Abstract
Parasitic nematodes have evolved to exploit a wide variety of hosts living in a range of marine environments. Benthimermithid nematodes occur deeper than any other nematode parasites (down to 5880 m depth) but are mostly known from free-living adult stages living in the sediments, and parasitic juveniles are seldom encountered. In the present study, the benthimermithid Trophomera cf. marionensis was discovered in the body cavity of the lysianassoid amphipod Hirondellea dubia sampled between 7018 and 10,005 m depths in the Kermadec Trench. The nematode specimens, which could be readily observed through the transparent exoskeleton of freshly caught amphipods, were up to twice the length of T. marionensis specimens described from the Atlantic and East Pacific Oceans but were otherwise morphologically identical. Because of its wide geographical and water depth distribution (almost 10,000 m), T. marionensis likely consists of several cryptic species. The prevalence of Trophomera parasites among the host population was estimated to be substantially less than 1 %; such a low proportion of parasitised hosts could help explain why so few Trophomera specimens have been obtained from their host so far. The present study demonstrates that parasites can occur throughout the entire ocean depth and that they likely occur in other hadal trenches where H. dubia and other lysianassoid amphipods also dominate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematode parasites exploit a wide range of vertebrate and invertebrate hosts in aquatic ecosystems. In the deep sea, nematode parasites have been described from macrourid fish hosts to depths of almost 4000 m (Campbell et al. 1980) and from zoarcid fish hosts at hydrothermal vents (Buron and Morand 2004; Justine et al. 2002). The deepest nematode parasites ever recorded were the free-living adults of the benthimermithid nematode Trophomera filiformis (Petter, 1983) in sediments of the western North Atlantic at 5880 m (Miljutin 2004). Until now, no parasitic nematodes had yet been observed in hadal trench environments (>6000 m depth), although free-living species have been recorded in the deepest part of Tonga Trench at 10,600–10,800 m (Belyaev 1972; Leduc 2015).
Benthimermithid nematodes are mainly found in deep-sea environments, but some species have also been described from shallow waters (Miljutin 2014). Their life cycle is similar to the freshwater mermithids and marine marimermithids, i.e. they parasitise marine invertebrate hosts as juveniles and emerge as adults (Tchesunov and Rozenberg 2011). Adults do not feed and survive in the sediments on stored food reserves in the trophosome, a modified midgut serving as storage organ. Male and female presumably reproduce in the sediments, where eggs are laid and subsequently ingested by their deposit-feeding host (Miljutin 2014). Benthimermithid nematodes are rare, and most specimens have been obtained from sediment samples; for most species, the host remains unknown (Miljutin 2006).
Deep-sea invertebrate and sediment samples are typically fixed or preserved immediately upon retrieval to that ensure specimens are in the best condition possible. This often changes the colour of the specimens and may render previously colourless structures opaque, thus making dissection of potential hosts the only means of ascertaining the presence of parasites. Visual observation of freshly caught invertebrates, such as deep-sea crustaceans with largely transparent exoskeletons, may provide a much more efficient way to identify parasitised individuals and help provide sufficient material for taxonomic and ecological investigations. This method was recently applied to samples of bait-attending amphipods from the Kermadec Trench, which yielded the first record of nematode parasites from a hadal environment.
Methods
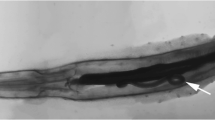
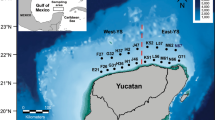
The Kermadec Trench is situated in the South-West Pacific Ocean and is formed by the subduction of the Pacific Plate under the Indo-Australian Plate. It extends from approximately 26 to 36° S near the north-eastern tip of New Zealand’s North Island. Samples were collected from the axis (7139–10,005 m depth) and slope (7018–7560 m) of the Kermadec Trench during Woods Hole Oceanographic Institute (WHOI) cruise TN309 (RV Thomas G Thompson) in May 2014 (Table 1). Amphipods were sampled using baited funnel traps. Each trap was 30 cm long by 12 cm in diameter with a funnel opening of 2.5 cm in diameter and was baited with locally sourced (previously frozen) mackerel. The traps were secured to the base or side of free-falling fish traps and benthic elevator before deployment to the seabed. Upon recovery of the traps, amphipods were inspected for the presence of parasitic nematodes, which can readily be seen through the amphipods’ exoskeleton (Fig. 1). Parasitised amphipods were fixed in 5 % buffered formalin for several months prior to dissection of the nematode parasites.
