Abstract
The concern about the harmful effects caused by synthetic pesticides has led to the search for safe and ecological alternatives for pest control. In this context, the neem tree (Azadirachta indica) stands out due to its repellent properties and effects on various arthropods, including ticks. For this reason, this study aimed to demonstrate the potential of neem as a control method for Rhipicephalus sanguineus ticks, important vectors of diseases in the veterinary point of view. For this, R. sanguineus semi-engorged females were subjected to treatment with neem seed oil enriched with azadirachtin, its main compound, and ovaries were assessed by means of morphological techniques in conventional light microscopy, confocal laser scanning microscopy, and transmission electron microscopy. Neem demonstrated a clear dose-dependent effect in the analyzed samples. The observed oocytes presented, especially in the groups treated with higher concentrations of neem oil, obvious signs of cytoplasmic disorganization, cellular vacuolization, nuclear and nucleolar irregularity, dilation in mitochondrial cristae, alterations in mitochondrial matrix, and swelling of rough endoplasmic reticulum. Intracellular microorganisms were observed in all analyzed groups, reinforcing the importance of ticks in the transmission of pathogens. A greater quantity of microorganisms was noted as the concentration of neem increased, indicating that the damaged oocytes may be more susceptible for their development. Such morphological alterations may promote future damages in reproductive performance of these animals and demonstrate the potential of neem seed oil for the control of R. sanguineus ticks, paving the way for new, cheaper, and safer methods of control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, ticks are targets of great concern due to their parasitic nature and high ability to transmit pathogens, being among the most important disease vectors affecting livestock, human, and companion animals, besides causing severe toxic conditions such as paralysis, toxicosis, irritation, and allergy (Oliver 1989; Jongejan and Uilenberg, 2004; Dantas-Torres 2010). From a veterinary point of view, Rhipicephalus sanguineus ticks are considered the most important species involved in the transmission of pathogens to dogs (Dantas-Torres 2010). Many of these diseases transmitted by them may even affect humans (Oyafuso et al. 2002).
As a result, many control methods for numerous species of ticks have been developed, with varied formulations and types of applications. Tick control may be accomplished by a great variety of synthetic acaricides, which eliminate infestations and prevent re-infestations for a certain period of time (Blagburn and Dryden 2009). However, the indiscriminate use of commercial acaricides is an emerging problem, leading to the selection of resistant strains of ticks, toxicity to humans and hosts, and damages to the environment (Blagburn and Dryden 2009; Rosado-Aguilar et al. 2010). The adverse effects of synthetic pesticides and the necessity for environmentally safe alternatives for pest control has led to the search for products extracted from plants, among which stand out the extracts from Annona squamosa (Magadum et al. 2009), Aegle marmelos, Andrographis lineata, Andrographis paniculata, Cocculus hirsutus, Eclipta prostrata (Elango and Rahuman 2011), Cuminum cyminum, Pimenta dioica (Martinez-Velazquez et al. 2011), Acorus calamus (Ghosh et al. 2011), and many others, including Azadirachta indica, the neem tree, which stands as one of the options with higher potential (Raizada et al. 2001; Brahmachari 2004).
In ticks, the use of neem products showed relevant effects for the control of Amblyomma variegatum (Ndumu et al. 1999), Hyalomma dromedarii (Al-Rajhy et al. 2003), Hyalomma anatolicum excavatum (Abdel-Shafy and Zayed, 2002), Rhipicephalus decoloratus (Choudhury 2009), Rhipicephalus (Boophilus) microplus (Srivastava et al. 2008; Broglio-Micheletti et al. 2010) and R. sanguineus (Denardi et al. 2010, 2011, 2012), affecting reproductive processes or even causing mortality.
Among the numerous active ingredients present in Azadirachta indica, azadirachtin is considered the most important and effective, being present predominantly in seeds, leaves, and other parts of the neem tree (Schmutterer 1990; Brahmachari 2004). It presents a broad action spectrum, including growth dysregulation, reduction in ecdysone levels, changes in development and reproduction, and damages in molt processes (Vietmeyer 1992; Brahmachari 2004).
Morphological analysis, evaluated using microscopy techniques, has been particularly useful in toxicological analysis, allowing the observation countless cellular alterations, including chromosomal aberrations, the occurrence of micronuclei, nuclear abnormalities, histopathological alterations, genotoxic and mutagenic effects, and alterations in cytoplasmic organelles (Fontanetti et al. 2010). In this sense, the use of morphology to evaluate vitellogenesis dynamics in ectoparasite females has been a tool of great importance, since it provides information that contributes to the development of studies accessing the cytotoxic potential of chemical agents in germ cells (Roma et al. 2010b).
Thus, the present study aimed to evaluate the morphological effects of the oil extracted from neem seeds (Azadirachta indica), with known concentrations of azadirachtin, in the ovary of R. sanguineus ticks by means of conventional light microscopy, laser scanning confocal microscopy, and transmission electron microscopy, in order to demonstrate the interference of neem compounds in the reproduction development of these animals.
Material and methods
R. sanguineus ticks
Unfed male and female ticks were obtained directly from the colony maintained in a biological oxygen demand (BOD) incubator under controlled conditions (25 ± 1 °C, 80 % humidity, in a 12-h photoperiod), in the Biosciences Institute vivarium of São Paulo State University (UNESP), Rio Claro/SP, Brazil.
Azadirachta indica (neem)
Neem oil was provided by Professor Moacir Rossi Forim, from Universidade Federal de São Carlos (UFScar). Neem seeds (2.5 kg) were ground in a Tecnal TE 631/2 mill and then subjected to an extraction process with hexane (five extractions with duration of 3 days each), in order to obtain the neem oil. After this, the seeds went through a methanol extraction by three sequent extractions performed using an Ultra-Turrax T-20. After filtration, the solvent was evaporated under reduced pressure to provide an extract rich in azadirachtin, which was subsequently mixed with the previous extracted oil in order to enrich it with azadirachtin. The measurement of azadirachtin concentration in the crude extract of neem oil was made through the same procedures described by Forim et al. (2010). A concentration of 1,000 ppm of azadirachtin in the crude extract was determined, and from this oil, the dilutions used in this experiment were made.
