Abstract
Mosquito-borne diseases represent a deadly threat for millions of people worldwide. The Culex genus, with special reference to Culex quinquefasciatus, comprises the most common vectors of filariasis across urban and semi-urban areas of Asia. In recent years, important efforts have been conducted to propose green-synthesized nanoparticles as a valuable alternative to synthetic insecticides. However, the mosquitocidal potential of carbon nanoparticles has been scarcely investigated. In this study, the larvicidal and pupicidal activity of carbon nanoparticle (CNP) and silver nanoparticle (AgNP) was tested against Cx. quinquefasciatus. UV–Vis spectrophotometry, Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray (EDX) spectroscopy, and Raman analysis confirmed the rapid and cheap synthesis of carbon and silver nanoparticles. In laboratory assays, LC50 (lethal concentration that kills 50 % of the exposed organisms) values ranged from 8.752 ppm (first-instar larvae) to 18.676 ppm (pupae) for silver nanoparticles and from 6.373 ppm (first-instar larvae) to 14.849 ppm (pupae) for carbon nanoparticles. The predation efficiency of the water bug Lethocerus indicus after a single treatment with low doses of silver and carbon nanoparticles was not reduced. Moderate evidence of genotoxic effects induced by exposure to carbon nanoparticles was found on non-target goldfish, Carassius auratus. Lastly, the plant extract used for silver nanosynthesis was tested for 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity. Overall, our results pointed out that AgNP and CNP can be a candidate for effective tools to reduce larval and pupal populations of filariasis vectors, with reduced genotoxicity and impact on behavioral traits of other aquatic organisms sharing the same ecological niche of Cx. quinquefasciatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes transmit a number of important diseases, such as malaria, filariasis, chikungunya, dengue, and Japanese encephalitis, causing millions of deaths every year (Mehlhorn et al. 2012; Benelli 2015a). Lymphatic filariasis, commonly known as elephantiasis, is a neglected tropical disease. More than 1.4 billion people in 73 countries are living in areas, where lymphatic filariasis is transmitted, and are at risk of being infected. Globally, an estimated 25 million men suffer with genital disease and over 15 million people are afflicted with lymphedema (WHO 2014). In this scenario, vector control of mosquito-borne diseases is a priority, particularly in situations of epidemic outbreaks. Recently, vector control using botanical products arising as a valuable alternative, due to their reduced toxicity towards vertebrates and high biodegradability (e.g., Mehlhorn et al. 2005; Amer and Mehlhorn 2006a, b, c, d; Hafeez et al. 2011; Ravikumar et al. 2011a, b; Benelli 2015b; Benelli et al. 2015).
The development of reliable green process for the synthesis of nanoparticles is an important aspect of current nanotechnology research. Nanotechnology research opens newer avenues for a wide array of applications in the fields of biomedical, sensors, antimicrobials, catalysts, electronics, optical fibers, agricultural, and biolabeling (Salam et al. 2012). Nanoparticles play an indispensable role in drug delivery, diagnostics, imaging, sensing, gene delivery, artificial implants, and tissue engineering (Morones et al. 2005). The synthesis of nanoparticles (NPs) using chemical and physical methods requires high pressure, energy, temperature, and toxic chemicals (Goodsell 2004). Microbes and plants are currently used for nanoparticle synthesis. The use of plants for the fabrication of NP is a rapid, low-cost, and eco-friendly method for biosynthesis process (Huang et al. 2007). A growing number of plant extracts and metabolites have been successfully used for efficient and rapid extracellular synthesis of mosquitocidal NP (Veerakumar et al. 2014; Murugan et al. 2015a, b, c; see Benelli 2016 for a review).
Carbon NPs (CNPs) are of great interest for both fundamental studies and practical applications. CNPs have been widely used in super capacitors (Laforguem et al. 2003), high-performance electrode materials in batteries (Dominko et al. 2003), and photoluminescent materials (Li et al. 2011). Specifically, CNPs with large surface areas can be readily functionalized, allowing an efficient binding of biomolecules. Compared with traditional quantum dots and organic dyes, photoluminescent carbon nanomaterials have advantages due to their chemical inertness and lower toxicity (Dominko et al. 2003). They have been also proposed as possible candidates to develop newer and safer mosquitocidals (see also Saxena et al. 2013), even if field evidences on a wider scale are lacking (Benelli 2016).
Furthermore, mosquito larval population can be controlled by a number of aquatic predators, including odonate young instars, water bugs, tadpoles, fishes, crabs, and copepods (Bowatte et al. 2013; Kalimuthu et al. 2014; Murugan et al. 2011, 2015a, b, c, d). Predation is reported as one of the most limiting factors causing a high level of mortality to immature stages of mosquitoes (Service 1973, 1977). Lethocerus indicus is a giant water bug in the family Belostomatidae; these large aquatic insects have the largest body size among Heteroptera and are reported as common inhabitants and major predators in many aquatic ecosystems (Perez-Goodwyn 2006).
As many chemicals with genotoxic potential are emitted to surface water, genotoxicity tests are gaining importance which led to the development of several techniques to detect directly DNA damage and to identify such pollutants. The relevance of detecting the genotoxic risks associated with water pollution was firstly perceived in the late 1970s. Since that time, several tests have been developed for evaluating DNA alterations in aquatic animals. The possibility of using changes in DNA integrity to the genetic material as markers of exposure and effect of genotoxicants has been investigated (McCarthy and Shugart 1990). The presence of DNA adducts has been taken as evidence of exposure to specific genotoxicants (Shugart 1999). These tests rely on the premise that any changes to DNA may have long-lasting and profound consequences (Lam and Gray 2003).
