetal bovine serum and antibiotic). Ten male BALB/c mice (3 weeks old) were lightly anesthetized and inoculated into the nostrils with the strains used in the study, according to Culbertson et al. (1959) which promotes infection in the CNS of mice. The mice were fed ad libitum, monitored daily, and received conventional care. Mice apparently healthy were sacrificed after 21 days. The brain, liver, lungs, and kidneys were placed on agar plates with non-nutritive-enriched medium (NNE) to recover the amoebae. These amoebae recently recovered from brain tissues were used in subsequent assays.
Experiments were based on protocol 002/02, approved by the Institutional Animal Care and Use Committee, in accordance with norm-062-Zoo-1999, based on the Guide for the Care and Use of Laboratory Animals, published in the Official Journal of the Federation (Mexico) 2001. Experimental animals were kept in a biotery in a temperature-controlled environment, with light-dark cycles, adequate food, and enough space for growth in optimal conditions. Three serial passages in BALB/c mice were carried out. A group of five mice was inoculated with culture medium without amoebae as the control group. The viability of the trophozoites was determined using trypan blue (0.4%).
Induction of GAE infection
Once the virulence of the strains was reactivated, the EAG model was implemented in order to describe the early events of the infection.
Two groups of 20 male BALB/c mice each (3 weeks old) were inoculated intra-nasally with 1 × 106 trophozoites of both strains as described above (20 male BALB/c mice for each strain). Subsequently, the groups were divided into four subgroups in order to be sacrificed with an intraperitoneally lethal dose of sodic pentobarbital (200 mg/kg) 24, 48, 72, and 96 h postinoculation. A group of five mice were inoculated with culture medium without amoebae as the control group.
At the end of the time point proposed, mice were perfused and fixed with 4% paraformaldehyde in PBS pH 7.2. Liver, lungs, spleen, kidneys, brain, and nasopharyngeal meatus were removed and kept in the fixative for 24 h. Afterwards, the nasopharyngeal meatus region was decalcified in a solution of 7% ethylenediaminetetraacetic acid (EDTA) for 7 days, changing the solution daily. The nasopharyngeal meatus region was cut sagittally before performing histological sections.
After profuse washing, all the organs were embedded in paraffin and sectioned at 4 μm, according to conventional histological technic.
Immunohistochemistry (DAB IHC)
Histological sections were deparaffinized, rehydrated, and washed with TBS-Tween 20 (0.1%) (TBS-T). Subsequently, antigenic recovery was performed by enzymatic digestion with proteinase K 0.1 mg/ml in TBS-T buffer with 1% CaCl2 for 15 min. Endogenous peroxidase was blocked with 3% H2O2 for 15 min. After washing, the slides were incubated for 2 h with 5% fetal bovine serum. Then incubated overnight with rabbit polyclonal antibodies anti-A. culbertsoni (1:400) and anti-A. castellanii (1:1000), obtained from amoebic extracts of a mixture of trophozoites (95%) and cysts (5%) which were lysed by thermal shock (5 cycles). Afterward, the samples were washed with TBS-T and incubated for 2 h with the secondary antibody (HRP-Rabbit MACH 2 Polymer Biocare Medical. USA). Peroxidase activity was revealed with diaminobenzidine-H2O2 (DAB Peroxidase Substrate. Burlingame CA. USA) and counter-stained with Harris hematoxylin. Finally, the samples were dehydrated and covered with synthetic resin. Negative controls were processed in the same manner but without the primary antibody.
The immunohistochemical description of the early events during the invasion of A. castellanii and A. culbertsoni in the murine model GAE included the following criteria: immunolocalization of trophozoites and cysts in different organs, histopathological findings, and the presence of inflammatory cells.
Control samples were processed following the same protocol, however, without the primary antibody.
Hematoxylin and eosin (H&E) staining
Briefly, slides obtained from extracted organs as described above were deparaffinized and hydrated through descending alcohols (100–80%), then stained with hematoxylin and eosin. After that, samples were dehydrated (80–100% alcohols), finally covered with synthetic resin. Samples were observed by light microscopy (Nikon, Eclipse E400, Japan).
Results
Reactivation of Acanthamoeba strains virulence
In order to describe the early morphological events during the invasion of Acanthamoeba strains in the murine model of GAE, the virulence of both strains in this study was reactivated through three serial passages in BALB/c mice.