Experimental procedures
Preparation of neem dilutions
The crude oil extracted from neem seeds was diluted at concentrations of 20, 40, and 60 % in a 10 % aqueous solution of ethanol, according to an adaptation of the methodology described by Ndumu et al. (1999). Thus, it was possible to obtain dilutions with specific concentrations of azadirachtin: 200 ppm (20 %), 400 ppm (40 %), and 600 ppm (60 %). The solutions were mixed on a magnetic stirrer and applied topically on ticks. The dilutions were made daily during the experiment and kept in a light-protected recipient, in order to avoid alterations of the acaricidal properties of neem oil.
Bioassay
A total of 60 couples of R. sanguineus were released inside special feeding chambers set on the back of naive New Zealand White female rabbits (without prior exposure to tick infestation), following the methodology described by Bechara et al. (1995). Five rabbits were used in this experiment, one for each group (CI, CII, TI, TII, and TIII). The procedures for application of neem dilutions were performed inside these feeding chambers, during engorgement of females.
Five groups were established in this experiment: control groups (CI and CII) and treatment groups (TI, TII, and TIII). In the treatment groups, the solutions of neem oil were applied topically on ticks attached on the back of rabbits with a soft brush for 3 days, twice a day. The applications began 24 h after attachment of tick to the host, at the concentrations of 20 % (TI), 40 % (TII), and 60 % (TIII). The ticks from the control groups (CI and CII) were subjected to the same procedure, with applications of distilled water and 10 % aqueous ethanol, respectively. Approximately 5 mL of solution was used in each application. On the fourth day of infestation, semi-engorged females were collected and maintained under controlled conditions in a BOD incubator before dissection for removal of samples. The collected ticks were dissected under a Leica EZ4 stereomicroscope in Petri dishes containing phosphate-buffered saline (PBS) solution (NaCl 0.13 M, Na2HPO4 0.017 M, KH2PO4 0.02 M, pH 7.2) for withdrawal of ovary samples.
All experimental procedures performed in this study were approved by the Ethics Committee in Animal Use, CEUA, UNESP, Rio Claro/SP, Brazil, protocol number 2206, decision number 021/2012.
Morphological analysis
Light microscopy
For histological analysis, the collected material was immediately fixed in 4 % paraformaldehyde solution for 72 h and then transferred to sodium phosphate buffer solution (pH 7.2) for 24 h. The samples were subsequently subjected to dehydration in an ascending series of ethanol (70, 80, 90, and 95 %, for 20 min in each solution), overnight infiltration in Leica historesin, followed by polymerization with Leica historesin plus a hardener agent.
The resin blocks containing the material were sectioned on a Leica RM2255 microtome, and the sections subjected to staining with hematoxylin and eosin technique (HE), for the observation and description of the general morphology of the tissue (Junqueira and Junqueira 1983). The microscopic slides obtained were mounted in Canada synthetic balsam, and the material was photographed in a Leica DM150 light photomicroscope, equipped with a Leica ICC50 HD camera, by means of the Leica LAS v.3.8 software.
Laser scanning confocal microscopy
The collected samples were immediately fixed in 4 % paraformaldehyde for 72 h, washed in PBS (pH 7.4) twice for 5 min and then permeabilized with 0.1 % Triton-X for 20 min. After two washes of 5 min each, the material was incubated with a solution of “Alexa Fluor 488 Phalloidin” for 30 min at room temperature in a covered container. The samples were washed in PBS (twice, 5 min each), stained with propidium iodide at 10 μg/mL for 30 min in the dark, washed again in PBS (twice, 5 min each), and mounted using mounting medium Prolong® Gold reagent. The images were obtained with a Leica TCS SP5II Laser Scanning Confocal Microscope and analyzed by means of the Leica LAS AF software.
Transmission electron microscopy
For ultrastructural analysis, the collected ovary samples were fixed in glutaraldehyde 2.5 % in sodium cacodylate buffer 0.1 M (pH 7.2) for 72 h. Next, the material was washed twice in 0.1 M sodium cacodilato buffer (15 min each time) and then postfixed in osmium tetroxide (OsO4) for 2 h. The samples were then washed twice again in 0.1 M sodium cacodilato buffer (15 min each time) and immersed in a 10 % aqueous ethanol solution for 15 min. The material was contrasted in 1 % uranyl acetate, dehydrated in an ascending series of acetone (50, 70, 90, and 100 %, for 5 min in each solution), and then dipped in a mixture of acetone and Epon-Araldite resin (1:1) for 12 h, embedded in Epon-Araldite resin for 24 h, and included in Epon-Araldite resin plus a catalyzer at 60 °C. Ultrathin sections, obtained using an ultramicrotome Leica Reichert Supernova were placed on copper grids and contrasted in uranyl acetate 4 % for 45 min and lead citrate for 10 min (Reynolds 1963). The material was observed and photographed in a Philips CM100 transmission electron microscope (TEM, operating at an accelerating voltage of 80 kV) equipped with a Veleta camera, by means of the iTEM software (v. 5.2).
Results
The histological evaluation of the ovary of R. sanguineus ticks showed the presence of oocytes in stages I and II and in the transition between them as well. However, fluorescence and ultrastructure observations allowed the visualization of oocytes only in the transition from type I to type II, which did not affect the analysis of the results. The histological and ultrastructural characteristics of the oocytes in the transition stage (I/II) were defined for each experimental group, as shown in Fig. 1.