In this research, the larvicidal and pupicidal activity of green-synthesized silver nanoparticle (AgNP) and CNP was tested for acute toxicity against Culex quinquefasciatus. UV–Vis spectrophotometry, Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy-dispersive X-ray (EDX) spectroscopy confirmed the rapid and cheap synthesis of AgNP and CNP. The predation efficiency of the water bug L. indicus against Cx. quinquefasciatus larvae, after a single treatment with low doses of AgNP and CNP, was evaluated. The possible genotoxic effects of CNP on non-target goldfish, Carassius auratus, were also investigated. Finally, the plant extract used for silver nanosynthesis was also tested for 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity.
Materials and methods
Collection of Moringa oleifera seeds and preparation of seed extracts
M. oleifera belongs to the family Moringacae and is commonly known as “miracle tree.” It is a multipurpose, rapidly growing crop indigenous to North West India (Cidamis et al. 2003). It is commonly planted in Africa as a living fence tree (Von Maydell 1986). These trees have a medicinal value for rural human communities, due to their antibiotic, anti-inflammatory, and analgesic properties (Vinoth et al. 2012). The leaves have been reported to be a rich source of carotene, protein, vitamin C, calcium, and potassium and act as a good source of natural antioxidants (Dillard and German 2000; Siddhuraju and Becker 2003). The seeds possess antimicrobial (Ali et al. 2004; Chuang et al. 2007), antitumor (Guevara et al. 1999; Bharali et al. 2003), anti-inflammatory, antispasmodic, and diuretic (Cárceres et al. 1992) properties. The use of traditional medicine is widespread, and plants still present a large source of natural antioxidants that might lead to the development of novel drugs (Perry et al. 1998).
In this study, seeds of M. oleifera were collected from the garden of the Bharathiar University (Coimbatore, India) and identified at the Department of Botany of Bharathiar University, Coimbatore, India. Voucher specimens were stored in our laboratories. M. oleifera seeds were washed with tap water and shade dried at room temperature. The dried seeds were powdered using an electrical blender; 500 g of the powder was macerated in 1.5 l of ethanol for 72 h. The crude plant extract was concentrated at reduced temperature using a rotary evaporator and stored at 22 °C. One gram of the seed residue was dissolved in 100 ml of acetone (fixative agent to separate the aqueous impurities altering the chemical composition of plant crude extract) and considered as 1 % stock solution. From this stock solution, experimental concentrations were prepared.
Synthesis and characterization of silver nanoparticles
The M. oleifera aqueous seed extract was prepared by adding 20 g of seeds washed with distilled water and was shade dried at room temperature (28 ± 2 °C) for 2 days. The air-dried materials were powdered separately using a commercial electrical blender. The plant seed material (5 g) in a 300-ml Erlenmeyer flask filled with 100 ml of sterilized double-distilled water was finely ground, and the mixture was boiled for 5 min, before finally decanting it. The extract was filtered using a Whatman N. 1 filter paper, stored at −4 °C, and tested within 5 days. The filtrate was treated with aqueous 1 mM silver nitrate in an Erlenmeyer flask and incubated at room temperature. A color change indicated the formation of AgNP, since aqueous silver ions were reduced by the M. oleifera seed extract generating stable AgNP in water (Sujitha et al. 2015). Silver nitrate (AgNO3) was purchased from the Precision Scientific Co. (Coimbatore, India).
As recently reported by Sujitha et al. (2015), the green synthesis of AgNP was confirmed by sampling the reaction mixture at regular intervals and the absorption maxima were scanned by UV–Vis spectrophotometry, at the wavelength of 200–800 nm in a UV-3600 Shimadzu spectrophotometer (1 nm resolution). Furthermore, the reaction mixture was subjected to centrifugation at 15,000 rpm for 20 min and the resulting pellet was dissolved in deionized water and filtered through a Millipore filter (0.45 μm). An aliquot of this filtrate containing AgNP was used for SEM, TEM, FTIR spectroscopy, XRD analysis, and EDX spectroscopy. In detail, the structure and composition of freeze-dried purified AgNP was analyzed using a 10-kV ultra high-resolution scanning electron microscope with 25 μl of the sample that was sputter coated on a copper stub and the morphology of AgNP was investigated using a FEI Quanta 200 SEM and HT7700 120 kV High-Contrast/High-Resolution Digital TEM. The surface groups of the AgNP were qualitatively confirmed by FTIR spectroscopy (Stuart 2002), with spectra recorded using a Perkin-Elmer Spectrum 2000 FTIR spectrophotometer. EDX assays confirmed the presence of metals in analyzed samples (Sujitha et al. 2015).
Synthesis and characterization of carbon nanoparticles
Citric acid and urea were purchased from HiMedia Chemicals; 1.0 M of citric acid and 1.0 M of urea were dissolved in 40 ml of DDW and transferred into a Teflon-lined autoclave. The reaction temperature was maintained at 180 °C for 3 h. Then, the autoclave was cooled naturally and a dark brownish solution, containing carbon quantum dots, was collected. The resultant solution was centrifuged at 10,000 rpm to remove the larger particles. The collected supernatants were subjected to nanocharacterization, following the methods described above for AgNP (Qu et al. 2014).
DPPH radical scavenging activity
The scavenging activity of M. oleifera seed extract against DPPH radical was assessed according to the method of Blois (1958) with minor modifications; 1 ml of M. oleifera seed extracts (0.01 mg dw ml−1) was mixed with 4 ml of 0.005 mg ml−1 DPPH methanol solution. The reaction mixture was vortexed and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured at 517 nm. Ascorbic acid and butylated hydroxytoluene (BHT) were used as references. The ability to scavenge DPPH radical was calculated using the following equation:
where Abscontrol is the absorbance of DPPH radical in methanol and Abssample is the absorbance of DPPH radical solution mixed with sample extract/standard. All determinations were performed in triplicate (n = 3).
