Abstract
Blastocystis spp., one of the most common parasites colonizing the human intestine, is an extracellular, luminal protozoan with controversial pathogenesis. The host’s immune response against Blastocystis spp. infection has also not been defined yet. Therefore, this research aimed to assess the potential pathogenicity of this parasite and its ability to modulate the immune response in experimental infected immunocompetent and immunosuppresed mice. These results demonstrated that the infected immunosuppressed mice were more affected than infected immunocompetent mice. Histopathological examination of the small intestine in the infected immunosuppressed mice showed that Blastocystis spp. infiltrated all the layers. Moreover, the epithelia showed exfoliation and inflammatory cell infiltration in submucosa compared to that of the infected immunocompetent mice. As well, examination of the large intestine of the infected immunosuppressed group showed severe goblet cell hyperplasia. Blastocystis spp. infiltrated all the large intestine layers compared to that of the infected immunocompetent group. Furthermore, there was a significant upregulation of the expression of proinflammatory cytokines: interleukin 12 (IL-12) and tumor necrosis factor alpha (TNF-α) in the infected immunosuppressed mice compared to that of the infected immunocompetent ones (p ≤ 0.004 and p ≤ 0.002, respectively). However, the expression of anti-inflammatory cytokines (IL-4 and IL-10) was significantly downregulated in the infected immunosuppressed group compared to that of the infected immunocompetent group one at 10 days postinfection (p ≤ 0.002 and p ≤ 0.001, respectively). The results of this study revealed that Blastocystis spp. affected the production of pro- and anti-inflammatory cytokines in both groups of mice compared to healthy normal (naive) group. Additionally, these data showed that there was a significant upregulation (p ≤ 0.005) of the locally synthesized antibody: secretary IgA (sIgA) in the gut of the infected immunocompetent mice when compared to that of the infected immunosuppressed ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis spp. are an enteric parasite that inhabits intestinal tract of humans and many animals. This parasite has a worldwide distribution (Sohail and Fischer 2005; Tan 2008; Stensvold et al. 2009). Blastocystis spp. showed a dramatic increase in recent years and were considered to be a member of normal intestinal flora in the past; recently, it has been accepted as a controversial pathogen (Al-kaissi and Al-Magdi 2009). Blastocystis spp. infect both immunocompetent and immunocompromised individuals. However, symptoms associated with Blastocystis spp. were more severe in immunocompromised patients, than those in immunocompetent individuals (Garavelli et al. 1988, 1991).
Although several reports have suggested that Blastocystis spp. could cause gastrointestinal disorders (Yakoob et al. 2011), Blastocystis species’ pathogenicity has not been defined yet. The various mechanisms suggested for Blastocystis spp.-mediated gastrointestinal symptoms include adherence of Blastocystis spp. to the gut epithelium, triggering a lysis mechanism and production of diarrheagenic toxin as what is clearly done by Entamoeba histolytica and Giardia lamblia (Yakoob et al. 2011). The clinical consequences of Blastocystis spp. infection are mainly diarrhea or abdominal pain with nonspecific gastrointestinal symptoms such as nausea, anorexia, vomiting, weight loss, lassitude, dizziness, and flatulence (Al-kaissi and Al-Magdi 2009).
Blastocystis spp. is a polymorphic parasite as it can be present in various forms such as presents in vacuolar, granular, multivacuolar, amoeboid, nonvacuolar, and cystic forms (Zierdt 1991; Miné and Rosa 2008). In general, the vacuolar and granular forms are the most predominant in the fresh fecal and in vitro culture samples (Sukthana 2001; Abdel-Hafeez et al. 2015). The amoeboid form is generally more frequently observed in the samples of in vitro culture than in fresh fecal materials. The amoeboid form has also been recently reported to be predominantly excreted in the stool samples of symptomatic patients (Tan and Suresh 2006).
The cyst form is mainly seen in the stool samples but rare in vitro cultures (Yoshikawa et al. 2003). Human infection with Blastocystis spp. occurs through ingestion of contaminated food or water with by cystic form which is then transformed into the vacuolar form in the human intestine (Tan 2004; Miné and Rosa 2008; Dogruman et al. 2010).
Case reports and series have suggested that the pathogenic role of Blastocystis spp. is thought to be through induction of intestinal inflammation. Some studies have also suggested that inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) are associated with Blastocystis spp. infection (Stark et al. 2007). Several clinical and epidemiological studies implicated Blastocystis spp. as a pathogen, while others dismissed it as a commensal (Chen et al. 2003; Leder et al. 2005; Rossignol et al. 2005; Tan and Suresh 2006). This controversial pathogenesis of Blastocystis spp. deserves consideration.