Schematic drawing of the general alterations observed in the oocytes of R. sanguineus ticks in each experimental group. a Control group I; b control group II; c treatment group I; d treatment group II; e treatment group III. bl basal lamina, chr chromatin, cy cytosol, dm damaged mitochondria, hz “halo zone,” ine irregular nuclear envelope, m mitochondria, mic microorganisms, mlb multilamellar bodies, n nucleus, ne nuclear envelope, nu nucleolus, pcy perinuclear cytoplasm, pm plasma membrane, vac vacuolation, vnu vacuolated nucleolus. Bars a–e 50 μm, details 1 μm
Conventional light microscopy
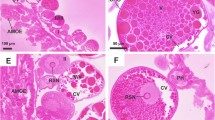
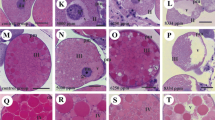
In group CI, the oocytes type I (Fig. 2a) presented smaller size, elliptic aspect, and round nuclei occupying great part of the center of the cells. The cytoplasm showed strong basophilia and purple color, with some acidophilous areas in pink. The perinuclear cytoplasm showed a higher degree of acidophilia containing a great number of basophilic particles transiting between the nucleus and the cytoplasm. The nucleus presented decondensed chromatin, weakly stained by hematoxylin. In general, one or two central nucleoli constituted by strong basophilic granulations were observed occupying great part of the nucleus. The oocytes type II (Fig. 2b) showed larger size and round shape, with a central round nucleus. The cytoplasm presented less intense basophilia in relation to the previous stage, and some acidophilous granulations—stained in pink by eosin—could be observed. The basal membrane was observed around the plasma membrane, presenting an intense pink staining. The perinuclear cytoplasm was less evident, apparently with a proportionally smaller number of particles in transport. The nucleus showed similar characteristics in relation to the previous stage, with the limits strongly stained by eosin and one or two nucleoli with granular aspect. In both oocytes, few basophilic structures were observed, involved by a slightly acidophilous halo, and distributed throughout the cytoplasm. These structures were posteriorly identified as intracellular microorganisms through fluorescence and ultrastructure.
Type I (a, c, e, g, i) and type II (b, d, f, h, j) oocytes of R. sanguineus ticks stained with hematoxylin and eosin and examined under conventional light microscopy. a–b Control group I; c–d control group II; e–f treatment group I; g–h treatment group II; i–j treatment group III. mic microorganisms, n nucleus, nu nucleolus, pc perinuclear cytoplasm, pdc pedicel cells, vac vacuolation. Bars a–i 50 μm
The individuals belonging to group CII showed the same characteristics observed in group CI. However, in oocytes I (Fig. 2c) and II (Fig. 2d) highly basophilic nucleoli were observed, with a more condensed and less granular appearance.
In group TI, the oocytes type I (Fig. 2e) presented similar characteristics in relation to the individuals from the control group. The oocytes type II (Fig. 2f) also showed similar morphology in relation to the control group. However, light pink acidophilous granulations could also be observed. In both oocytes, a large number of microorganisms were detected, generally covered by a whitish halo.
In group TII, the oocytes type I (Fig. 2g) presented fewer variations in relation to group TI. The oocytes type II (Fig. 2h) presented more relevant alterations. The cytoplasm showed non-stained regions, in small number but very evident, representing possible vacuolation areas. The nucleus presented a slightly irregular morphology. A great number of microorganisms were observed in both oocytes with whitish regions around them.
In group TIII, the oocytes type I (Fig. 2i) showed cytoplasmic areas with acidophilous characteristics in a proportionally larger amount in relation to the rest of the cytoplasm. However, more intense alterations were observed in oocytes type II (Fig. 2j). The composition of the cytoplasm was clearly different from the one observed in the control individuals, with evident disorganization. Some regions with higher acidophilia were observed, in addition to vacuolated regions that formed non-stained areas. The nuclear membrane showed slight irregularity, in addition to a decrease in the intensity of coloration by the hematoxylin. In many oocytes, vacuoles or lighter halos were detected in the interior of the nucleoli. In both types of oocytes, large groups of microorganisms were observed, with noticeably vacuolated regions around them, a characteristic that was not observed in any other group.
Confocal laser scanning microscopy
No differences were observed between the groups CI (Fig. 3a, b) and CII (Fig. 3c, d). The oocytes presented irregular shape, and the cytoplasm was strongly marked by the propidium iodide, showing an intense reddish color. The nucleus was more lightly marked by the propidium iodide, presenting regular shape and one or two round and intact nucleoli, more intensely marked in red. The reaction with phalloidin did not show the presence of cytoskeleton molecules; these molecules were marked only around the oocytes, possibly belonging to the cells of the ovary duct or the pedicel.
Oocytes of R. sanguineus ticks labeled with Alexa Fluor 488 Phalloidin (green) and stained with propidium iodide (red) examined under laser scanning confocal microscopy. a–b Control group I; c–d control group II; e–f treatment group I; g–h treatment group II; i–j treatment group III. ac actin filament, cy cytoplasm, icl irregular cell limit, mic microorganisms, n nucleus, nu nucleolus, vac vacuolation. Bars a–j 30 μm
In the individuals of group TI (Fig. 3e, f), the cytoplasm of the oocytes was less intensely marked by the propidium iodide and the cytoplasmic limits were strongly marked by phalloidin, showing irregularity in the surface of the oocyte. The microorganisms, generally in the cellular periphery, were marked by the propidium iodide, presenting strong red color.
The oocytes of the individuals of group TII (Fig. 3g, h) presented similarity to the ones belonging to group TI. The cellular limits were also irregular and strongly marked by phalloidin. In relation to group TI an apparently higher proportion of red-stained microorganisms could be visualized.
The cytoplasm and nuclei of the oocytes of the individuals belonging to group TIII (Fig. 3i, j) presented similar marking in relation to groups TI and TII, as well as similar irregularity of the cellular limit, strongly marked by phalloidin. However, most cells presented nucleoli with less stained and round-shaped areas, corresponding to the vacuoles or halos found in the histological analysis. The presence of microorganisms was apparently similar to group TII, being more significant in some cases.