ABTS radical scavenging assay
Here, we followed the method by Arnao et al. (2001) with minor modifications; stock solutions included 7 mM ABTS solution and 2.4 mM potassium per sulfate solution. Then, the experimental solution was prepared by mixing the two stock solutions (1:1, v/v) and allowing them to react for 14 h at room temperature in the dark. The solution was then diluted by mixing 1 ml ABTS solution with 60 ml methanol to obtain an absorbance of 0.706 ± 0.01 units at 734 nm using a spectrophotometer. Fresh ABTS solution was prepared for each assay. Seed extracts (1 ml) were allowed to react with 1 ml of the ABTS solution, and the absorbance was taken at 734 nm after 7 min using a spectrophotometer. The ABTS scavenging capacity of the extract was compared with that of BHT and ascorbic acid, and the percentage inhibition was calculated as follows:
where Abscontrol is the absorbance of ABTS radical in methanol and Abssample is the absorbance of ABTS radical solution mixed with the sample extract/standard. All determinations were performed in triplicate (n = 3).
Cx. quinquefasciatus rearing
The mosquitoes tested in this study were laboratory reared and pathogen free. The rearing was originally established as described by Murugan et al. (2015d). Eggs of Cx. quinquefasciatus were provided by the National Centre for Disease Control (NCDC) field station of Mettupalayam (Tamil Nadu, India). Batches of 100–110 eggs were transferred to 18 cm (L) × 13 cm (W) × 4 cm (D) enamel trays containing 500 ml of water where they were allowed to hatch at laboratory conditions [27 ± 2 °C; 7–85 % R.H.; 14:10 (L:D) photoperiod]. Larvae were fed daily with 5 g of ground dog biscuits (Pedigree, USA) and brewer’s yeast (Sigma-Aldrich, Germany) in a 3:1 ratio. First- to fourth-instar larvae and recently pupated individuals (i.e., pupation occurred within 24 h) were collected and used in the experiments (Murugan et al. 2015d).
Larvicidal and pupicidal experiments against Cx. quinquefasciatus
Twenty-five Cx. quinquefasciatus larvae (first, second, third, or fourth instar) or pupae were placed for 24 h in a glass beaker filled with 250 ml of dechlorinated water plus the desired concentration of the M. oleifera seed extract, AgNP or CNP. Larval food (0.5 mg) was provided for each tested concentration. Each concentration was replicated five times against all instars. Control mosquitoes were exposed for 24 h to the corresponding concentration of the solvent. Percentage mortality was calculated as follows:
Impact of nanoparticles on water bug predation
L. indicus were collected from rural ponds in Coimbatore (Tamil Nadu, India) and maintained in laboratory conditions. Water bugs are cannibalistic among the five stages, here only second-instar nymphs were used for the trials. In this experiment, the predation efficiency of second-instar nymphs of L. indicus was assessed against second- and third-instar larvae of Cx. quinquefasciatus. Five hundred mosquitoes were introduced, with two water bugs, into a glass arena (5 l) containing dechlorinated water. Mosquito larvae were replaced daily with new ones. Both for second- and third-instar mosquito larvae, five replicates were conducted. The control was 5 l of dechlorinated water without water bugs. All experimental arenas were checked every 6 h, and the number of prey consumed by water bug was recorded. Predatory efficiency was calculated using the following formula:
Following the same method described above, the predation efficiency of L. indicus adults was assessed against Cx. quinquefasciatus larvae after a single mosquitocidal treatment with AgNP and CNP. In each treatment, 500 second- or third-instar larvae were introduced with two water bugs into a 5-l plastic filled with 5 l of dechlorinated water and 1 ml of the desired concentration of AgNP or CNP [i.e., one third of the LC50 (lethal concentration (ppm) that kills 50 % of the exposed organisms) calculated against first-instar larvae of Cx. quinquefasciatus]. Five replicates were conducted. Predation efficiency was calculated using the abovementioned formula.
Genotoxicity analysis
The goldfish, C. auratus, was chosen for this study due to its wide presence in common freshwater habitats and to its sensitivity to genotoxic chemicals (Deguchi et al. 2007). Juvenile individuals with average weight and length of 5 ± 1 g and 6 ± 1 cm, respectively, were purchased from a local market in Coimbatore and identified by the authors. Before the experiments, they were acclimated under laboratory conditions for 3 weeks at a population density of 15 specimens for l aquarium. Fishes were fed once a day with commercial fish food and exposed to 5, 10, 15, 20, and 25 ppm of CNP for 24, 48, 72, and 96 h.