Therefore, this research aimed to assess the potential pathogenicity of Blastocystis spp. parasite and its ability to modulate the host’s immune response. Moreover, the ability of this organism to elicit an immune response through expression of proinflammatory cytokines (IFN-γ, IL-12, and TNF-α) and anti-inflammatory cytokines (IL-4 and IL-10) had been examined. Also, production of sIgA had been assessed.
Material and methods
Type of the study
The study was an experimental one and was conducted in the period from June 2014 to March 2015 in the Parasitology Department, Faculty of Medicine, Minia University, Minia, Egypt.
Source of strains of Blastocystis spp. isolates
Strains of Blastocystis spp. were isolated from the stool specimens obtained from IBS patients (Rossignol et al. 2005; Yakoob et al. 2010b). Patients were selected from wards of Tropical Medicine Department, Minia University, and those attending outpatient clinics. Those patients had been clinically diagnosed as IBS patients (Poirier et al. 2012; Nagel et al. 2014). Stool samples were collected in clean sterile cups and immediately subjected to direct parasitological examination in Parasitology Department, Faculty of Medicine, Minia University. However, Blastocystis spp. isolates obtained by stool culture have not been subjected to molecular genotyping for identification of its subtypes (Stensvold et al. 2009). In Egypt, two recent studies have identified the subtypes of Blastocystis spp. isolated from symptomatic patients in some Egyptian localities to be subtypes (ST1, ST3, ST6, and ST7). They concluded that the most predominant subtype is ST3 (Hussein et al. 2008; Fouad et al. 2011). So, we can expect that the predominant subtype in our study could be ST3, but this guess requires further future work to be confirmed.
Ethical considerations
Verbal consent was obtained from the patients. All procedures were conducted according to the ethical standards approved by the Institutional Human Ethics Committee, Faculty of Medicine, Minia University, Egypt.
Stool examination
Microscopic examination
Direct wet smear methods, both saline and lugols iodine wet mount, were applied. Giemsa stain was also used according to Garcia (2001) for confirmation of Blastocystis spp. Positive samples were used in culture.
Stool cultivation
Approximately, 50 mg of positively detected stool sample for Blastocystis spp. was cultivated in 5-ml screw caped tube containing Locke egg serum medium (LE) according to Saksirisampant et al. (2010). The medium was sterilized by autoclaving for 20 min at 121 °C and stored in 500-ml bottles at 4 °C until use. Under sterile hood conditions, 10 % heat-inactivated (56 °C for 30 min) horse serum (Invitrogen, Groningen, Netherlands), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) were added to the cooled medium. The inoculated medium was incubated at 37 °C for 2–3 days. The cultures were screened for Blastocystis spp. by standard light microscopy every 12 h and subcultured for an additional 2–3 days in fresh medium. Subcultivation was performed every 72 h when it was further needed. The organism could be maintained for more than 3 months (Saksirisampant et al. 2010).
Animals
Eight-week-old male BLAB/c mice weighing 18–20 g each were obtained from experimental house, Faculty of Medicine, Minia University. The mice were housed in standard animal house conditions. The mice had free access to standard rodent chow and water. All experimental procedures were conducted according to the ethical standards approved by the Institutional Animal Ethics Committee guidelines for animal care and use, Minia University, Egypt.
Experimental design
Forty animals were randomly divided into four groups, ten mice each, the first was apparently healthy normal group (naive), the second was infected-immunocompetent group, the third was noninfected immunosuppressed group, and the fourth was infected-immunosuppressed group. Stool samples of all mice were subjected to direct parasitological examination by wet mount and lugols iodine-staining to detect the presence of Blastocystis or other parasites. The mice of the second group “infected-immunocompetent” were infected intragastrically with 2 × 106/ml of Blastocystis spp. in sterile saline on the tenth day of the experiment. The mice of the third group “immunosuppressed” were immunosuppressed by administration of dexamethazone (DEX p) (Decadron 0.5 mg, Merk Sharb and Dom UK) 33 μg/ml in their drinking water during the whole period of the experiment. The mice of the fourth “infected-immunosuppressed” were immunosuppressed by administration of dexamethazone (DEX p) (Decadron 0.5 mg, Merk Sharb and Dom UK) 33 μg/ml in their drinking water during the whole period of the experiment, and they were infected intragastrically with 2 × 106/ml of Blastocystis spp. in sterile saline on the tenth day after the start of immunosuppression by dexamethazone.