Transmission Electron Microscopy
No differences were observed between the oocytes of the individuals belonging to groups CI and CII. The oocytes analyzed showed from round to elliptic shape, without irregularities in the plasma membrane, as well as in the shape and size of nucleus. From one to two nucleoli were observed, with granulate and electron-dense aspect. The chromatin generally presented a decondensed aspect (Figs. 4a, d and 5a). Many electron-dense bodies, corresponding to basophile particles in conventional light microscopy (CLM), could be observed in the perinuclear cytoplasm (Fig. 4b). The mitochondria presented elongated shape, with a more electron-dense mitochondrial matrix in relation to the cytoplasm, internal and external membranes evidentiating the intermembrane space and elongated cristae, transversal to the organelle (Figs. 4e and 5d, e). In some oocytes, well-preserved and intact cisternae of the rough endoplasmic reticulum (RER) could be observed. In some regions of the cytosol, round to egg-shaped structures, larger than the mitochondria and with variable electron density could be visualized, being identified as intracellular microorganisms (Figs. 4f and 5c, f). These structures presented a highly electron-dense spot in their interior, in addition to several membranes around. They were inserted in a completely electron-lucid region (“halo zone”), in which many multilamellar bodies formed by concentric membrane circles were present. Multivesicular bodies formed by several small and round structures inside a large vesicle were also observed in the cytosol. The basal lamina showed electron density relatively similar to cytosol, however with a more granular aspect (Figs. 4c and 5b).
Oocytes of R. sanguineus ticks from control group I, observed under transmission electron microscopy. a Detail of oocyte nucleus; b detail of cytoplasm near the oocyte nucleus; c oocyte periphery; d detail of nucleolus; e–f detail of cytoplasmic organelles. bl basal lamina, chr chromatin, cy cytosol, eu euchromatin, het heterochromatin, hz “halo zone,” ld lipid droplet, m mitochondria, mic microorganism, mvb multivesicular odies, ne nuclear envelope, nu nucleolus, pcy perinuclear cytoplasm, pm plasma membrane. Bars a 5 μm, b 2 μm, c–f 1 μm
Oocytes of R. sanguineus ticks from control group II, observed under transmission electron microscopy. a Detail of oocyte nucleus; b–c oocyte periphery; d–f detail of cytoplasmic organelles. bl basal lamina, chr chromatin, cy cytosol, hz “halo zone,” ld lipid droplet, m mitochondria, mic microorganism, mlb multilamellar bodies, mvb multivesicular bodies, ne nuclear envelope, nu nucleolus, pcy perinuclear cytoplasm, pm plasma membrane, ves vesicle. Bars a 2 μm, b–d, f 1 μm, e 500 nm
In the oocytes belonging to group TI, the cytosol showed slight disorganization, with the emergence of vacuolated regions and apparent decrease in the electron density (Fig. 6a, c–e). The microorganisms were also observed, generally with large electron-lucid spaces around the halo zone, similarly to a process of cytoplasmic vacuolation (Fig. 6b, f).
Oocytes of R. sanguineus ticks from treatment group I, observed under transmission electron microscopy. a Detail of oocyte nucleus; b detail of cytoplasm near the oocyte nucleus; c–d oocyte periphery; e–f detail of cytoplasmic organelles. bl basal lamina, chr chromatin, cy cytosol, dcy damaged/disorganized cytosol, eu euchromatin, ld lipid droplet, hz “halo zone,” m mitochondria, mic microorganism, mlb multilamellar bodies, mv microvillus, mvb multivesicular bodies, ne nuclear envelope, nu nucleolus, pcy perinuclear cytoplasm, pm plasma membrane, vac vacuole. Bars a 5 μm, b 1 μm, c–d 500 nm, e 1 μm, f 2 μm
In the oocytes of the individuals belonging to group TII, several regions of cytoplasmic disorganization and vacuolation were frequently visualized throughout the cytosol as strongly electron-lucid and irregular spaces in which no organelles were observed (Fig. 7a–c). The organelles did not show uniformity in their characteristics. In some cases, enlarged cisternae of the RER were observed (Fig. 7d). Moreover, many oocytes presented visibly altered mitochondria, with evident enlargement of the intermembrane spaces, specifically in the interior of the mitochondrial cristae, in addition to an increase in the electron density of the mitochondrial matrix (Fig. 7e). Large regions of vacuolation and cytoplasmic disorganization could be observed around the microorganisms, similarly to group TI (Fig. 7f).
Oocytes of R. sanguineus ticks from treatment group II, observed under transmission electron microscopy. a Detail of oocyte nucleus; b detail of vacuolated cytoplasm; c oocyte periphery, d–f detail of cytoplasmic organelles. bl basal lamina, chr chromatin, cy cytosol, dcy damaged/disorganized cytosol, drer damaged/dilated rough endoplasmic reticulum, ims intermembrane space, hz “halo zone,” ld lipid droplet, m mitochondria, mic microorganism, mlb multilamellar bodies, ne nuclear envelope, nu nucleolus, pcy perinuclear cytoplasm, pm plasma membrane, vac vacuole. Bars a 2 μm, b–e 1 μm, f 500 nm
The most expressive effects were observed in group TIII. In some cases, nuclei with evident irregularity in the nuclear membrane could be visualized (Fig. 8a). The presence of fragmented nucleoli or presenting vacuoles as observed in CLM and confocal laser scanning microscopy (CLSM) was not detected here. Many regions of disorganization and cytoplasmic vacuolation visualized as non-uniform and highly electron-lucid portions were evidenced in the extension of the cytosol, similarly to group TII (Fig. 8b). In most cases, evident enlargements in the RER cisternae were visualized (Fig. 8f), in addition to mitochondria presenting highly electron-dense matrix and significant enlargements in the intermembrane spaces of the mitochondrial cristae (Fig. 8g, h). Large groups with three or more intracellular microorganisms dispersed throughout the cytosol were found, similarly to the analysis under CLM and CLSM. Intense vacuolation was observed around these structures (Fig. 8c–e).