Micronucleus assay
Experimental subjects were sacrificed in the ethical rules and regulations (see “Compliance with ethical standards” section). The frequency of micronuclei in the peripheral erythrocytes was evaluated according to the criteria described by Countryman and Heddle (1976) and Fenech (1993). Immediately after sampling, a drop of blood was smeared on clean slides (two per fish) and then dried at laboratory temperature. After 24 h, the samples were fixed using pure methanol for 10 min. Afterwards, they were stained with 4 % Giemsa solution for 10 min, air-dried, and prepared for permanent use. Cytological analysis was done under an optical microscope (×1000). A total of 2000 erythrocyte cells were examined per fish in coded slides. The presence of nuclear erythrocyte abnormalities was analyzed. The MN frequency was calculated as
Single cell gel electrophoresis/comet assay
In fishes, about 98 % of total blood cells are erythrocytes. Therefore, no cell separation was performed and, hereafter, cells in the whole blood are referred to as erythrocytes (Theodorakis et al. 1994; De Miranda Cabral Gontijo et al. 2003). The alkaline comet assay was performed according to the method of Tice et al. (2000). The blood samples collected from gills of fish were diluted with 1 ml of PBS. Sixty microliters of the diluted sample was mixed with 200 μl of 0.65 % low-melting-point (LMP) agarose. Seventy-five microliters of the mixture was then layered on the slides pre-coated with 0.5 % normal-melting-point (NMP) agarose and immediately covered with a cover slip and then kept for 10 min in a refrigerator to solidify. After gently removing the cover slips, the slides were covered with a third layer of 90 μl low-melting-point agarose and covered with cover slips again. After solidification of the gel, the cover slips were removed and the slides were immersed in cold lysing solution (i.e., 2.5 M NaCl, 100 mM Na2–EDTA, 10 mM Tris, pH 10, with 10 % DMSO and 1 % Triton X-100 added fresh) and refrigerated at 4 °C for 2 h. After lysis, the slides were placed on a horizontal electrophoresis box side by side. The tank was filled with fresh electrophoresis solution (1 mM Na–EDTA, 300 mM NaOH, and pH 13.5) to a level approximately 0.25 cm above the slides. The slides were left in the solution for 20 min to allow the unwinding. Electrophoresis was performed using the same solution at 25 V and 300 mA for 25 min. The slides were then neutralized with 0.4 M Tris buffer at pH 7.5 and stained with 75 μl ethidium bromide (20 μg ml−1). Then, the slide was photographed by fluorescent microscopy.
Data analysis
Mosquito mortality data were analyzed by probit analysis, calculating LC50 and LC90 (lethal concentration (ppm) that kills 90 % of the exposed organisms) following the method by Finney (1971). Water bug predation data were analyzed using a generalized linear model with two fixed factors: y = Xβ + ε where y is the vector of the observations (i.e., the number of consumed preys), X is the incidence matrix, β is the vector of fixed effects (i.e., treatment and targeted instar), and ε is the vector of the random residual effect. A probability level of P < 0.05 was used for the significance of differences between values. Antioxidant data were transformed into arcsine proportion values and then analyzed by one-way ANOVA. The means were separated using Tukey’s honest significant difference (HSD) test. P < 0.05 was used for the significance of differences between means. JMP 7 was used for all analyses.
Results and discussion
Characterization of silver nanoparticles
The biosynthesis of AgNPs using the M. oleifera aqueous seed extract has been recently described in detail by Sujitha et al. (2015). In this paragraph, we confirmed the biosynthetical route by adding TEM analysis and briefly focusing on the biophysical characterization of M. oleifera-fabricated AgNPs. In our experiments, the biosynthesis of AgNPs was confirmed within 120 min and, after that the M. oleifera seed extract was added to the AgNO3 solution. The color changed from pale yellow to dark brown. The absorption spectra of AgNP at different time intervals showed highly symmetric single-band absorption peaks. A maximum absorption peak was observed at 450 nm; broadening of the peak indicated that the particles are polydispersed (Prasad and Elumalai 2011), and this may be due to excitation of surface plasmon vibrations in green-synthesized AgNPs (Shankar et al. 2004; Dinesh et al. 2015). SEM micrographs showed that the biosynthesized AgNPs were mostly spherical, highlighting a large distribution of sizes, mainly ranging from 35 to 55 nm. It is known that the shape of metal NP can considerably change their optical and electronic properties (Xu and Kall 2002; Chandran et al. 2006; Udayasoorian et al. 2011). TEM supported SEM results, highlighting that the green-synthesized AgNPs showed spherical and cubic shapes, with size ranging from 39 to 57 nm (Supplementary Material Fig. S1). Comparable TEM results were obtained for polydispersed AgNPs chemically reduced by Lee et al. (2004) or biosynthesized by Huang et al. (2007). Notably, biomolecules may act as reducing and capping agents, protecting AgNPs from aggregation (Shankar et al. 2004; Song and Kim 2009). EDX spectroscopy revealed a strong signal in the Ag region, confirming the presence of elemental silver. An XRD pattern of M. oleifera-synthesized AgNPs highlighted the presence of four high diffraction peaks indexing Bragg’s reflection planes (111), (200), (220), and (311), respectively (Sudha Lakshmi 2011; Kumar et al. 2014; Phanjom and Ahmed 2015). These peaks may be due to the organic compounds which are present in the extract and responsible for the reduction of Ag ions and stabilization of AgNPs (Roopan et al. 2013). Thus, the XRD pattern showed that the AgNPs tested in this study are crystalline. An FTIR spectrum showed main peaks at 3336.758 cm−1 (–O–H stretch), 1637.519 cm−1 (aldehyde C–H stretch), and 597.916 cm−1 (C–Br bonds). The broad band appearing close to 3450 cm−1 can be assigned for O–H stretching vibration, indicating the presence of hydroxyl groups in the reducing agent (Das et al. 2013). Bands denote stretching vibration bands responsible for compounds like flavonoids and terpenoids (Huang et al. 2007).