Differences in the degree of virulence between both isolates were observed. A. castellanii did not kill any of the experimental animals, whereas 50% of mice infected with A. culbertsoni died. However, it is important to mention that A. castellanii trophozoites were recovered in NNE agar plates from the brain, lung, liver, and kidney in 100% of the samples; conversely, A. culbertsoni was recovered only from brain and lung in performed assays.
Immunohistochemistry and H&E
The results of the histopathological and immunohistochemical analysis of A. castellanii and A. culbertsoni infection were consistent in all samples and time points evaluated. During the macroscopic analysis of target organs, no evident pathological changes such as edema, necrosis, or hemorrhagic areas were observed.
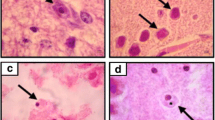
The sequence of immunohistochemical events observed during the invasion of amoebae is described below: trophic forms of A. castellanii invaded and penetrated through the nasal cavity toward the respiratory epithelium 24 h postinoculation. Immunolocalized trophozoites had adhered to the surface of the respiratory epithelium (Fig. 1a), provoking paracellular rupture of the epithelium (Fig. 1b) suggesting that amoebae migrate through cell junctions (Fig. 1c). Importantly, trophozoites were observed adhered to the stratified epithelium resembling the process of trophozoite invasion in the hamster as well as human cornea (Fig. 1d), showing the characteristic vacuole. Posteriorly, amoebae penetrated to the nasal stroma (Fig. 1e); moreover, vascular permeation of trophozoites was observed, suggesting hematogenous spreading (Fig. 1f). It is important to highlight the absence of inflammatory infiltrate.
a–i A. castellanii invasion through the nasal cavity 24 h postinoculation. a Adhesion to the surface respiratory epithelium (arrow). b Paracellular break. c Income through the cell junctions of the epithelium (arrow). d Adherence to the stratified epithelium (arrow). e Entering the nasal stroma (arrowhead). f Vascular permeation suggesting hematogenous spread of amoebae (arrowhead). Note the absence of inflammatory infiltrate. DAB IHC. g, h An A. culbertsoni trophozoite was observed adhered onto the nasal epithelium 48 h postinoculation. DAB IHC (arrowheads). i Control. Respiratory epithelium sample without first antibody. DAB IHC. Bars 10 μm
A similar sequence of events was observed during the interaction of A. culbertsoni; however, the amoebae were detected until 48 h postinoculation, in which amoebae were observed attached to the nasal epithelium (Fig. 1g, h).
As expected, no stained trophozoites were observed in control samples. Besides, the analysis of organs and tissues of control animals that were processed by immunohistochemistry and H&E showed normal morphology with no evidence of damage or inflammation.
Similar images as described before were observed at 72 h postinoculation in both strains in which several trophozoites were observed adhering and penetrating through the respiratory and olfactory epithelium, without loss of epithelium continuity, showing damage only in areas close to the trophozoites.
After 96 h, amoebae of both species were immunolocalized in the olfactory bulb near an olfactory glomerulus surrounded by light inflammatory infiltrates (Fig. 2a, b), as well as in the brain; numerous trophozoites were observed near the granular layer of the dentate gyrus of the hippocampus (Fig. 2c). Characteristic structures of these protozoa such as vacuoles, nucleus, and nucleolus are clearly observed (Fig. 2c, d). Trophozoites were immunolocalized in the cerebral cortex (Fig. 2e, f) where detachment of the meninges was observed. There was no evidence of inflammation, necrosis, or hemorrhage into the surrounding tissue, except in the olfactory bulb where light inflammatory infiltrate was observed surrounding some amoebae (Fig. 2b).
a–f Immunolocalization of A. culbertsoni in nervous tissue 96 h postintranasal instillation. a, b Amoeba immunolocalized in olfactory bulb surrounded by scarce inflammatory infiltrate (arrow) DAB IHC. c Numerous trophozoites are located near hippocampus (arrow) DAB IHC, tissue undamaged is observed. d Trophozoite at higher magnification in the hippocampus area (arrow). Characteristic structures of these amoebae such as vacuoles, nucleus, and nucleolus are clearly observed DAB IHC. e, f Trophozoite adhered on the cerebral cortex without tissue damage is observed. DAB IHC. Bars 10 μm
The immunohistochemical analysis of amoebae invasion to different organs revealed that in the times evaluated in this study, A. culbertsoni migrated only to the brain, immunolocalized in cerebral tissue until 96 h postinoculation, whereas A. castellanii trophozoites migrated toward brain tissue (72 h), lung and spleen (24 h), and liver and kidney (72 h).