a Subsample of the amphipod catch (mainly Hirondellea dubia) at site WT03 (7225-m depth); b parasitised H. dubia specimen from the same station, photographed shortly after trap retrieval and before fixing in formalin; multiple Trophomera cf. marionensis parasites can be seen coiled in the body cavity. Scale bar = 5 mm
Ten nematode specimens (from 7139 to 7251 m depth; stations EL06, TR16 and WT03) were used for scanning electron microscopy (SEM), and the remainder were processed for light microscopy. Specimens for SEM were transferred to a 2 % glutaraldehyde solution with sodium cacodylate buffer overnight and transferred to a 4 % osmium tetroxide solution for 2 h. Specimens were then gradually transferred to pure ethanol using a graded ethanol series, critical point dried and mounted onto stubs before coating with gold using a sputter coater. Observations were made using a Hitachi TM3000 tabletop SEM at high vacuum mode. Specimens for light microscopy were transferred to glycerol and mounted onto permanent slides using the method of Somerfield and Warwick (1996); all specimens were identified, and nine complete and well-preserved specimens representing the entire water depth range sampled were chosen for detailed morphological analyses.
Unless otherwise stated, all measurements are in micrometre, and all curved structures are measured along the arc. The terminology used for describing the arrangement of morphological features such as setae follows Coomans (1979). Specimens are held in the NIWA Invertebrate Collection (Wellington). Abbreviations in the text are as follows: a, body length/maximum body diameter; abd, anal body diameter; b, body length/oesophagus length; c, body length/tail length; cbd, corresponding body diameter; %V, vulva distance from anterior end of body × 100 / total body length.
Results
A total of 40 nematode parasites from 32 amphipod hosts were found among the several thousand individuals that were visually inspected (Table 1). Up to four nematode parasites were found in each host, but the majority of hosts (28) contained only one nematode. All parasitised amphipod hosts were identified as Hirondellea dubia Dahl, 1959, and all parasitic nematodes belonged to the same morphospecies, Trophomera cf. marionensis (Petter, 1983). At site TR13 (7948-m depth), one T. cf. marionensis specimen was found in the trap among the amphipods and had presumably emerged out of its host during retrieval of the sample.
Family Benthimermithidae Petter, 1980
Genus Trophomera Rubtsov & Platonova, 1974
(Synonyms: Benthimermis Petter, 1980; Abos Rubtsov, 1980)
Trophomera cf. marionensis (Petter, 1983)
Material examined
Four immature females (NIWA 99750-2, 99758), collected 13 May 2014 (WHOI cruise TN309, station WT03, 7225-m depth); one immature female (NIWA 99759), collected 6 May 2014 (WHOI cruise TN309, station TR13, 7948-m depth); two immature females (NIWA 99753-4), collected 7 May 2014 (WHOI cruise TN309, station TR14, 9198-m depth); two immature females (NIWA 99755-6), collected 8 May 2014 (WHOI cruise TN309, station TR15, 10,005-m depth).
Measurements
See Table 2.
Description
Body long, cylindrical tapering slightly near both body extremities. Cuticle with faint transverse striations, most obvious in head region, difficult to observe with light microscopy (Fig. 4f); cuticle thickness 2–5 μm in head region, 1–3 μm elsewhere. Six labial sensillae, pore-like, only visible with SEM (Fig. 4e); four stout cephalic setae, 4–6 μm long. Somatic papillae arranged in four pairs of more or less regular longitudinal rows: one pair ventrally, one pair dorsally and two pairs laterally (Figs. 2a and 4c); papillae becoming sparse and irregularly arranged posterior to amphideal fovea but reappearing in tail region. Each somatic papilla connected to subcuticular gland with large nucleus (Fig. 2b). Amphideal fovea round or oval, small, slightly cuticularised, slightly larger than amphideal aperture of same shape (Fig. 4f). Mouth opening vestigial, pore-like (Figs. 3a and 4e). Pharynx, a non-musculuar mass without lumen, surrounded by large, clear cells (Fig. 3a). Intestine absent; instead, a large trophosome consisting of rows of numerous large cells with numerous lipid-containing vesicles is present; trophosome with vestigial lumen, only visible using SEM (Fig. 5a, b). Anus absent in all but one specimen.