Characterization of carbon nanoparticles
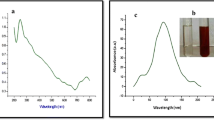
The UV–Vis spectrum of CNPs was reported in Fig. 1. The formation of CNPs was confirmed through the presence of an absorption peak at 235 and 335 nm, probably due to surface plasmon resonance (see also Murugan et al. 2015f) formed. In TEM assays, CNPs showed a mean size of about 3.3 nm and spherical morphology (Fig. 2). This indicates that low quantities of the extract can easily reduce carbon but do not protect most of the quasi-spherical nanoparticles from aggregation, and this is probably due to the deficiency of biomolecules acting as capping agents (Kiruba Daniel et al. 2011). The XRD pattern of CNP is showed in Fig. 3; the peaks are indexed as (002) planes of FCC carbon by comparing with JCPDS data (Dubey et al. 2005). FTIR spectrum of CNP showed peaks at 3235, 1688, 1613, 1200–1400, and 850 cm−1 (Fig. 4); they denoted stretching in O–H, C=O, and C–O bonds of carboxylic acids and 850-out-of-plane C–H vibration, respectively. Following Srinivas Naik et al. (2013), peaks at about 1019 cm−1 may be assigned to –C–O; peaks close to 1642 cm−1 may be assigned to chelated carbonyl group or –OH from carboxylic group in the sennosides. These absorbance bands are known to be associated with the stretching vibrations for –C–C–C–O, –C–C– [(in-ring) aromatic], –C–C– [(in-ring) aromatic], C–O (esters, ethers), and C–O (polyols), respectively (Das et al. 2013; see also Benelli 2016). The Raman spectrum of CNP showed two prominent peaks at 1340 and 1580 cm−1 which corresponded to the E2g (G band) and disorder-induced (D band) modes of graphite, respectively (Fig. 5). Similarly, the Raman spectrum of soxhlet-purified mustard soot has two prominent peaks at 1590 and 1347 cm−1 which corresponds to the E2g (G band) and disorder-induced (D band) modes of graphite (Dubey et al. 2005).
DPPH and ABTS radical scavenging and total antioxidant activity
We compared the radical scavenging activity of the M. oleifera seed extract (0.01 mg ml−1) with BHT and ascorbic acid at the same concentration and expressed it as a percentage of inhibition against DPPH and ABTS, respectively (Table 1). Interestingly, the M. oleifera seed extract significantly reduced DPPH and ABTS (91.43 ± 0.68 and 94.15 ± 0.42, respectively) if compared to ascorbic acid and BHT. Natural products are widely known for their antioxidant properties. For instance, Dimitrova et al. (2010) pointed out that Hypericum species also exhibited radical scavenging activity, with special reference to methanolic extracts of Hypericum cerastoides, Hypericum perforatum, and Hypericum maculatum. Furthermore, Liu et al. (2008) reported that the total phenolic content seems to be strongly related to the antioxidant potential of botanical preparations. Further research is urgently required to shed light on the compound(s) responsible of the high antioxidant activity of the M. oleifera seed extract, since this may have important connections with nutraceutical issues and human health.
Mosquitocidal activity
In laboratory conditions, the M. oleifera seed extract was toxic against the larval instars (I–IV) and pupae of Cx. quinquefasciatus. LC50 values were 112.151 ppm (I), 137.216 ppm (II), 146.540 ppm (III), 184.681 ppm (IV), and 240.111 ppm (pupae) (Table 2). A dose-dependent effect was found, as reported for a growing number of botanicals (Amer and Mehlhorn 2006c, d; Murugan et al. 2015a, b, c; Govindarajan and Benelli 2016; Govindarajan et al. 2016). Recently, Sujitha et al. (2015) reported that the M. oleifera seed extract is effective against young instars of the dengue vector Aedes aegypti.
AgNPs are highly toxic against Cx. quinquefasciatus larvae and pupae, with LC50 of 8.752 ppm (I), 9.875 ppm (II), 11.719 ppm (III), 14.125 ppm (IV), and 18.676 ppm (pupae) (Table 3). Higher toxicity was obtained testing CNPs, since LC50 values were 6.373 ppm (I), 7.710 ppm (II), 8.703 ppm (III), 11.482 ppm (IV), and 14.849 ppm (pupae) (Table 4). The mosquitocidal potential of CNPs has been scarcely investigated. To the best of our knowledge, only Saxena et al. (2013) highlighted that fluorescent water-soluble carbon nanoparticles (wsCNPs) formed from the carbonization of wood wool followed by oxidative treatment may impact on the life cycle of mosquitoes. In detail, they showed that at 3 mg l−1 of wsCNPs, the growth of the Aedes, Anopheles, and Culex mosquitoes from the larval stage to adulthood is blocked. On the other hand, M. oleifera-synthesized AgNPs have been recently found toxic against Ae. aegypti larvae and pupae, with LC50 ranging from 10.24 ppm (I) to 21.17 ppm (pupae) (Sujitha et al. 2015). Interestingly, these AgNPs also inhibited the growth of dengue virus, serotype DEN-2. More generally, in latest years, a growing number of green-fabricated AgNPs have been reported as effective pesticides against different mosquito vectors of medical and veterinary importance (e.g., Suganya et al. 2014; Veerakumar et al. 2014; Murugan et al. 2015a, b, c, d; see Benelli 2016 for a review).
Impact of nanoparticles on water bug predation
In standard laboratory conditions, the predation efficiency of L. indicus towards Cx. quinquefasciatus was 38.5 % (larva II) and 61.25 % (larva III). Post-treatment with AgNPs, L. indicus predation rates were boosted to 50.5 and 77.0 %, respectively. Post-treatment with CNPs, predation rates were boosted to 63.5 and 85.7 %, respectively (Table 5). Scarce information is available about how ultra-low dosages of nanoparticles impact behavioral traits of aquatic organisms sharing the same ecological niche as mosquitoes (reviewed by Benelli 2016; see also Murugan et al. 2015g). Pokhrel and Dubey (2012) studied the effect of AgNPs on the predator–prey relationship between Daphnia magna and dragonfly young instars, showing that these AgNPs reduced the ability of D. magna to detect predators. Later on, some investigations revealed fascinating scenarios. In agreement with the present research, Murugan et al. (2015b) showed that very low doses (i.e., 1 ppm) of lemongrass-synthesized gold nanoparticles may control malaria and dengue mosquitoes by boosting copepod predation on early-instar mosquito larvae in a gold nanoparticle-contaminated environment. We hypothesize that low doses of green-synthesized AgNPs reduce the motility of mosquito larvae, thus enhancing the predation success of mosquito natural enemies (Murugan et al. 2015c, d).