Body weight of the mice
The body weight of the mice of the various studied groups was recorded on the day of infection “day 0” and on the second, fourth, sixth, eighth, and tenth days postinfection.
Evaluation of Blastocystis spp. infection in mice
Detection of cysts shedding in feces
Feces from all mice were examined microscopically at different periods (2, 4, 6, 8, and 10 days) postinfection. Quantitative estimation of the infection intensity in the stool samples of Blastocystis spp.-infected mice was performed according to the method described by Shlim et al. (1995). Cysts of Blastocystis spp. were counted in at least three fields with estimation of the average number/high power field (HPF) (Shlim et al. 1995).
Blood collection
Blood samples were collected from the medial canthus of the eye of mice in nonheparinized 5-ml centrifuge tube on the tenth day postinfection. Blood samples were used to separate sera by centrifugation at 1500 rpm for 10 min. Serum samples were stored at −20 °C until used.
Histolopathological examination
On the tenth days postinfection, all mice from each group were sacrificed. Tissue samples from walls of small intestine, caecum, and colon of scarifying animals were collected then fixed in 10 % neutral buffered formalin. The organs were routinely processed and sectioned at 4- to 5-μm thickness. The obtained tissue sections were collected on glass slides, deparaffinized, and stained with hematoxylin and eosin stain. The sections were then examined and observed under light microscope at ×10, ×40, and ×100 magnifications (Moe et al. 1997; Bancroft and Gamble 2008).
Cytokines measurement
IFN-γ, IL-12, TNF-α, IL-4, and IL-10 concentrations in the sera of the different groups of mice were measured at tenth day postinfection. These concentrations were assayed by a two-site sandwich enzyme-like immunosorbent assay (ELISA) using ELISA kits according to the manufacturer’s instructions (Wuhan Elabscience Biotechnology Co., Ltd) (Schumacher et al. 1988).
SIgA measurement
On the tenth day postinfection, tissue samples from walls of small intestine of all scarifying animals were thoroughly rinsed in ice-cold PBS (0.01 M, pH = 7.4) to remove excess blood. Tissue specimens were weighed and then homogenized in PBS (the volume depends on the weight of the tissue) with a glass homogenizer on ice. To further breakdown the cells, sonication of the suspension with an ultrasonic cell disrupter had been done. The tissue homogenates were then centrifuged at 5000×g for 5 min. Then, the supernatants were separated. The concentration of sIgA in these supernatants was assayed by a two-site sandwich enzyme-like immunosorbent assay (ELISA) using ELISA kits according to the manufacturer’s instructions (Wuhan Elabscience Biotechnology Co., Ltd) (Schumacher et al. 1988).
Statistical analysis
Data were presented as means ± standard deviation (SD) using Statistical SPSS for Windows, issue 15.8. Statistical significance was determined using t tests (Mann-Whitney), chi-square tests, and one-way analysis of variance. A p value less than 0.05 was considered significant.
Results
Survival rate
The infected immunosuppressed mice exhibited slow locomotion, lethargy, and losing body weight. Although the change in the body weight of mice of the various studied groups was statistically nonsignificant, there was a decrease in body weight of the mice of both infected immunocompetent and immunosuppressed groups on the tenth day postinfection (Table 1). Additionally, the survival rate of the infected immunocompetent and infected immunosuppressed mice was similar until the third day postinfection. Starting from the fourth day postinfection, the survival rate of the infected immunosuppressed mice was statistically less than that of the infected immunocompetent mice (p value ≤0.03) as shown in Fig. 1.
Survival rate of the mice of the various studied groups. Closed triangles represent the data of the naive group. Closed circles represent the data of the noninfected immunosuppressed group. Closed squares represent the data of the infected immunocompetent group. Open circles represent the data of the infected immunosuppressed group. Data are presented as the mean ± SD
Detection of Blastocystis spp. in stools
By light microscopy, the vacuolar form of Blastocystis spp. was the most commonly detected in the mice’s stool samples, followed by cystic and granular forms (Fig. S1a–c). On the contrary, the amoeboid form was the least detected one. The average number of Blastocystis spp. forms/HPF in the stool of the infected mice is shown in Table 2. Blastocystis spp. shedding in all mice were initially detected on second day postinfection and continued in shedding until the end of the tenth day of the experiment. However, throughout the experiment, the intensities of Blastocystis spp. shedding were significantly greater in the infected immunosuppressed than those of the infected immunocompetent mice (Table 2).