A. castellanii trophozoites were observed in the alveolar space 24 h postinoculation, some of which were surrounded by alveolar macrophages (Fig. 3a, b). In the spleen, trophozoites and cystic forms were observed 24 h postinoculation without evidence of inflammation (Fig. 3c, d). In the kidney after 72 h, trophozoites were immunolocalized and shown to be penetrating and invading renal tissue through renal tubules, although no evidence of tissue destruction was observed. It was clearly seen that amoebae migrate through the cell junctions (Fig. 3e, f). Also, trophozoites attached to liver hepatocytes in the space of Disse as well as to hepatic sinusoidal endothelium were observed (Fig. 3g, h).
a–h Photomicrograph showing the presence of cysts and trophozoites of A. castellanii (arrow) 24 h postinoculation. a, b In alveolar tissue with inflammatory infiltrate around Acanthamoeba, primarily by macrophages (arrows). Trophozoite identified H&E and DAB IHC respectively. c, d Spleen. Trophozoite (arrow) and cystic forms (arrowhead) with H&E and DAB IHC respectively. e, f 72 h postinoculation. Kidney: in the basement membrane of proximal tubule and collecting tubule trophozoites were observed apparently located between cell junctions (arrows) DAB IHC. g, h Trophozoites attached to liver hepatocytes (arrows) H&E in space of Disse attached to hepatic sinusoidal endothelium H&E. Bars 10 μm
Discussion
This study describes the histopathologic and immunohistochemical findings during initial steps of GAE induced by intranasal instillation of A. culbertsoni and A. castellanii trophozoites into BALB/c mice. Amoebae studied belong to genotype T4, which is associated with GAE cases in immunocompromised patients and amoebic keratitis in contact lens wearers. Since the pathogenic potential of these amoebae had been confirmed, several authors have described the histopathological findings of experimental infection by Acanthamoeba (Culbertson et al. 1966; Martinez and Janitschke 1985; Górnik and Kuźna-Grygiel 2005; Alves et al. 2016). Nevertheless, most of these findings have been described in time periods ranging from 3 to 5 days to 7 months postinfection (Culbertson et al. 1966). Even though it is widely accepted that the portal of entry of these amoebae to the brain can occur via hematogenous dissemination or through the olfactory neuroepithelium (Martinez et al. 1975), there are no reports in which the earliest stages of the invasion are described, as we show in this report.
A. castellanii and A. culbertsoni strains evaluated in this work are considered pathogens since they were isolated from severe amoebic keratitis cases; however, these amoebae were maintained in axenic culture for several months. Therefore, intranasal inoculation in mice was carried out in order to reactivate their virulence properties. Although both species were invasive in the animal model, A. castellanii did not kill any of the inoculated mice; trophozoites were recovered from the different target organs evaluated, whereas A. culbertsoni caused the death of 50% of mice recovering only brain and lung. The invasion of amoebae in the evaluated organs was consistent with the reactivation assays of virulence, as well as in the experimental groups because their presence in the same organs was confirmed. Our results are in accordance with previous studies by Kasprzak et al. (1974), who reported that despite several passages in mice, Acanthamoeba trophozoites maintained their tropism toward the same organs (brain and lung).
The early morphological events during the invasion of amoebae described in this study consisted of trophozoites penetrating different epithelias: olfactory, respiratory, alveolar space, and renal tubule, which resemble the process of amoebae invasion described in corneal tissue (Omaña-Molina et al. 2013). In this study, we suggest that trophozoites, after reaching the nasal epithelium, continued invasion by separating and lifting the most superficial cells, then migrating and penetrating between the cell junctions without causing a cytolytic effect on adjacent cells (Figs. 1 and 3). These results reaffirm the idea that dependent mechanisms of contact are relevant for amoebae of Acanthamoeba genus regardless of the invasion site.
Through light microscopy, it was not possible to determine phagocytic processes of the host cells. It would be desirable through electron microscopic studies to confirm that the pathogenic mechanisms that have been suggested for the invasion of the corneal epithelium are also being carried out in the epithelia of different target organs.