Oocytes of R. sanguineus ticks from treatment group III, observed under transmission electron microscopy. a–b Detail of cytoplasm near the oocyte nucleus; c, f oocyte periphery; d–e, g–h detail of cytoplasmic organelles. bl basal lamina, chr chromatin, cy cytosol, dcy damaged/disorganized cytosol, drer damaged/dilated rough endoplasmic reticulum, dm damaged/dilated mitochondria, hz “halo zone,” ims intermembrane space, ine irregular nuclear envelope, ld lipid droplet, m mitochondria, mic microorganism, mlb multilamellar bodies, mm mitochondrial matrix, mvb multivesicular bodies, ne nuclear envelope, pcy perinuclear cytoplasm, pm plasma membrane, vac vacuole. Bars a, c–h 1 μm, b 5 μm
Discussion
Products derived from plants have been widely used as an alternative method to control parasites, aiming to decrease the development of resistance and obtain low-cost biodegradable parasiticides (Chagas et al. 2012). A CO2 extract of the seeds of Vitex agnus castus at concentrations of 1–3 %, for example, can be used as a repellent spray for Ixodes ricinus and R. sanguineus ticks from animals and humans (Mehlhorn et al. 2005). Moreover, a high antiparasitic activity against R. microplus was detected in leaf ethyl acetate extracts of Andrographis paniculata and Cocculus hirsutus and methanolic extracts of Aegle marmelos, Andrographis lineata, and Eclipta prostrate (Elango and Rahuman 2011). Essential oils obtained from Cuminum cyminum and Pimenta dioica resulted in 100 % mortality in almost all concentrations against R. microplus, indicating that they can be used as an effective alternative against this tick species (Martinez-Velazquez et al. 2011). Furthermore, Azadirachta indica extracts demonstrated high level of efficacy (80 %) in R. microplus ticks, also reducing the egg-laying properties of the survived ticks (Srivastava et al. 2008).
Substances having pesticide properties can be found in practically all the parts of the neem tree (Azadirachta indica); however, the highest concentration is found in the seeds (Brahmachari 2004). Thus, insects and other plagues of agricultural, medical, and veterinary interest can be controlled through repulsion, feeding inhibition or even death (Atawodi and Atawodi 2009), in addition to development and reproductive impairment (Vietmeyer 1992; Brahmachari 2004). Furthermore, the oil obtained from the neem seeds is able to cover the body of the animal, blocking the tracheal openings and causing suffocation (Brahmachari 2004). The neem derivatives do not necessarily lead the individuals to death but cause extremely subtle alterations which impair their biological success (Vietmeyer 1992).
In this context, the structural modifications observed in the reproductive system cells can provide important information which might not be observed in other methods of study. This is particularly noticeable in many studies which aimed to evaluate the morphological effects of substances with acaricide potential on ticks, especially R. sanguineus, considering its great veterinary importance. Synthetic compounds such as fipronil (Oliveira et al. 2008, 2009) and permethrin (Roma et al. 2010a,b, 2011), and natural ones such as andiroba oil (Vendramini et al. 2012; Roma et al. 2013a), the esters of ricinoleic acid of castor oil (Arnosti et al. 2011; Sampieri et al. 2012) and neem leaves extract (Denardi et al. 2010, 2011, 2012) caused expressive structural alterations in the reproductive system of these ticks, such as irregularity of the oocytes, cytoplasmic vacuolation, alterations in the organelles, nuclear and nucleolar damages, and modifications in the yolk synthesis.
The neem active ingredients are absorbed by the body of the insects and other organisms like hormones, due to their structural similarity with these compounds, blocking the endocrine system and causing alterations which inhibit reproduction (Vietmeyer 1992). When considering a topic application of the product on R. sanguineus ticks, it is suggested that the toxic agents crossed the tegumental system penetrating the hemolymph, from where they would be transported to the internal organs. Similarly to the ovary, other organs may have been directly or indirectly affected. Considering the large amount of azadirachtin in the extracts used and its importance in controlling arthropods (Schmutterer 1990; Vietmeyer 1992; Brahmachari 2004), it can be inferred that great part of the effects found here is related to the action of these molecules.
In this study, a dose-dependent effect of the neem oil on the reproductive system of R. sanguineus was clearly observed, proved by the use of different microscopy techniques. The morphological damages visualized in higher concentrations were more evident, indicating alteration in the future development and viability of the embryos. The application of aqueous extracts of neem leaves on R. sanguineus ticks in the concentrations of 10 and 20 % also showed evident dose-dependent histological effects on the ovary (Denardi et al. 2010), although this fact has not been demonstrated in histochemical or ultrastructural analyses (Denardi et al. 2011, 2012). The large amount of azadirachtin in the neem-enriched oil as well as its chemical composition can have been responsible for the differences observed in relation to the leaf extracts.
According to the cytoplasmic aspect, location of the nucleus, number and constitution of the yolk granules, and the presence of chorion, the oocytes can be classified in five types (Oliveira et al. 2005; Roma et al. 2013b). In most analyses carried out here, the oocytes were visualized in stages I, II, or in the transition between them. Therefore, aiming to facilitate the understanding, these alterations were treated in a general way in the discussion, so that the effects of neem on the oocytes as a whole in this feeding stage could be evaluated and discussed. Such effects tend to be progressive, i.e., they are intensified as the oocyte develops. This fact becomes evident in oocytes type II, where the effects were, in general, more intense in relation to type I.
The individuals belonging to both groups did not show significant differences in relation to each other and showed a morphology corresponding to previous descriptions for the species (Oliveira et al. 2005; Roma et al. 2013b). However, the histological analysis revealed differences in the characteristics of the nucleoli of the individuals subjected to aqueous ethanol at 10 %. The functional meaning of this change is not clear yet, considering that such characteristic has not been confirmed by the images in fluorescence and ultrastructure, in which the nucleoli presented completely normal aspect. No other alteration was observed, and this characteristic has probably not affected the development of oocytes in these ticks. Thus, it can be suggested that the use of ethylic alcohol diluted in water at 10 % did not interfere in the results obtained by the use of neem, only helping in the process of homogenization and dilution of this oil in aqueous solution.
No alterations in the morphological characteristics of the nucleus of the treated individuals were observed, except for the treatment with a higher concentration of the product, where the nuclear membrane was irregular, corroborating the results obtained by Denardi et al. (2010, 2011) for the effects of neem leaf extracts on R. sanguineus. The irregularity of the nuclear membrane was also observed in oocytes of R. sanguineus treated with the synthetic acaricide permethrin (Roma et al. 2011), demonstrating that the effects here observed are similar to the ones caused by products recognizably efficient in the control of ticks.