Histolopathological examination
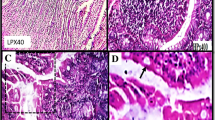
Histopathological examination of the whole small intestine showed that Blastocystis spp. (mainly granular form) were observed in the luminal content of the terminal part of the ileum. However, it was fewer in the infected immunocompetent mice than that of the infected immunosuppressed one (Fig. 2b, c). In both groups, villius exhibited shortening or attenuation (Fig. 2b, c). Though, the intestine of the infected immunosuppressed showed many areas of the mucosal layer with epithelial desquamation, minute ulcerations, and Blastocystis spp. infiltrating enterocytes (Fig. 2c). Furthermore, Blastocystis sp. was dispersed throughout all the intestinal layers (mucosal layer, the lamina propria, and the submucosal layer) and was accompanied with intense inflammatory-cell infiltration and intensive eosinophilia in the infected immunosuppressed intestine (Figs. 2c, d and S2).
Histological section of ileum stained with H&E, ×100. a A photomicrograph of intestinal villus in ileum of naïve mice is showing columnar epithelial liningon thin lamina propria. b A photomicrograph of intestinal villus in ileum of the infected immunocompetent mice is showing few luminal Blastocystis (blue arrow) with minute area of sloughed epithelial lining (black arrow). The lamina propria contains a very few number of eosinophils (green arrow). c A photomicrograph of intestinal villus in ileum of the infected immunosuppressed mice is showing many luminal Blastocystis (blue arrow) with large area of sloughed epithelial lining (black arrow). The lamina propria shows abundant eosinophils (green arrow). d A photomicrograph of intestinal villus in ileum of the infected immunosuppressed mice is showing that the Blastocystis was dispersed throughout the lamina propria and the submucosal layers (blue arrow) with a large number of eosinophils (green arrow)
Histological examination of the caecum and colon of the noninfected immunosuppressed mice showed normal columnar epitheial linning (Fig. S3). Histological examination of the caecum and colon of the infected immunosuppressed group showed edematous lamina propria and severe congestion (Fig. 3a, b). Blastocystis spp. dispersed throughout the muscularis mucosa layer (Fig. 3c). However, sections of infected immunocompetent ones showed minute edema and congestion (Fig. 3d). The luminal content of Blastocystis spp. of the infected immunosuppressed mice (Fig. 3e) was greater than that of the infected immunocompetent ones (Fig. 3f). There was severe goblet cells hyperplasia in the cecal and colonic sections of the infected immunosuppressed mice (Fig. 4a, b), while mild goblet cells hyperplasia were observed in the cecal and colonic sections of the infected immunocompetent mice (Fig. 4d). Furthermore, Blastocystis spp. were dispersed through all the layers of the intestine of the infected immunosuppressed mice (Fig. 4a–c).
Histological section of the intestinal crypts of the colon stained with H&E, ×100 (a). A photomicrograph of the intestinal crypts of the colon of infected immunosuppressed mice is showing severe edema and congestion of blood vessels (black arrow), ×10 (b). A photomicrograph of the intestinal crypts of the colon of infected immunosuppressed mice is showing the lamina propria containing many Blastocystis (blue arrow) (c). A photomicrograph of the intestinal crypts of the colon of infected immunosuppressed mice is showing that the Blastocystis was dispersed throughout the muscularis mucosa (d). A photomicrograph of the intestinal crypts of the colon of infected immunocompetent mice is showing mild edema and congestion of blood vessels (black arrow). The lamina propria contains Blastocystis (blue arrow) (e). A photomicrograph of the luminal contents of the colon of infected immunosuppressed mice is showing that the number of Blastocystis was greater than that of infected immunocompetent ones (f)
Photomicrographs of the intestinal crypts of the colon showing goblet cells stained with H&E, ×100. a A photomicrograph of the intestinal crypts of the colon of infected immunosuppressed is showing severe hyperplasia of goblet cells (black arrow) and the lamina propria contains many Blastocystis (blue arrow). b Blastocystis was dispersed throughout the muscularis mucosa (blue arrow). There are numerous inflammatory cells (green arrow) (a–c). d A photomicrograph of the intestinal crypts of the colon of infected immunocompetent mice is showing mild hyperplasia of goblet cells (black arrow)
Cytokine production
Cytokine responses are shown in Fig. 5a. There was significant upregulation of the expression of proinflammatory cytokines (IL-12) and TNF-α in the infected immunosuppressed group compared to that of the infected immunocompetent group. However, the expression of anti-inflammatory cytokines (IL-4 and IL-10) was significantly downregulated in the infected immunosuppressed group compared to that of the infected immunocompetent one on the tenth days postinfection (p values were ≤0.002 and ≤0.001, respectively).