During the invasion of Acanthamoeba trophozoites, it is probable that many amoebae have been removed by the nasal mucus during the first hours after intranasal inoculation. Rojas-Hernández et al. (2004) reported that Naegleria fowleri trophozoites as well as inflammatory cells were observed embedded in mucus in the lumen of the nasal cavity 8 h postinoculation, suggesting that mucus production prevents adherence and subsequent penetration of the amoebae as a first line of defense. On the other hand, it is certain that other trophozoites evade this initial immune response, beginning the invasion process after 24 and 48 h postinoculation, since immunolocalized trophozoites of both species were observed penetrating the olfactory and respiratory epithelium and migrating to deeper areas of the nasopharyngeal meatus to reach nasal turbinates. Subsequently, trophozoites migrated through the brain, particularly in cerebral cortex and hippocampus 96 h postinoculation (Figs. 1 and 2).
The route of invasion of Acanthamoeba trophozoites evaluated in the current study appears to be similar to that of N. fowleri. Rojas-Hernández et al. (2004) reported that 30 h postinoculation, N. fowleri trophozoites were found in the olfactory bulb with little inflammatory exudate, but tissue damage and severe inflammatory reaction was observed in the brain at 96 h. However, even though the course of invasion seems to be the same with both genera, N. fowleri causes severe inflammation and acute damage, in contrast to observations of Acanthamoeba samples, most of which showed no inflammatory reaction. After analyzing tissue sections with H&E and immunohistochemistry of all tissues at the times proposed in this study, no evidence of inflammation, necrosis, or hemorrhage was observed except on the olfactory bulb and in lung tissue where limited inflammatory infiltrate was observed (Figs. 2b and 3b). Moreover, some amoebae were found inside blood vessels in addition to the presence of amoebae in different organs such as the spleen 24 h postinoculation, suggesting hematogenous spread.
We believe that only the initial steps of invasion of Acanthamoeba and N. fowleri are very similar since the manner by which both trophozoites penetrate through the different epithelia is analogous; however, the process is slower and apparently contact dependent in Acanthamoeba, which explains the chronic course of GAE infection in humans and experimental animals.
It has been suggested that brain damage caused by N. fowleri is substantially due to toxins and enzymes produced by this amoeba; however, Baig (2015) assessed that “the in-fection results in an extensive brain damage that in fact is substantially caused by the host immune response rather than the amoeba,” including Acanthamoeba. In our studies, at least at the periods evaluated, a significant inflammatory response was not observed. Górnik and Kuźna-Grygiel (2005) sug-gested that the absence of an inflammatory reaction in neigh-boring areas to a single trophozoite or cyst is likely due to the absence of a high level of antigen needed to activate the im-mune system.
In conclusion, the current study which was developed with two different strains of Acanthamoeba genotype T4 in a murine CNS infection model supports previous evidences of lack of inflammatory response during the early stages of infection and also that invasion of the CNS by Acanthamoeba is a slow and contact-dependent process. Further studies should be carried out in order to elucidate the key proteins/receptors involved in these processes in order to fully understand the pathogenesis of Acanthamoeba CNS infections and also to develop novel drug targets which could improve the current arsenal of anti-amoebic agents.
References
Abd H, Saeed A, Jalal S, Bekassy AN, Sandström G (2009) Ante mortem diagnosis of amoebic encephalitis in a haematopoietic stem cell transplanted patient. Scand J Infect Dis 41(8):619–622. doi:10.1007/s00436-015-4871-7
Alves DS, Moraes AS, Alves LM, Gurgel-Gonçalves R, Lino Junior RS, Cuba-Cuba CA, Vinaud MC (2016) Experimental infection of T4 Acanthamoeba genotype determines the pathogenic potential. Parasitol Res 115(9):3435–3440. doi:10.1007/s00436-016-5105-3