Genotoxicity assay
C. auratus had normal erythrocytes, elliptical in shape with the centrally placed nucleus in the cytoplasm. These erythrocytes were observed in the blood taken from control individuals (Fig. 6a). After exposure to CNPs at doses lower than 25 ppm, no effects were found. After 24 and 48 h of exposure at 25 ppm, the cell membrane was bulged and some micronuclei were found without membrane (Fig. 6b, c). After 72 h, the cell membrane was damaged and numerous micronuclei were found without membrane (Fig. 6d). After 96 h, the cell membrane was fully damaged (Fig. 6e) and micronuclei were observed in the erythrocytes of treated individuals. Concerning the comet assay, no impact on erythrocytes was observed at CNP doses lower than 25 ppm. After 24 h of exposure to 25 ppm, little erythrocyte damage was found (Fig. 7a, b). After 48 h of exposure, medium damage was found (Fig. 7c). After 72 and 96 h, high damage was reported (Fig. 7d, e). In agreement with our aim, Singh (2000) used the comet assay as a very sensitive tool for environmental monitoring which can detect ultra-low levels of DNA damage. Furthermore, Cavaş and Könen (2007) suggested that dose-dependent treatments with pollutants increase the frequencies of micronuclei, nuclear abnormalities, and DNA strand breaks.
Conclusions
This research focused on two eco-friendly nanosynthetical routes to fabricate mosquitocidal CNPs and AgNPs. UV–Vis spectrophotometry, FTIR spectroscopy, XRD analysis, SEM, TEM, EDX spectroscopy, and Raman spectroscopy confirmed the rapid and cheap synthesis of AgNPs and CNPs. The predation efficiency of the water bug L. indicus after a single treatment with low doses of AgNP and CNP was boosted. Moderate evidence of genotoxic effects induced by exposure to high doses of CNPs was found on non-target goldfishes. The plant extract used for silver nanosynthesis showed excellent DPPH and ABTS radical scavenging activity. In summary, our results pointed out that AgNPs and CNPs can be proposed as effective tools to reduce larval and pupal populations of filariasis vectors (see also Benelli 2016), with moderate genotoxicity and impact on behavioral traits of other aquatic organisms sharing the same ecological niche of Cx. quinquefasciatus. However, extensive field assays based on the employ of carbon and metal nanoparticles capped with plant-borne metabolites against mosquito vectors are urgently required.
References
Ali GH, El-Taweel GE, Ali MA (2004) The cytotoxicity and antimicrobial efficiency of Moringa oleifera seeds extracts. Int J Environ Stud 61:699–708
Amer A, Mehlhorn H (2006a) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Amer A, Mehlhorn H (2006b) The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol Res 99:491–499
Amer A, Mehlhorn H (2006c) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006d) Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol Res 99:473–477
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73:239–244
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res. doi:10.1007/s00436-015-4586-9
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2016) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. doi:10.1007/s00436-015-4800-9
Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M (2015) Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res 114:391–397
Bharali R, Tabassum J, Azad MR (2003) Chemomodulatory effect of Moringa oleifera Lam. on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J Cancer Prev 4:131–139
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–2000
Bowatte G, Perera P, Senevirathne G, Meegaskumbura S, Meegaskumbura M (2013) Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol Control 67:469–474
Cárceres A, Saraiva A, Rizzio S, Zabala L, De Leon E, Navy F (1992) Pharmacological properties of Moringa oleifera: screening for antispasmodic, anti-inflammatory and diuretic activity. J Ethnopharmacol 36:233–237
Cavaş T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22(4):263–268
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577–583
Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM (2007) Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol 98:232–236
Cidamis AB, Panga JT, Sarwatt SV, Chore BE, Shayo NB (2003) Nutrient and antinutrient contents in raw and cooked young leaves and immature pods of Moringa oleifera, Lam. Ecol Food Nutri 42:399–411
Countryman PI, Heddle JA (1976) The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res 41:321–332
Das J, Paul Das M, Velusamy P (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta A Mol Biomol Spectrosc 104:265–270
De Miranda Cabral Gontijo AM, Barreto RE, Speit G, Valenzuela Reyes VA, Volpato GL, Favero Salvadori DM (2003) Anesthesia of fish with benzocaine does not interfere with comet assay results. Mutat Res 534(1-2):165–172
Deguchi Y, Toyoizumi T, Masuda S, Yasuhara A, Mohri S, Yamada M, Inoue Y, Kinae N (2007) Evaluation of mutagenic activities of leachates in landfill sites by micronucleus test and comet assay using goldfish. Mutat Res 627:178–185
Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health: a review. J Sci Food Agric 80:1744–1756
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529
Dominko R, Gaberscek M, Drofenik J, Bele M, Jamnik J (2003) Influence of carbon black distribution on performance of oxide cathodes for Li ion batteries. Electrochim Acta 48:3709–3716