Trophomera cf. marionensis immature female. a Sublateral view of head showing position of amphid, cephalic setae and somatic setae; b sublateral view of head showing glands associated with somatic setae; c sublateral view of head showing vestigial pharynx and posterior portion of trophosome; d dorsal view of head showing vestgia pharynx, anterior portion of trophosome and glands; e, g posterior body region; f developing reproductive system. Arrows show the position of the amphideal fovea. Scale bar = 100 μm
Trophomera cf. marionensis immature female, scanning electron micrographs. a Entire immature female; b sublateral view of head; c lateral view of anterior body region; d posterior body region; e anterior body apex showing head sensillae and vestigial mouth opening; f details of amphideal aperture, cuticle ornamentation and somatic setae. a amphideal aperture, cs cephalic seta, ls labial sensilla, vm vestigial mouth opening. Scale bar: a 1250 μm, b 30 μm, c 44 μm, d 144 μm, e 11 μm, f 9 μm
Female reproductive system didelphic-amphidelphic, occupying about a quarter of body length, with compact uterus and long oviducts leading to short, reflected ovaries; reproductive system poorly developed in some specimens. Vulva situated at midbody or slightly posterior to midbody, surrounded by numerous large cells regularly arranged around the opening (Fig. 2f). Vagina short, perpendicular to body wall. Posterior end conical, short, narrowing into terminal spine with cytoplasmic core and granular inclusions (Fig. 3b).
Discussion
Kermadec Trench Trophomera
The majority of Trophomera species are known either only from male or female specimens (Miljutin 2006), which limits the number of morphological characters that can be used to differentiate among species. This limitation is particularly true for species for which only females have been described, since females typically have fewer characters than males. No male specimens have been described for T. marionensis, which is thus primarily differentiated from other species of the genus by the shape of the tail (a terminal conical spine with a cytoplasmic core). The Kermadec Trench specimens are identical in most respects to the original description of T. marionensis based on Indian Ocean specimens (31–110-m depth), as well as re-descriptions of the species by Tchesunov (1988) from the South Atlantic (1729 m), by Miljutin (2011) from the Equatorial Atlantic, by Miljutin (2004) from the West Atlantic (4440 m) and by Bussau (1993) and Miljutin and Miljutina (2009) from the East Pacific (4154–4800 m). Given the extremely broad depth distribution of the species (31–4800 m, present study excluded) as well as wide geographical distribution (across three oceans), it seems likely that T. marionensis consists of several cryptic species.
The only difference between the Kermadec Trench specimens and specimens from other regions appears to be the substantially larger body size of the former (15.36–32.71 vs 9.03–16.37 mm). Body size alone is not a reliable trait to use differentiating among species, because body size of free-living adult stages of benthimermithids may depend largely on host body size (Tchesunov and Rozenberg 2011). The large body size of T. cf. marionensis specimens from the Kermadec Trench could reflect the relatively large size of their H. dubia host, but too little is known about the hosts of Trophomera species to conduct comparisons among species. The present study differs from most other studies of the genus in that it is based on specimens dissected out of their hosts, and which are therefore probably not yet fully grown. The largest specimen we measured was a specimen found outside its presumed amphipod host and likely emerged during trap retrieval. Thus, the difference in body length between parasitic specimens from Kermadec Trench and free-living adults from elsewhere is considered to be a conservative estimate of the difference between adults of these geographically disparate populations.
Labial sensillae are rarely observed in Trophomera species. Labial sensillae, which sometimes appear to be intracuticular, have been observed using light microscopy in Trophomera iturupiensis (Rubtzov & Platonova, 1974), Trophomera conchicola Holovachov et al., 2013 and Trophomera megala (Petter, 1987). Scanning electron microscopy has also revealed the presence of labial sensillae in Trophomera litoralis Miljutin, 2006 and T. cf. marionensis specimens from the Kermadec Trench. The cuticle of Trophomera is usually described as smooth but is also sometimes faintly striated (e.g. Trophomera arnaudi (Petter, 1983) and T. megala (Miljutin & Tchesunov, 2001)). The use of SEM has also revealed the presence of cuticle striations in T. litoralis Miljutin, 2006 and T. cf. marionensis.