Arnalich-Montiel F, Martín-Navarro CM, Alió JL, López-Vélez R, Martínez-Carretero E, Valladares B, Piñero JE, Lorenzo-Morales J (2012) Successful monitoring and treatment of intraocular dissemination of Acanthamoeba. Arch Ophthalmol 130:1474–1475
Baig AM (2015) Pathogenesis of amoebic encephalitis: are the amoebae being credited to an 'inside job' done by the host immune response? Acta Trop 148:72–76. doi:10.1016/j.actatropica.2015.04.022
Blanco-Vidal MJ, Catalán-Uribarrena G, López JI, Del Águila C, Magnet A, Pomposo-Gaztelu I, Montejo M (2013) Hemiparesia izquierda en un paciente diabético: encefalitis granulomatosa crónica por Acanthamoeba. Rev Neurol 56:187–188
Booton GC, Kelly DJ, Chu YW, Seal DV, Houang E, Lam DS, Byers TJ, Fuerst PA (2002) 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J Clin Microbiol 40:1621–1625
Chávez-Munguía B, Salazar-Villatoro L, Omaña-Molina M, Espinosa-Cantellano M, Ramírez-Flores E, Lorenzo-Morales J, Martínez-Palomo A (2016) Acanthamoeba culbertsoni: electron-dense granules in a highly virulent clinical isolate. J Eukaryot Microbiol. doi:10.1111/jeu.12321
Culbertson CG, Smith JW, Cohen HK, Minner JR (1959) Experimental infection of mice and monkey by Acanthamoeba. Am J Pathol 35:185–197
Culbertson CG, Ensminger PW, Overton WM (1966) Hartmannella (Acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. Am J Clin Pathol 46(3):305–314
Das S, Saha R, Rani M, Goyal R, Shah D, Asish JK (2016) Central nervous system infection due to Acanthamoeba: a case series. Trop Parasitol 6(1):88–91. doi:10.4103/2229-5070.175130
Doan N, Rozansky G, Nguyen HS, Gelsomino M, Shabani S, Mueller W, Johnson V (2015) Granulomatous amebic encephalitis following hematopoietic stem cell transplantation. Surg Neurol Int 6:S459–S462. doi:10.4103/2152-7806.166788
Fung KT, Dhillon AP, McLaughlin JE, Lucas SB, Davidson B, Rolles K, Patch D, Burroughs AK (2008) Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl 14:308–312
Górnik K, Kuźna-Grygiel W (2005) Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol (Warsz) 64(3):161–167
Grunnet ML, Cannon GH, Kushner JP (1981) Fulminant amebic meningoencephalitis due to Acanthamoeba. Neurology 31(2):174–176
Kasprzak W, Mazur T, Rucka A (1974) Studies on some pathogenic strains of free-living amoebae isolated from lakes in Poland. Ann Soc Belg Med Trop 54(4–5):351–357
Kaushal V, Chhina DK, Kumar R, Pannu HS, Dhooria HP, Chhina RS (2008) Acanthamoeba encephalitis. J Med Microbiol 26:182–184
Khan NA, Siddiqui R (2009) Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int J Parsitol 39:1611–1616
Kinde H, Read DH, Daft BM, Manzer M, Nordhausen RW, Kelly DJ, Fuerst PA, Booton G, Visvesvara GS (2007) Infections caused by pathogenic free-living amebas (Balamuthia mandrillaris and Acanthamoeba sp.) in horses. J Vet Diagn Investig 19(3):317–322
Koide J, Okusawa E, Ito T, Mori S, Takeuchi T, Itoyama S, Abe T (1998) Granulomatous amebic encephalitis caused by Acanthamoeba in a patient with systemic lupus erythematosus. Clin Rheumatol 17(4):329–332
Lorenzo-Morales J, Ortega-Rivas A, Martínez E, Khoubbane M, Artigas P, Periago MV, Foronda P, Abreu-Acosta N, Valladares B, Mas-Coma S (2006) Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile delta region. Acta Trop 100:63–69
Ma P, Visvesvara GS, Martinez AJ, Theodore FH, Daggett PM, Sawyer TK (1990) Naegleria and Acanthamoeba infections: review. Rev Infect Dis 12(3):490–513
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 2:273–307
Martinez AJ (1982) Acanthamoebiasis and immunosuppression. Case report. J Neuropathol Exp Neurol 41(5):548–557
Martinez JA (1991) Infection of the central nervous system due to Acanthamoeba. Rev Infect Dis 13(Suppl 5):S399–S402
Martinez AJ, Janitschke K (1985) Acanthamoeba, an opportunistic microorganism: a review. Infection 13(6):251–256
Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunistic amebas. Brain Pathol 7(1):583–598
Martinez JA, Markowitz SM, Duma JR (1975) Experimental pneumonitis and encephalitis caused by Acanthamoeba in mice: pathogenesis and ultrastructural features. J Infect Dis 131:692–699
Massilamany C, Marciano-Cabral F, Rocha-Azevedo BD, Jamerson M, Gangaplara A, Steffen D, Zabad R, Illes Z, Sobel RA, Reddy J (2014) SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PLoS One 9(5):e98506. doi:10.1371/journal.pone.0098506