SIgA production
SIgA production is shown in Fig. 5b. There was a significant upregulation of the locally synthesized antibody of the sIgA class in the gut of the infected immunocompetent group compared to that of the infected immunosuppressed group (p ≤ 0.005). This played a significant immunomodulatory role in the clearance of Blastocystis spp. infection from the gut of the infected immunocompetent group.
Discussion
Since symptoms associated with Blastocystis spp. infection were generally more severe in immunocompromised patients than in immunocompetent individuals (Garavelli et al. 1988, 1991), the main focus of this study was to establish an immunosuppressed mouse model for studying this parasite and comparing immunopathological changes in immunosuppressed and immunocompetent mice following Blastocystis spp. infection.
Furthermore, many researchers reported that clinical symptoms of blastocystosis varied among individuals as some patients are asymptomatic, while others display severe abdominal cramps, diarrhea, and fatigue. Researchers also linked Blastocystis spp. with IBS (Stark et al. 2007; Boorom et al. 2008; Stensvold et al. 2009). Others concluded that a clear understanding of the parasite pathogenicity still requires further studies (Yakoob et al. 2004).
In this research, the loss of body weight, lethargy, and slow movement was the common clinical symptoms in immunosuppressed mice following Blastocystis spp. infection. These clinical symptoms were obvious when compared them with those of the infected immunocompetent mice. This result confirmed the role of immunosuppression in increasing the susceptibility of mice for Blastocystis spp. infection. Additionally, the survival rate of the infected immunocompetent mice was higher than that of infected immunosuppressed ones.
Consistent with increased severity of Blastocystis spp. infection in immunosuppressed mice, the histopathological examination of the small intestine showed that Blastocystis spp. infiltrated the lamina propria, the submucosa, and the muscle layers. Moreover, there was severe edema, hyperemia, and congestion. The epithelia of small intestine showed exfoliation, and inflammatory cell infiltration was observed in the submucosa. These data were in agreement with Abou-El Naga and Negm (2001), Yao et al. (2005), Zhang et al. (2006), Iguchi et al. (2009), and Elwakil and Hewedi (2010).
As well, histological examination of the large intestine showed inflammatory-cell infiltration, edematous lamina propria, and mucosal sloughing. These findings were more intense in the intestine of infected immunosuppressed mice. This could be explained on the basis assuming that Blastocystis sp. is an invasive pathogen and capable of causing pathogenesis in BALB/c mice. The pathogenesis was more severe in immunosuppressed mice.
Moreover, the large intestine of infected immunosuppressed mice showed that Blastocystis spp. infiltrated the lamina propria, the submucosa, and the muscle layers forming collection of vacuolar forms. We speculate that this may be due to two reasons. The first reason is the release of cysteine protease by Blastocystis spp. which leads to evasion or modulation of the immune system by degradation of host immune molecules (Puthia et al. 2005). Some studies have demonstrated that cysteine proteases can increase epithelial permeability by modulating the tight junction complex (Mirza et al. 2012).
The second one is the release of hyaluronidase enzyme which leads to the degradation of extracellular matrix proteins namely hyaluronic acid facilitating the invasion by Blastocystis spp. into colonic epithelium (Chandramathi et al. 2010). Chandramathi et al. (2010) found elevation of hyaluronidase in urine of mice infected with Blastocystis spp. which was an indirect evidence of invasion of colonic epithelium by Blastocystis spp.
However, immunohistochemical studies have to be done to elucidate and confirm that these Blastocystis forms identified in the studied mice’s tissues are invasive (Fayer et al. 2015).
Parasites were found mainly in the caecum and colon of the infected immunocompetent mice and in the whole intestine of the infected immunosuppressed mice. The later observation was also reported by Yao et al. (2005) who found Blastocystis spp. in the whole gut of experimentally infected mice. As well, severe edema, hyperemia, and congestion were observed in the tissues of caecum and colon of the infected immunosuppressed mice.
In the present work, vacuolar forms of Blastocystis spp. were the most common form found in the intestine; it can be postulated that the vacuolar forms developed from the inoculated cysts which are the most resistant forms able to withstand gastric digestion. This observation was also reported by El-Gebaly and Zaki (2012). This was also seen in vitro culture, where all noncyst forms arose from cysts within 24 and 48 h Abdel-Hafeez et al. (2015).