Dubey P, Muthukumaran D, Dash S, Mukhopadhyay R, Sarkar S (2005) Synthesis and characterization of water-soluble carbon nanotubes from mustard soot. J Phys 65(4):681–697
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Goodsell DS (2004) Bionanotechnology: lessons from nature. Wiley, Hoboken
Govindarajan M, Benelli G (2016) Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol Res. doi:10.1007/s00436-015-4817-0
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Mehlhorn H, Barnard DR, Benelli G (2016) Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitol Res. doi:10.1007/s00436-015-4794-3
Guevara AP, Vargas C, Sakurai H, Fujiwara Y, Hashimoto K, Maoka T, Kozuka M, Ito Y, Tokuda H, Nishino H (1999) An antitumor promoter from Moringa oleifera Lam. Mutat Res 440:181–188
Hafeez F, Akram W, Abdul E, Shaalan S (2011) Mosquito larvicidal activity of citrus limonoids against Aedes albopictus. Parasitol Res 109:221–229
Huang Q, Li D, Sun Y, Lu Y, Su X, Yang H, Wang Y, Wang W, Shao N, Hong J, Chen C (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:105104–105114
Kalimuthu K, Lin SM, Tseng LC, Murugan K, Hwang JS (2014) Bio-efficacy potential of seaweed Gracilaria firma with copepod, Megacyclops formosanus for the control larvae of dengue vector Aedes aegypti. Hydrobiologia 741:113–123
Kiruba Daniel SCG, Kumar R, Sathish V, Sivakumar M, Sunitha S, Anitha Sironmani T (2011) Green synthesis (Ocimum tenuiflorum) of silver nanoparticles and toxicity studies in zebra fish (Danio rerio) model. Int J Nano Sci Nanotech 2(2):103–117
Kumar DA, Palanichamy V, Roopan SM (2014) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochimica Acta A Molecul Biomolecul Spect 127:168–171
Laforgue A, Simon P, Fauvarque JF, Mastragostino M, Soav F, Sarrau JF, Lailler P, Conte M, Rossi E, Saguatti S (2003) Activated carbon/conducting polymer hybrid super capacitors. J Electrochem Soc 150:A645–A651
Lam PKS, Gray JS (2003) The use of biomarkers in environmental monitoring programmes. Mar Pollut Bull 46:182–186
Lee GJ, Shin S, Kim YC, Oh SG (2004) Preparation of silver nanorods through the control of temperature and pH of reaction medium. Mater Chem Phys 84:197–204
Li HT, He XD, Liu Y, Huang H, Lian SY, Lee ST, Kang ZH (2011) One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 49:605–609
Liu H, Qiu N, Ding H, Yao R (2008) Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Foodserv Res Int 41:363–370
McCarthy JF, Shugart LR (1990) Biological markers of environmental contamination. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis, Boca Raton, pp 3–14
Mehlhorn H, Schmahl G, Schmidt J (2005) Extract of the seeds of the plant Vitex agnus castus proven to be highly efficacious as a repellent against ticks, fleas, mosquitoes and biting flies. Parasitol Res 95:363–365
Mehlhorn H, Al-Rasheid KAS, Al-Quraishy S, Abdel-Ghaffar F (2012) Research and increase of expertise in arachno-entomology are urgently needed. Parasitol Res 110:259–265
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramfrez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotech 16:2346–2353
Murugan K, Hwang JS, Kovendan K, Prasanna Kumar K, Vasugi C, Naresh Kumar A (2011) Use of plant products and copepods for control of the dengue vector, Aedes aegypti. Hydrobiologia 666:331–338
Murugan K, Benelli G, Ayyappan S, Dinesh D, Panneerselvam C, Nicoletti M, Hwang JS, Mahesh Kumar P, Subramaniam J, Suresh U (2015a) Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasit Res 114:2243–2253
Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D, Nicoletti M, Hwang JS, Suresh U, Madhiyazhagan P (2015b) Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol 153:129–138
Murugan K, Priyanka V, Dinesh D, Madhiyazhagan P, Panneerselvam C, Subramaniam J, Suresh U, Chandramohan B, Roni M, Nicoletti M, Alarfaj AA, Higuchi A, Munusamy MA, Khater HF, Messing RH, Benelli G (2015c) Enhanced predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector Aedes aegypti in an aquatic environment treated with mosquitocidal nanoparticles: towards “boosted” biological control? Parasitol Res. doi:10.1007/s00436-015-4582-0
Murugan K, Eugine Venus JS, Panneerselvam C, Bedini S, Conti B, Nicoletti M, Kumar Sarkar S, Hwang JS, Subramaniam J, Madhiyazhagan P, Mahesh Kumar P, Dinesh D, Suresh U, Benelli G (2015d) Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environ Sci Pollut Res. doi:10.1007/s11356-015-4920-x
Murugan K, Aarthi N, Kovendan K, Panneerselvam C, Chandramohan B, Mahesh Kumar P, Amerasan D, Paulpandi M, Chandirasekar R, Dinesh D, Suresh U, Subramaniam J, Higuchi A, Abdullah A, Alarfaj NM, Mehlhorn H, Benelli G (2015e) Mosquitocidal and antiplasmodial activity of Senna occidentalis (Cassiae) and Ocimum basilicum (Lamiaceae) from Maruthamalai hills against Anopheles stephensi and Plasmodium falciparum. Parasitol Res. doi:10.1007/s00436-015-4593-x
Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, Chandramohan B, Subramaniam J, Madhiyazhagan P, Dinesh D, Rajaganesh R, Alarfaj AA, Nicoletti M, Kumar S, Wei H, Canale A, Mehlhorn H, Benelli G (2015f) Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol Res. doi:10.1007/s00436-015-4638-1
Murugan K, Aamina Labeeba M, Panneerselvam C, Dinesh D, Suresh U, Subramaniam J, Madhiyazhagan P, Hwang JS, Wang L, Nicoletti M, Benelli G (2015g) Aristolochia indica green-synthesized silver nanoparticles: a sustainable control tool against the malaria vector Anopheles stephensi? Res Vet Sci 102:127–135