Trophomera and their hosts
Most of the 40 or so known Trophomera species are only known from adult specimens obtained from sediment samples. Species for which the host is known are Trophomera australis (Petter, 1983) (nematode host, Deontostoma sp.), Trophomera bathycola (Rubtzov, 1980) (priapulid host, Chaetostephanus cirratus), Trophomera granovitchi Tchesunov & Rozenberg, 2011 (amphipod, ostracod, tanaid and isopod hosts) and Trophomera conchicola (chemosymbiotic bivalve host, Idas modiolaeformis). The few data available to date suggest that Trophomera species are able to parasitise a wide range of invertebrates and are not specific in their choice of host (e.g. T. granovitchi). This flexibility is likely to be advantageous in deep-sea environments where population densities of both hosts and parasites are low compared to more productive shallow water habitats.
The lysianassoid amphipod Hirondellea dubia is distributed across the western Pacific trenches, including the Kermadec, Tonga and New Hebrides trenches (Blankenship et al. 2006; Ritchie et al. 2015). H. dubia has also recently been found at abyssal depths near the Mariana Trench, revealing a wide bathymetric range of over 4500 m for this species, from depths of 5678 m (Ritchie et al. 2015) to 10,782 m (Blankenship et al. 2006). H. dubia is highly prolific and is often the sole species recovered from baited traps beyond 9000-m depth (Blankenship et al. 2006; Jamieson et al. 2010). This species may be capable of migrating across abyssal depths, thus substantially increasing the range of any parasites they host (Ritchie et al. 2015). H. dubia has been shown to supplement necrophagy with detritivory (Blankenship and Levin 2007), particularly in juveniles. This greater reliance on detritus in early instars probably increases the probability of parasite transmission in younger individuals. The impact of parasites on their hosts in the food-limited hadal environment is likely to be severe, particularly in individuals that are supporting multiple parasites. It is likely that benthimermithid parasites lead to delayed maturity and reproduction of their hosts before eventually killing them as they emerge.
The prevalence of Trophomera parasites among the H. dubia population was not quantified but is estimated to be substantially less than 1 %. Such a low proportion of parasitised hosts, if typical of Trophomera populations elsewhere, would help explain why so few parasitic juvenile Trophomera specimens have been obtained in previous studies. In the present study, parasitised amphipods could be easily identified through visual inspection of fresh trap samples (often containing thousands of amphipods) prior to fixation in formalin or ethanol. The exoskeleton of fixed amphipod specimens rapidly becomes opaque, thus requiring time-consuming dissection of specimens to ascertain the presence of parasites. In addition, the condition of Trophomera parasites in amphipods preserved in ethanol is particularly poor, which makes dissection and identification difficult (D. Leduc pers. obs.). Given the worldwide distribution of benthimermithids and the dominance of lysianassoid amphipod hosts in hadal environments, visual observation of fresh trap samples from trenches is likely to provide an effective way to obtain more data on the taxonomy, phylogeny and ecology of nematode parasites in this extreme environment. Elucidating the identity of morphologically similar populations will require a molecular approach, which has not yet been applied to this group.
References
Belyaev GM (1972) Hadal bottom fauna of the world ocean. Zenkevich LA (ed.) Jerusalem: Israel Program for Scientific Translations.