The mucous layer is an important barrier between protozoa and host epithelial cells (Ponce-Macotela et al. 2008). Thus, goblet cell hyperplasia had occurred after Blastocystis spp. infection.
Other researchers recorded that protein-losing enteropathy may accompany Blastocystis spp. infections and may be related to increased intestinal permeability (Dogruman and Hokelek 2007). An association with an alteration of goblet cell response and mucin production is observed in different intestinal infections induced by bacteria, viruses, and parasites (Boshuizen et al. 2005; Khan 2008; Hansson 2012).
Many studies demonstrated that the mechanisms of the protective role of goblet cells and mucins against parasites include the demonstration of trapping of worms in the mucus and inhibition of parasite motility and feeding capacity. In addition to enhancing the mucus barrier, goblet cells may play a role in immune activation by presenting luminal antigens to lamina propria dendritic cells (Miller 1987; Khan 2008; McDole et al. 2012).
There was a significant upregulation of the expression of proinflammatory cytokines (IL-12 and TNF-α) in the infected immunosuppressed group compared to that of the infected immunocompetent group (p values ≤0.004 and ≤0.002, respectively). These results suggest that Blastocystis spp. infection in mice induced to some extent an extensive local host response to the exposed antigens. These results were matching with the results obtained by Sinigaglia et al. (1999) and Iguchi et al. (2009). The infected immunosuppressed mice produced more proinflammatory cytokines than the infected immunocompetent one, and the differences were statistically significant. These data suggested that the colonization of Blastocystis spp. parasites provoked the activation (or influx) of T cells, monocytes/macrophages, and/or natural killer cells in local tissues (Iguchi et al. 2009).
Type 1 cytokines have been shown to be important for protective cell-mediated immune responses against a variety of intracellular pathogens (Sinigaglia et al. 1999), but it remains to be elucidated whether IFN-γ and IL-12 play a pivotal role in mucosal defense against Blastocystis spp. infection.
However, the expression of anti-inflammatory cytokines (IL-4 and IL-10) was significantly downregulated in the mice of the infected immunosuppressed group compared to that of the mice of the infected immunocompetent (p values ≤0.002 and ≤0.001, respectively). The infected immunocompetent mice activated production of both Th1 and Th2 cytokines. The synthesized IL-10 might be produced by the host’s immune system in response to infection. IL-10 is thought to act as one of the regulatory cytokines that reduce inflammatory response Antibodies against Blastocystis spp. are one of the potential host defense mechanisms against parasitic infection. They have been suggested to play an important role against parasitic infection (Santos and Rivera, 2009). In mice infected with Blastocystis spp., sIgA was the predominant antibody isotype in intestinal secretions (Santos and Rivera 2009). In Blastocystis spp. infection, an increase in sIgA response was also observed. These data were matching with the results recorded by Mahmoud and Saleh (2003).
Conclusion
Blastocystis spp. infection in immunosuppressed mice was characterized by invasion of that parasite in all intestinal layers with mucosal sloughing, inflammatory cell infiltration, and severe goblet cell hyperplasia. Additionally, there was an elevation of proinflammatory cytokine (IL-12 and TNF-α), which may trigger the inflammatory process. There was a significant upregulation of the locally synthesized antibodies sIgA in the gut of the mice of the infected immunocompetent group compared to that of the mice of the infected immunosuppressed one. This played a significant immunomodulatory role in the clearance of Blastocystis spp. infection from the gut of the infected immunocompetent mice.