The treatment with neem oil in R. sanguineus was responsible for the appearance of vacuolated regions in the nucleoli of the oocytes type II, indicating degenerative processes that can lead to the death of the cells. Similar alterations were observed in R. sanguineus ticks treated with neem leaf extracts, in which the nucleoli formed a compact ring-shaped mass with a central vacuole, observed both in the histological and ultrastructural analyses, indicating a process of cell death (Denardi et al. 2010, 2011). Such alterations were also reported for the oocytes of R. sanguineus ticks treated with permethrin (Roma et al. 2010b) and andiroba oil (Vendramini et al. 2012), suggesting the occurrence of degeneration of the genetic material of the cells, which could affect their future development.
These nucleolar vacuoles were detected through histological evaluation only in the oocytes type II of the individuals subjected to the treatment with higher concentration of neem oil. The observation in fluorescence with propidium iodide, which allows the visualization of nucleic acids present in the cell (Suzuki et al. 1997), also showed these alterations. However, in the ultrastructure, the nucleoli of the oocytes did not show visible changes, implying that these structural effects are evident only in oocytes in more advanced development stages, once the oocytes observed under electron microscopy were still in intermediate stages, between types I and II.
In general, the oocytes of R. sanguineus treated with neem oil did not show alterations in their shape when evaluated histologically and ultrastructurally, differently from what was observed in experiments with other natural compounds such as esters of ricinoleic acid (Arnosti et al. 2010), andiroba seed oil (Vendramini et al. 2012), and neem aqueous extracts (Denardi et al. 2011, 2012). However, when evaluated under confocal microscopy, the individuals of all the groups showed oocytes with evident irregularity, possibly because a total mounting was made. Thus, these characteristics were not considered as alterations caused by the treatment.
The preparations on fluorescence also allowed the evaluation of the cytoskeleton architecture of the ovarian cells by phalloidin, which attaches to the actin filaments (Cooper 1987). The intense staining in the periphery of the oocytes of the treated individuals can indicate a possible alteration in the composition and the presence of elements from the cytoskeleton in these cells and has also been demonstrated for R. sanguineus treated with aqueous neem extracts, which presented disorganized elements in the cytoskeleton, especially at the concentration of 10 % (Denardi et al. 2012).
The morphological damages observed in this study represent strong evidence that some of the neem components caused direct effects on the oocytes. In literature, there are two main assumptions to explain the possible entrance route of the chemical, synthetic, and natural compounds to the interior of the cells: they can directly cross the oocyte wall (Oliveira et al. 2009; Vendramini et al. 2012) or be transferred into these cells through the pedicel cells (Roma et al. 2010a, 2011; Denardi et al. 2010, 2011). Regarding the neem oil compounds, the way the substances are absorbed is unknown; however, as observed in R. sanguineus ticks exposed to fipronil (Oliveira et al. 2009) and andiroba oil (Vendramini et al. 2012), the vulnerability of the oocytes in the initial stages of development may indicate that the neem active ingredients have crossed their wall. The basal lamina and the plasma membrane have possibly been crossed without having suffered any harmful effect evident under microscopy. In the analyzed stages, the deposition of the chorion, the membrane which protects the eggs against mechanical shocks, temperature variations, and dehydration, in addition to helping in the gaseous exchanges was not observed (Oliveira et al. 2008). Its deposition begins in the oocytes in stage III and is completed in stage IV of the ovarian development (Coons and Alberti 1999), resulting in an extra protection against the entrance of strange substances in the oocytes (Oliveira et al. 2009).
The vacuolation regions observed in the histological analysis of R. sanguineus oocytes were visualized in ultrastructure as completely electron-lucid portions indicating extended disorganization and cytoplasmic degradation, suggesting significant damages in the cell as a whole. These characteristics became more intense as the concentration of the product increased. Similar ultrastructural effects were also observed in R. sanguineus exposed to fipronil (Oliveira et al. 2009), permethrin (Roma et al. 2010b), and to the extracts of neem leaves (Denardi et al. 2012). These regions may be damaged portions of the cytosol or even regions where the cytoplasmic components are being degraded or recycled through autophagic processes in order to maintain the cellular integrity and assure the viability of the oocyte (Roma et al. 2011; Denardi et al. 2012). Such mechanisms, in an uncontrolled way, can lead to autophagic cell death, a form of morphologically programmed death, differently from apoptosis (Levine and Yuan 2005).
In the oocytes of R. sanguineus treated with fipronil (Oliveira et al. 2009) and neem leaf extract (Denardi et al. 2012), the mitochondria presented irregular morphology, with disorganized or absent cristae. Mitochondrial alterations were also observed in the oocytes of the individuals treated with neem oil. These damages can lead to energetic impairments able to affect the metabolism of these cells, considering their great energetic demand for the production of yolk. This fact, allied to the alterations in the rough RER, can drastically affect the vitellogenesis over the embryonic development. The alterations observed in these organelles can also indicate a process of cell death (Oliveira et al. 2009; Denardi et al. 2012).
In the Malphigian tubules and salivary glands of naturally infected R. sanguineus, Rickettsia rhipicephali bacteria were identified and showed in ultrastructural analysis, presenting trilaminar or quadrilaminar wall with an electron-lucid halo (halo zone) around and also ribosomes distributed in a variable way throughout the cytoplasm, immerse in a finely granular and amorphous substance (Hayes and Burgdorfer 1979). Santos et al. (2002) demonstrated the morphological characteristics of R. conorii bacteria in the salivary glands of R. sanguineus, calling the electron-lucid halo “periplasmic space.” Internally, electron-dense material consistent with the prokaryotic chromatin was observed. These ultrastructural characteristics are very similar to those observed in this study, suggesting that the microorganisms observed may be intracellular bacteria. In this case, the electron-dense stain in their interior can correspond to the genetic material dispersed in the cytoplasm. The fluorescence images reinforce this hypothesis, once these structures were strongly stained by the propidium iodide, indicating the presence of genetic material (Suzuki et al. 1997).