Blankenship LE, Levin LA (2007) Extreme food webs: foraging strategies and diets of scavenging amphipods from the ocean’s deepest five kilometers. Limnol and Oceanog 52:1685–1697
Blankenship LE, Yayanos AA, Cadien DB, Levin LA (2006) Vertical zonation patterns of scavenging amphipods from the Hadal zone of the Tonga and Kermadec Trenches. Deep Sea Res I 53:48–61
Buron I, Morand S (2004) Deep-sea hydrothermal vent parasites: why do we not find more? Parasitology 128:1–6
Bussau C (1993) Taxonomische und ökologische untersuchungen an nematoden des Peru-Beckens. PhD thesis, University of Kiel, Germany
Campbell RA, Haedrich RL, Munroe TA (1980) Parasitism and ecological relationships among deep-sea benthic fishes. Marine Biol 57:301–313
Coomans A (1979) A proposal for a more precise terminology of the body regions in the nematode. Annales de la Societe Royale de Zoologie de Belgique 108:115–117
Jamieson AJ, Fujii T, Mayor DJ, Solan M, Priede IG (2010) Hadal trenches: the ecology of the deepest places on Earth. Trends Ecol Evol 25:190–197
Justine J, Cassone J, Petter A (2002) Moravecnema segonzaci n. sp., n. gen. (Nematoda:Cystidicolidae) from Pachycara thermophilum (Zoarcidae), a deep-sea hydrothermal vent fish from the Mid-Atlatic Ridge. Folia Parasitol 49:299–303
Leduc D (2015) One new genus and five new nematode species (Mohysterida, Xyalidae) from Tonga and Kermadec Trenches, Southwest Pacific. Zootaxa 3964:501–525
Miljutin DM (2004) New findings of deep-sea nematodes of genus Benthimermis Petter, 1980 (Nematoda, Benthimermithidae) with description of seven new species. Zoosystema 26:21–48
Miljutin DM (2006) The genus Trophomera Rubstov & Platonova, 1974 with description of T. litoralis sp. n. (Nematoda: Benthimermithidae) from the tidal zone of the Kuril Archipelago and proposal of Benthimermis Petter, 1980 as a junior synonym. Nematology 8:411–423
Miljutin DM (2011) Deep-sea parasitic nematodes of the genus Trophomera Robstov & Platonova, 1974 (Benthimermithidae) from the Equatorial Atlantic, with the descriptions of the two new species. Helgoland Marine Res 65:245–256
Miljutin DM (2014) Order Benthimermithida Tchesunov, 1995. In: Shmidt-Rhaesa A (ed) Handbook of Zoology Gastrotricha, Cyclioneura and Gnathifera, vol 2, Nematoda. De Gruyter, Hamburg, pp 179–186
Miljutin DM, Miljutina MA (2009) Description of Bathynema nodinauti gen. n., sp., n. and four new Trophomera species (Nematoda: Benthimermithidae) from the Clarion-Clipperton Fracture Zone (Eastern Tropic Pacific), supplemented with the keys to genera and species. Zootaxa 2096:173–196
Ritchie H, Jamieson AJ, Piertney SB (2015) Phylogenetic relationships among hadal amphipods of the Superfamily Lysianassoidea: Implications for taxonomy and biogeography. Deep-Sea Res I Oceanogr Res Pap 105:119–131
Somerfield PJ, Warwick RM (1996) Meiofauna in marine pollution monitoring programmes: a laboratory manual. Ministry of Agriculture, Fisheries and Food, Lowestoft, pp 71
Tchesunov AV (1988) New finds of deep-sea nematodes of the family Benthimermithidae in the South Atlantic with description of a new species. Vestnik Zoologii 5:12–22, In Russian
Tchesunov AV, Rozenberg AA (2011) Data on the life cycle of parasitic benthimermithid nematodes with the description of a new species discovered in marine aquaria. Russian J Nematol 19:139–150
Acknowledgments
Funding was provided by NIWA’s Coasts and Oceans Centre Research Programme ‘Marine Biological Resources’ and the project ‘Impact of resource use on vulnerable deep-sea communities’ (CO1X0906). We are grateful to Tim Shank (WHOI), Alan Jamieson (University of Aberdeen), Ashley Rowden and Malcolm Clark (both NIWA), principal investigators of the HADES project (HADal Ecosystem Studies, funded by the National Science Foundation, NSF-OCE 1130712, 1130494 and 1131620) and to the officers, crew and scientific personnel of RV Thomas G. Thompson (voyage TN309) and ROV Nereus engineers and technicians. We thank an anonymous reviewer for providing constructive criticisms on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leduc, D., Wilson, J. Benthimermithid nematode parasites of the amphipod Hirondellea dubia in the Kermadec Trench. Parasitol Res 115, 1675–1682 (2016). https://doi.org/10.1007/s00436-016-4907-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4907-7