References
Abdel-Hafeez EH, Ahmed AK, Abdelgelil NH, Abdellatif MZM, Kamal AM, Mohamed RM (2015) In-vitro effect of some Egyptian herbal extracts against Blastocystis spp. J Egypt Soc Parasitol 45:93–100
Abou-El Naga I, Negm A (2001) Morphology, histochemistry and infectivity of Blastocystis hominis cysts. J Egypt Soc Parasitol 31:627–635
Al-kaissi E, Al-Magdi KJ (2009) Pathogenicity of Blastocystis hominis in Relation to Entropathogens in Gastroenteritis Cases in Baghdad. Eur J Sci Res 25:606–613
Bancroft JD, Gamble A (2008) Theory and practice of Histological techniques. 6th Ed., Churchill-Livingstone, Edinburgh, London, Melbourne, New York
Boorom KF, Smith H, Nimri I, Viscogliosi E, Spanakos G, Parkar U (2008) Oh my aching gut: Irritable bowel syndrome, Blastocystis and asymptomatic infection. Parasit Vectors 1(1):40. doi:10.1186/1756-3305-1-40
Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Bouma J, Gerwig GJ, Koopmans MP, Büller HA, Dekker J, Einerhand AW (2005) Homeostasis and function of goblet cells during rotavirus infection in mice. Virol J 337:210–221
Chandramathi S, Suresh KG, Mahmood AA, Kuppusamy UR (2010) Urinary hyaluronidase activity in rats infected with Blastocystis hominis—evidence for invasion? Parasitol Res 106:1459–1463
Chen TL, Chan CC, Chen HP, Fung CP, Lin CP, Chan WL, Liu CY (2003) Clinical characteristics and endoscopic findings associated with Blastocystis hominis in healthy adults. Am J Trop Med Hyg 69:213–216
Dogruman ALF, Hokelek M (2007) Blastocystis hominis is an opportunistic pathogen. Turk J Parasitol 31:28–36
Dogruman ALF, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, Kustimur S, Altinbas A (2010) Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One 5:e15484
El-Gebaly NSM, Zaki MM (2012) Ultrastuctural intestinal pathology induced by human Blastocystis in experimentally infected mice. P U J 5:127–134
Elwakil HS, Hewedi IH (2010) Pathogenic potential of Blastocystis hominis in laboratory mice. Parasitol Res 107:685–9
Fayer R, Esposito DH, Dubey JP (2015) Human infections with Sarcocystis species. Clin Microbiol Rev 28:295–311
Fouad SA, Basyoni MM, Fahmy RA, Kobaisi MH (2011) The pathogenic role of different Blastocystis hominis genotypes isolated from patients with irritable bowel syndrome. Arab J Gastroenterol 12:194–200
Garavelli PL, Orsi P, Scaglione L (1988) Blastocystis hominis infection during AIDS. Lancet 332:1364
Garavelli PL, Scaglione L, Bicocchi R, Libanore M (1991) Pathogenicity of Blastocystis hominis. Infection 19:185
Garcia LS (2001) Diagnostic Medical Parasitology, 4th edn. ASM Press, Washington DC, USA, pp 87–97
Hansson GC (2012) Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15:57–62
Hussein EM, Hussein AM, Eida MM, Atwa MM (2008) Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102:853–860
Iguchi A, Yoshikawa H, Yamada M, Kimata I, Arizono N (2009) Expression of interferon gamma and proinflammatory cytokines in the cecal mucosa of rats experimentally infected with Blastocystis spp. strain RN94-9. Parasitol Res 105:135–140
Khan WI (2008) Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitolo 135:671–682
Leder K, Hellard ME, Sinclair MI, Fairley CK, Wolfe R (2005) No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J Gastroenterol Hepatol 20:1390–1394
Mahmoud MS, Saleh WA (2003) Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J Egypt Soc Parasitol 33:13–30
McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483:345–349
Miller HR (1987) Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitolo 94:77–100
Miné JC, Rosa JA (2008) Frequency of Blastocystis spp. hominis and other intestinal parasites in stool samples examined at the Parasitology Laboratory of the School of Pharmaceutical Sciences at the São Paulo State University, Araraquara. Rev Soc Bras Med Trop 41:565–569
Mirza H, Wu Z, Teo JD, Tan KS (2012) Statin-pleiotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by Blastocystis cysteineproteases. Cell Microbiol 14:1474–1484
Moe KT, Singh M, Howe J, Ho LC, Tan SW, Chen XQ, Ng GC, Yap EH (1997) Experimental Blastocystis hominis infection in laboratory mice. Parasitol Res 83:319–325
Nagel R, Bielefeldt-Ohmann H, Traub R (2014) Clinical pilot study: efficacy of triple antibiotic therapy in Blastocystis positive irritable bowel syndrome patients. Gut Pathogens 6:1–9
Poirier PH, Wawrzyniak I, Vivare’ CP, Delbac F, El Alaoui H (2012) New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. Plos Pathog 8:1–4