Memari F, Niyyati M, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z (2015) Ocurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res 114(5):197–212
Omaña-Molina M, González-Robles A, Salazar-Villatoro L, Lorenzo-Morales J, Cristóbal-Ramos AR, Hernández-Ramírez VI, Talamás-Rohana P, Méndez-Cruz AR, Martínez-Palomo A (2013) Reevaluating the role of Acanthamoeba proteases in tissue invasion: observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed Res Int. doi:10.1155/2013/461329
Omaña-Molina M, Vanzzini-Zago V, Hernandez-Martinez D, Gonzalez-Robles A, Salazar-Villatoro L, Ramirez-Flores E, Oregon-Miranda E, Lorenzo-Morales J, Martinez-Palomo A (2016) Acanthamoeba genotypes T3 and T4 as causative agents of amoebic keratitis in Mexico. Parasitol Res 115(2):873–878. doi:10.1007/s00436-015-4821-4
Page FC (1988) A new key to freshwater and soil Gymnamoebae with instructions for culture. Culture Collections of Algae and Protozoa. Freshwater Biological Association. Ambleside, Cumbria, England, pp 92–96
Petry F, Torzewski M, Bohl J, Wilhelm-Schwenkmezger T, Scheid P, Walochnik J, Zöller L, Bhakdi S, Lackner KJ (2006) Early diagnosis of Acanthamoeba infection during routine cytological examination of cerebrospinal fluid. J Clin Microbiol 44:1903–1904. doi:10.1128/JCM.44.5.1903-1904.2006
Rojas-Hernández S, Jarillo-Luna A, Rodríguez-Monroy M, Moreno-Fierros L, Campos-Rodríguez R (2004) Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parasitol Res 94(1):31–36
Salameh A, Bello N, Becker J, Zangeneh T (2015) Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with kidney transplant: a case report. Open Forum Infect Dis 2(3). doi:10.1093/ofid/ofv104
Satlin MJ, Graham JK, Visvesvara GS, Mena H, Marks KM, Saal SD, Soave R (2013) Fulminant and fatal encephalitis caused by Acanthamoeba in a kidney transplant recipient: case report and literature review. Transpl Infect Dis 15:619–626
Shukla D, Rumpa S, Mayuri R, Ritika G, Dheeraj S, Jhajjar KA (2016) Central nervous system infection due to Acanthamoeba: a case series. Trop Parasitol 6(1):88–91
Siddiqui R, Emes R, Elsheikha H, Khan NA (2011) Area 51: how do Acanthamoeba invade the central nervous system? Trends Parasitol 27(5):185–189
Singhal T, Bajpai A, Kalra V, Kabra SK, Samantaray JC, Satpathy G, Gupta AK (2001) Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J 20(6):623–627
Thamtam VK, Uppin MS, Pyal A, Kaul S, Rani JY, Sundaram C (2016) Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a newly diagnosed patient with systemic lupus erythematosus. Neurol India 64:101–104
Visvesvara GS (2013) Infections with free-living amebae. Handb Clin Neurol 114:153–168
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-livingamoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. Immunol Med Microbiol 50(1):1–26
Walochnik J, Haller-Schober EM, Kölli H, Picher O, Obwaller A, Aspöck H (2000) Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J Clin Microbiol 38(11):3932–3936
Young AL, LeBoeuf NR, Tsiouris SJ, Husain S, Grossman ME (2010) Fatal disseminated Acanthamoeba infection in a liver transplant recipient immunocompromised by combination therapies for graft-versus-host disease. Transpl Infect Dis 12:529–537
Acknowledgements
This work was funded by the UNAM-FESI grant no. FESI-DIP-PAPCA-2014-13. We deeply appreciate the valuable support of Lizbeth Salazar Villatoro for her technical assistance in amoebae cultures, Miriam Romero Grijalva for her assistance in handling mice, and Carmen Guadalupe Mondragon Huerta and Leticia Verdín Terán for their immunohistochemistry assistance. Finally we thank Maria Rosa Avila responsible for the Neuromorphology Laboratory, of the School of Superior Studies Iztacala, National Autonomous University of Mexico, for providing the equipment to carry out the histological processes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omaña-Molina, M., Hernandez-Martinez, D., Sanchez-Rocha, R. et al. In vivo CNS infection model of Acanthamoeba genotype T4: the early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol Res 116, 725–733 (2017). https://doi.org/10.1007/s00436-016-5338-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5338-1