Perez-Goodwyn PJ (2006) Taxonomic revision of the subfamily Lethocerinae Lauck & Menke (Heteroptera: Belostomatidae). Stuttgarter Beiträge zur Naturkunde A (Biologie) 695:1–71
Perry AS, Yamamoto I, Ishaaya I, Perry RY (1998) Insecticides in agriculture and environment. Springer, Berlin
Phanjom P, Ahmed G (2015) Biosynthesis of silver nanoparticles by Aspergillus oryzae (MTCC No. 1846) and its characterizations. Nanosci Nanotech 5(1):14–21
Pokhrel LR, Dubey B (2012) Potential impact of low-concentration silver nanoparticles on predator-prey interactions between predatory dragonfly nymphs and Daphnia magna as a prey. Environ Sci Tech 46:7755–7762
Prasad TNVKV, Elumalai EK (2011) Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed 1:439–442
Qu D, Zheng M, Zhang L, Zhao H, Xie Z, Jing X, Hadda RE, Fan H, Sun Z (2014) Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci Rep 4(5294):1–9
Ravikumar S, Ramanathan G, Inbaneson SJ, Ramu A (2011a) Antiplasmodial activity of two marine polyherbal preparations from Chaetomorpha antennina and Aegiceras corniculatum against Plasmodium falciparum. Parasitol Res 108:107–113
Ravikumar S, Ali MS, Beula JM (2011b) Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asia Pac J Trop Biomed 1:S143–S146
Roopan RSM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod 43:631–635
Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, Sivaraj R (2012) Plants: green route for nanoparticles synthesis. Int Res J Biol Sci 1:85–90
Saxena M, Sumit Kumar Sonkar S, Sarkar S (2013) Water soluble nanocarbons arrest the growth of mosquitoes. RSC Adv 3:22504
Service MW (1973) Mortalities of the larvae of the Anopheles gambiae Giles complex and detection of predators by the precipitin test. Bull Entomol Res 62:359–369
Service MW (1977) Mortalities of the immature stages of species B of the Anopheles gambiae complex in Kenya: comparison between rice fields and temporary pools, identification of predators. J Med Entomol 13(4-5):535–545
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502
Shugart LR (1999) Structural damage to DNA in response to toxicant exposure. In: Forbes VE (ed) Genetics and ecotoxicology. Taylor & Francis, London, pp 151–167
Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). J Agric Food Chem 15:2144–2155
Singh NP (2000) Microgel for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res 455:111–127
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extract. Bioprocess Biosyst Eng 32:79–84
Srinivas NL, Paul MK, Sree vennela P, Venkata RD (2013) Green synthesis of silver nanoparticles using strawberry leaf extract (Arbutus unedo) and evaluation of its antimicrobial activity—a novel study. Int J Nanomat Biostructures pp. 47-50
Stuart BH (2002) Polymer analysis. Wiley, United Kingdom
Sudha Lakshmi Y (2011) Green synthesis of silver nanoparticles from Cleome viscosa: synthesis and antimicrobial activity. International conference on bioscience, biochemistry and bioinformatics IPCBEE, 5th edn. IACSIT, Singapore, pp 334–337
Suganya G, Karthi S, Shivakumar MS (2014) Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. Parasitol Res 113(5):1673–1679
Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, Nicoletti M, Higuchi A, Madhiyazhagan P, Subramaniam J, Dinesh D, Vadivalagan C, Chandramohan B, Alarfaj AA, Munusamy MA, Barnard DR, Benelli G (2015) Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res. doi:10.1007/s00436-015-4556-2
Theodorakis CW, D’Surney SJ, Shugart LR (1994) Detection of genotoxic insult as DNA strand breaks in fish blood cells by agarose gel electrophoresis. Environ Toxicol Chem 13:1023–1031
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Udayasoorian C, Kumar RV, Jayabalakrishnan M (2011) Extracellular synthesis of silver nanoparticles using leaf extract of Cassia auriculata. Dig J Nanomater Biostruct 6(1):279–283
Veerakumar K, Govindrajan M, Rajeswary M, Muthukumaran U (2014) Low-cost and eco-friendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol Res 113:1775–1785
Vinoth B, Manivasagaperumal R, Balamurugan S (2012) Phytochemical analysis and antibacterial activity of Moringa oleifera Lam. Int J Res Biol Sci 2:98–102
Von Maydell HJ (1986) Trees and shrubs of the Sahel, their characteristics and uses. Deutsche Gesellschaft fur Technische Zusammenarbeit, Federal Republic of Germany, pp 334–337
WHO (2014) Lymphatic filariasis. Fact sheet no. 102
Xu H, Kall M (2002) Surface-plasmon-enhanced optical forces in silver nanoaggregates. Phys Rev Lett 89:246802
Zheleva-Dimitrova D, Nedialkov P, Kitanov G (2010) Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn Mag 6:74–78
Acknowledgments
The authors would like to thank the financial support rendered by King Saud University, through the Vice Deanship of Research Chairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no competing interests. G. Benelli is an Editorial Board Member of Parasitology Research. This does not alter the authors’ adherence to all the Parasitology Research policies on sharing data and materials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 122 kb)
Rights and permissions
About this article
Cite this article
Murugan, K., Nataraj, D., Madhiyazhagan, P. et al. Carbon and silver nanoparticles in the fight against the filariasis vector Culex quinquefasciatus: genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol Res 115, 1071–1083 (2016). https://doi.org/10.1007/s00436-015-4837-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4837-9