Ponce-Macotela M, González-Maciel A, Reynoso-Robles R, Martínez-Gordillo MN (2008) Goblet cells: are they an unspecific barrier against Giardia intestinalis or a gate? Parasitol Res 102:509–513
Puthia M, Vaithilingam A, Lu J, Tan K (2005) Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol Res 97:286–289
Rossignol JF, Kabil SM, Said M, Samir H, Younis AM (2005) Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol 3:987–991
Saksirisampant W, Nuchprayoon S, Pradniwat P, Lamchuan D (2010) Boeck and Drbohlav Locke egg serum medium for detection of Blastocystis hominis. Chula Med J 54:527–536
Santos HJ, Rivera WL (2009) Kinetic analysis of antibody responses to Blastocystis hominis in sera and intestinal secretions of orally infected mice. Parasitol Res 105:1303–1310
Schumacher JH, O’Garra A, Shrader B, van Kimmenade A, Bond MW, Mosmann TR, Coffman RL (1988) The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol 141:1576–1581
Shlim DR, Hoge CW, Rajah R, Rabold JG, Echeverria P (1995) Is Blastocystis hominis a cause of diarrhea in travelers? A prospective controlled study in Nepal. Clin Infect Dis 21:97–101
Sinigaglia F, D’Ambrosio D, Panina-Bordignon P, Rogge L (1999) Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev 170:65–72
Sohail MR, Fischer PR (2005) Blastocystis hominis and travelers. Travel Med Infect Dis 3(1):33–38
Stark D, van Hal S, Marriott D, Ellis J, Harkness J (2007) Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 37:11–20
Stensvold CR, Lewis HC, Hammerum AM, Porsbo LJ, Nielsen SS, Olsen KE, Arendrup MC, Nielsen HV, Molbak K (2009) Blastocystis: unraveling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol Infect 137:1655–1663
Sukthana Y (2001) Is Blastocystis hominis a Human Pathogenic Protozoan? J Trop Med Parasitol 24:16–22
Tan KS (2004) Blastocystis spp. in human and animals: new using modern methodologies. Vet Parasitol 126:121–144
Tan KS (2008) New insights on classification, identification and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21:639–665
Tan TC, Suresh KG (2006) Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol Res 98:189–193
Yakoob J, Jafri W, Jafri N, Khan R, Islam M, Beg MA, Zaman V (2004) Irritable bowel syndrome in search of etiology: roles of Blastocystis hominis. Am Soc Trop Med Hyg 70:383–385
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010b) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107:679–684
Yakoob J, Abbas Z, Beg MA, Naz S, Awan S, Hamid S, Jafri W (2011) In vitro sensitivity of Blastocystis hominis to garlic, ginger, white cumin, and black pepper used in diet. Parasitol Res 9:379–385
Yao FR, Qiao JY, Zhao Y, Yang JH, Li XQ (2005) Experimental infection of mice with Blastocystis hominis. Chin J Parasitol Parasit Dis 23:444–448
Yoshikawa H, Nagashima M, Morimoto K, Yamanouti Y, Yap EH, Singh M (2003) Freezefracture and cytochemical studies on the in vitro cyst form of reptilian Blastocystis spp. pythoni. J Eukaryot Microbiol 50:70–75
Zhang HM, Li W, Yan QY, He LJ, Su YP (2006) Impact of Blastocystis hominis infection on ultrastructure of intestinal mucosa in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 24:187–191
Zierdt CH (1991) Blastocystis spp. hominis—past and future. Clin Microbiol Rev 4:61–79
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Photomicrographs of various forms of Blastocystis spp. in axenic culture and mice’s feces (x100). a. The axenic culture shows many forms of Blastocystis spp.; the vacuolar form (red arrow) and the granular form (blue arrow). b. In axenic culture, one of the granular form shows multiplication by budding (blue arrow). c. A direct smear of mice’s feces sample shows different forms of Blastocystis spp.; the vacuolar (red arrow), the granular form (blue arrow) and the cyst form (green arrow) (JPG 164 kb)
Figure S2
A photomicrograph of intestine of the infected immunosuppresed mice is showing that Blastocystis (7- 8μm) was dispersed throughout the lamina propria (blue arrow) with numerous eosinophils (green arrow) (JPG 236 kb)
Figure S3
A photomicrograph of the intestinal crypts of the colon of the mice of non-infected immunosuppressed group is showing normal columnar epitheial lining H&E, x 10 (JPG 218 kb)
Rights and permissions
About this article
Cite this article
Abdel-Hafeez, E.H., Ahmad, A.K., Abdelgelil, N.H. et al. Immunopathological assessments of human Blastocystis spp. in experimentally infected immunocompetent and immunosuppresed mice. Parasitol Res 115, 2061–2071 (2016). https://doi.org/10.1007/s00436-016-4951-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4951-3