In the individuals treated with neem oil, a gradually more intense occurrence of these supposed bacteria, usually in large groups and presenting an electron-lucid halo around, was observed. In this case, for having harmed and affected the sensitivity of the oocytes, the neem components might have made them more susceptible to the microbial development. The cellular metabolism is probably able to control the development and reproduction of these microorganisms in healthy individuals, and this mechanism of control may have been impaired in the oocytes of treated individuals. The presence of large groups of bacteria in the treatment with higher concentrations reinforces this hypothesis. The electron-lucid portions confirm the occurrence of cytoplasmic degeneration in this region, which were more intense as the number of microorganisms increased. Anyhow, their presence did not interfere in the emergence of damages caused by neem, once they were also observed in distant regions and were not detected in the control individuals which were also infected.
Conclusion
The methodology used in this study conciliated the results obtained and a field application of neem. Furthermore, the use of ticks in intermediate feeding stages increased the reproductive cell susceptibility to the compounds used, once these cells were not protected by the chorion. Thus, a progressive application initiated at the first signs of infestation tends to me more efficient in the control of this species. The neem components, especially azadirachtin, interfered directly in the metabolism and in the morphology of the oocytes, with more expressive effects in higher concentrations of the compounds. These alterations suggest that this treatment can affect the viability of the embryos, contributing for the reproductive control of R. sanguineus, a way of control that is less harmful to the environment and also safer to the hosts.
References
Abdel-Shafy S, Zayed AA (2002) In vitro acaricidal effect of plant extract of neem seed oil (Azadirachta indica) on egg, immature, and adult stages of Hyalomma anatolicum excavatum (Ixodoidea: Ixodidae). Vet Parasitol 106:89–96
Al-Rajhy DH, Alahmed AM, Hussein HI, Kheir SL (2003) Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae). Pest Manag Sci 59:1250–1254
Arnosti A, Brienza PD, Furquim KCS, Chierice GO, Bechara GH, Calligaris IB, Camargo-Mathias MI (2011) Effects of ricinoleic acid esters from castor oil of Ricinus communis on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Exp Parasitol 127(2):575–580
Atawodi SE, Atawodi JC (2009) Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev 8:601–620
Bechara GH, Szabó MPJ, Ferreira BR, Garcia MV (1995) Rhipicephalus sanguineus tick in Brazil: feeding and reproductive aspects under laboratorial conditions. Rev Bras Parasitol Vet 4(2):61–66
Blagburn BL, Dryden MW (2009) Biology, treatment and control of flea and tick infestations. Vet Clin Small Anim 39:1173–1200
Brahmachari G (2004) Neem—an omnipotent plant: a retrospection. Chem BioChem 5:408–421
Broglio-Micheletti SMF, Dias NS, Valente ECN, Souza LA, Lopes DOP, Santos JM (2010) Ação do extrato e óleo de nim no controle de Rhipicephalus (Boophilus) microplus (Canestrini, 1887) (Acari: Ixodidae) em laboratório. Rev Bras Parasitol Vet 19(1):44–48
Chagas ACS, Barros LD, Cotinguiba F, Furlan M, Giglioti R, Oliveira MCS, Bizzo HR (2012) In vitro efficacy of plant extracts and synthesized substances on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol Res 110:295–303
Choudhury MK (2009) Toxicity of neem seed oil against the larvae of Boophilus decoloratus, a one-host tick in cattle. Indian J Pharm Sci 5:562–563
Coons LB, Alberti G (1999) The acari-ticks. In: Harrison FW, Foelix R (eds) Microscopic anatomy of invertebrates, Chelicerate Arthropoda. Wiley-Liss, NewYork, pp 267–514
Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478
Dantas-Torres F (2010) Biology and ecology of the Brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3(26):1–11
Denardi SE, Bechara GH, Oliveira PR, Camargo-Mathias MI (2010) Azadirachta indica A. Juss (neem) induced morphological changes on oocytes of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) tick females. Exp Parasitol 126:462–470
Denardi SE, Bechara GH, Oliveira PR, Camargo-Mathias MI (2011) Inhibitory action of neem aqueous extract (Azadirachta indica A. Juss) on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Microsc Res Techniq 74:889–899
Denardi SE, Bechara GH, Oliveira PR, Camargo-Mathias MI (2012) Ultrastructural analysis of the oocytes of female Rhipicephalus sanguineus (Latreille,1806) (Acari: Ixodidae) tick subjected to the action of Azadirachta indica A. Juss (neem). Ultrastruct Pathol 1:56–67
Elango G, Rahuman AA (2011) Evaluation of medicinal plant extracts against ticks and fluke. Parasitol Res 108:513–519
Fontanetti CS, Christofoletti CA, Pinheiro TG, Souza TS, Pedro-Escher J (2010) Microscopy as a tool in toxicological evaluations. In: Méndez-Vilas A, Díaz J (eds) Microscopy: Science, technology, applications and education. Badajoz, Formatex, pp 1002–1007
Forim MR, Silva MFGF, Cass QB, Fernandes JB, Vieira PC (2010) Simultaneous quantification of azadirachtin and 3-tigloylazadirachtol in Brazilian seeds and oil of Azadirachta indica: application to quality control and marketing. Anal Method 2:860–869
Ghosh S, Sharma AK, Kumar S, Tiwari SS, Rastogi S, Srivastava S, Singh M, Kumar R, Paul S, Ray DD, Rawat AKS (2011) In vitro and in vivo efficacy of Acorus calamus extract against Rhipicephalus (Boophilus) microplus. Parasitol Res 108:361–370
Hayes SF, Burgdorfer W (1979) Ultrastructure of Rickettsia rhipicephali, a new membr of the spotted fever group Rickettsiae in tissues of the host vector Rhipicephalus sanguineus. J Bacteriol 137(1):605–613
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14
Junqueira LCU, Junqueira LMMS (1983) Técnicas Básicas de Citologia e Histologia. Editora Santos, São Paulo
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Magadum S, Mondal DB, Ghosh S (2009) Comparative efficacy of Annona squamosa and Azadiracta indica extracts against Boophilus microplus Izatnagar isolate. Parasitol Res 105:1085–1091
Martinez-Velazquez M, Castillo-Herrera GA, Rosario Cruz R, Flores-Fernandez JM, Lopez-Ramirez J, Hernandez-Gutierrez R, Lugo-Cervantes EC (2011) Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dióica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol Res 108:481–487
Mehlhorn H, Schmahl G, Schmidt J (2005) Extract of the seeds of the plant Vitex agnus castus proven to be highly efficacious as a repellent against ticks, fleas, mosquitoes and biting flies. Parasitol Res 95:363–365
Ndumu PA, George JBD, Choudhury MK (1999) Toxicity of neem seed oil (Azadirachta indica) against the larvae of Amblyomma variegatum a three-host tick in cattle. Phytother Res 13:532–534
Oliveira PR, Bechara GH, Denardi SE, Nunes ET, Camargo-Mathias MI (2005) Morphological characterization of the ovary and oocytes vitellogenesis of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Exp Parasitol 110:146–156
Oliveira PR, Bechara GH, Camargo-Mathias MI (2008) Evaluation of cytotoxic effects of fipronil on ovaries of semi-engorged Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) tick female. Food Chem Toxicol 46:2459–2465
Oliveira PR, Bechara GH, Marin-Morales MA, Camargo-Mathias MI (2009) Action of the chemical agent fipronil on the reproductive process of semi-engorged females of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Ultrastructural evaluation of ovary cells. Food Chem Toxicol 47:1255–1264
Oliver JH (1989) Biology and systematics of ticks (Acari: Ixodida). Annu Rev Ecol Syst 20:397–430
Oyafuso MK, Dagnone AS, Vidotto O, Morais HSA (2002) Characterization of ticks infecting dogs in a hospital population in North Paraná, Brazil. Semina: Ciências Agrárias 23:71–74
Raizada RB, Srivastava MK, Kaushal RA, Singh RP (2001) Azadirachtin, a neem biopesticide: subchronic toxicity assessment in rats. Food Chem Toxicol 39:477–483
Reynolds ES (1963) The use of lead citrate at high pH eletron-opaque stain in electron microscopy. J Cell Biol 208–212
Roma GC, Furquim KCS, Bechara GH, Camargo-Mathias MI (2010a) Permethrin-induced morphological changes in oocytes of Rhipicephalus sanguineus (Acari: Ixodidae) semi-engorged females. Food Chem Toxicol 48:825–830
Roma GC, Bechara GH, Camargo-Mathias MI (2010b) Permethrin-induced ultrastructural changes in oocytes of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females. Ticks Tick Borne Dis 1:113–123
Roma GC, Furquim KCS, Bechara GH, Camargo-Mathias MI (2011) Cytotoxic effects of permethrin in oocytes of Rhipicephalus sanguineus (Acari: Ixodidae) fully engoged females: I. Direct or indirect action of the acaricide in germ cells? Exp Appl Acarol 53:287–299
Roma GC, Vendramini MCR, Camargo-Mathias MI, Nunes PH, Faria AU, Bechara GH (2013a) Action of andiroba oil and permethrin on the central nervous and reproductive systems of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) tick females. A confocal study. Res Vet Sci 95:529–536
Roma GC, Sanches GS, Oliveira PR, Denardi SE, Camargo-Mathias MI (2013b) Sistema reprodutor feminino. In: Camargo-Mathias MI (ed) Guia básico de morfologia interna de carrapatos ixodídeos. 1ª Edição, Editora UNESP, pp.83–108
Rosado-Aguilar JA, Aguilar-Caballero A, Rodriguez-Vivas RI, Borges-Argaez R, Garcia-Vazquez Z, Mendez-Gonzalez M (2010) Acaricidal activity of extracts from Petiveria alliacea (Phytolaccaceae) against the cattle tick, Rhipicephalus (Boophilus) microplus. Vet Parasitol 168:299–303
Sampieri BR, Arnosti A, Nunes PH, Furquim KCS, Chierice GO, Camargo-Mathias MI (2012) Ultrastructural changes in the ovary cells of engorged Rhipicephalus sanguineus female ticks treated with esters of ricinoléico acid from castor oil (Ricinus communis). Microsc Res Techniq 75:683,690
Santos AS, Bacellar F, Santos-Silva M, Formosinho P, Grácio AJ, Franca S (2002) Ultrastructural study of the infection process of Rickettsia conorii in the salivary glands of the vector tick Rhipicephalus sanguineus. Vector Borne Zoonotic Dis 2(3):165–177
Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu Rev Entomol 35:271–297
Srivastava R, Ghosh S, Mandal DB, Azhahianambi P, Singhal PS, Pandey NN, Swarup D (2008) Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol Res 104:149–153
Suzuki T, Fujikura K, Higashiyama T, Takatam K (1997) DNA staining for fluorescence and laser confocal microscopy. J Histochem Cytochem 45(1):49–53
Vendramini MCR, Camargo-Mathias MI, Faria AU, Bechara GH, Oliveira PR, Roma GC (2012) Cytotoxic effects of andiroba oil (Carapa guianensis) in reproductive system of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females. Parasitol Res 111:1885–1894
Vietmeyer ND (1992) Neem: a tree for solving global problems. Report of an ad hoc panel of the Board on Science and Technology for International Development. National Academy Press, Washington, D.C., 141 pp
Acknowledgments
The authors are thankful to Prof. Moacir Rossi Forim, Prof. Odair Correa Bueno, and Marcela Ceccato for providing neem oil for the development of this work. This research was financially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflict of interest
No conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remedio, R.N., Nunes, P.H., Anholeto, L.A. et al. Morphological effects of neem (Azadirachta indica A. Juss) seed oil with known azadirachtin concentrations on the oocytes of semi-engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol Res 114, 431–444 (2015). https://doi.org/10.1007/s00436-014-4200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4200-6