Abstract
This study was conducted to evaluate the phytochemical composition and larvicidal effect of leaf essential oil from Murraya exotica against early fourth-instar larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. Gas chromatography (GC) and gas chromatography mass spectrometry (GC-MS) analyses revealed that the essential oil contained 27 components. The major chemical components identified were β-humulene (40.62 %), benzyl benzoate (23.96 %), β-caryophyllene (7.05 %) and α-terpinene (5.66 %). The larval mortality was observed after 12 and 24 h of exposure period. The results revealed that essential oil showed varied levels of larvicidal activity against A. aegypti, A. stephensi and C. quinquefasciatus. After 12 h of exposure period, the larvicidal activities were LC50 = 74.7 and LC90 = 152.7 ppm (A. aegypti), LC50 = 56.3 and LC90 = 107.8 ppm (A. stephensi ), and LC50 = 74.4 and LC90 = 136.9 ppm (C. quinquefasciatus) and the larvicidal activities after 24 h of exposure period were LC50 = 35.8 and LC90 = 85.4 ppm (A. aegypti), LC50 = 31.3 and LC90 = 75.1 ppm (A. stephensi), and LC50 = 43.2 and LC90 = 103.2 ppm (C. quinquefasciatus). These results suggest that leaf essential oil from M. exotica is a promising and eco-friendly source of natural larvicidal agent against A. aegypti, A. stephensi and C. quinquefasciatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases are illnesses caused by pathogens and parasites in human populations. Every year, more than one billion people are infected and more than one million people die from vector-borne diseases including malaria, dengue, schistosomiasis, leishmaniasis, Chagas disease, yellow fever, lymphatic filariasis and onchocerciasis (WHO 2014). Mosquitoes represent a significant threat because of their ability to vector pathogens that cause diseases that afflict millions of people worldwide (WHO 2010). Several species belonging to genera Aedes, Anopheles and Culex are vectors for the pathogens of various diseases like dengue fever, dengue hemorrhagic fever, malaria, Japanese encephalitis and filariasis (Borah et al. 2010; Rahuman et al. 2009). Dengue is prevalent in more than 100 countries and threatens the health of approximately 2.5 billion people. Around 50 million people are infected annually, characterizing a pandemia (WHO 2002).
In recent years, the emphasis to control the mosquito populations has steadily shifted from the use of conventional chemicals towards more specific and environmentally friendly materials which are generally of botanical origin. For this purpose, many phytochemicals from various plant species have been tested for their larvicidal and repellent actions against mosquitoes (Ciccia et al. 2000; Ansari and Razdan 2000).
Aedes aegypti is generally known as a vector for an arbovirus responsible for dengue fever, which is endemic to southeast Asia, the pacific island area, Africa and America. This mosquito is also the vector of yellow fever in central South America and West Africa. Dengue fever has become an important public health problem as the number of reported cases continues to increase, especially with more severe forms of the disease, dengue hemorrhagic fever and dengue shock syndrome, or with unusual manifestations such as central nervous system involvement (Pancharoen et al. 2002). Culex quinquefasciatus, a vector of lymphatic filariasis, is widely distributed in tropical zones with around 120 million people being infected worldwide and 44 million people having a common chronic manifestation (Bernhard et al. 2003). A. stephensi Liston is the primary vector of malaria in India and other west Asian countries. Malaria remains one of the most prevalent diseases in the tropical world with 200 million to 450 million infections annually worldwide; it causes up to 2.7 million deaths (WHO 2010).
Essential oils from plants may be an alternative source of mosquito larval control agents, since they constitute a rich source of bioactive compounds that are biodegradable into nontoxic products and potentially suitable for use in integrated management programmes. In fact, many researchers have reported on the effectiveness of plant essential oils against mosquito larvae, and the recent examples are studied by (Jantan et al. 2005; Thomas et al. 2004). Among them, essential oils were the first preservatives used by man, originally in their natural state within plant tissues and then as oils obtained by water distillation (Bakkali et al. 2008). The essential oil extracted from fresh leaves of Hyptis suaveolens and its main constituents were evaluated for larvicidal and repellent activity against the Asian tiger mosquito, Aedes albopictus (Conti et al. 2012).
Murraya exotica L. (family Rutaceae) is a handsome evergreen shrub or small tree 3–4 m in height with a spreading crown and short, often crooked, trunk found almost throughout India and in the Andaman Islands. The plant is commonly grown in gardens for its glossy green foliage and large clusters of fragrant flowers. It is a popular hedge plant and is well adapted for topiary work. Propagation may be done by seeds, cuttings or layering. The leaves are stimulant and astringent. The leaves and bark are reported to be used for diarrhoea and dysentery in the Philippines and China (Chopra 1956; Li et al. 1988; Anonymous 1964). The acetone extract of M. exotica leaves showed an antifeedant activity against the early third-stage larvae of Spodoptera litura (Wang et al. 2009).
The purpose of the present investigation was made to explore the larvicidal activity of leaf essential oil from M. exotica against A. aegypti, A. stephensi and C. quinquefasciatus larvae in search for effective and affordable natural products to be used in the control of vectors.
Materials and methods
Plant material
The leaves of M. exotica were collected from Annamalainagar (Lat. 11.39° N; Long. 79.71° E), Cuddalore District, Tamil Nadu, India, during April, 2012. The plant was identified by former professor Dr. R. Selvaraj, Department of Botany, Annamalai University. Herbarium specimen was deposited in the Department of Botany, Annamalai University.
Extraction of the essential oil
Healthy and well-grown fresh leaves of M. exotica were collected and immediately brought to the laboratory using polythene bags. The fresh leaves were cut into small pieces and subjected to hydro-distillation using the Clevenger X77 type of apparatus for 4 h. The obtained essential oil was dried over anhydrous sodium sulphate, and the purified essential oil was stored in an amber colour vial (sealed with parafilm) at 4 °C for further analysis and larvicidal assay.
Chemical analysis of essential oil
Gas chromatography (GC) analysis was carried out using Thermo GC-Trace Ultra Ver: 5.0, Thermo MS DSQ II. The chromatograph was fitted with DB 5-MS capillary non-polar column. The injector temperature was set at 300 °C and the oven temperature initially at 80 °C then programmed to 200 °C at the rate of 5 °C/min and held at 200 °C for 10 min. Then the temperature was increased to 300 °C at the rate of 20 °C/min, finally held at 300 °C for 5 min. Helium was used as a carrier gas with the flow rate of 1.0 ml/min. The sample was injected in the split mode in the ratio of 1:100. The percentage of composition of the essential oil was calculated by the GC peak areas.
GC-MS analysis
GC and gas chromatography-mass spectrometry (GC-MS) analyses of essential oils were performed by using Varian 3800 Gas Chromatography equipped with Varian 1200 C single quadrupole mass spectrometer. GC conditions were the same as reported for GC analysis, and the same column was used. The mass spectrometer was operated in the electron impact (EI) mode at 70 eV. Ion source and transfer line temperature was kept at 300 °C. The mass spectrum was obtained by centroid scan of the mass range from 40 to 800 amu. Identification of components of the essential oil was matching their recorded spectra with the data bank mass spectra of NIST and WILEY library provided by the instrument software, and the components were confirmed by comparing with previous literature.
Mosquito larvicidal bioassay
The eggs of A. aegypti and A. stephensi were received from the Field Station, Centre for Research in Medical Entomology (ICMR-Government of India), Viruthachalam, and the egg rafts of C. quinquefasciatus were collected from drainage of local residential area of Annamalai Nagar (11° 23′ 17 N, 79° 42′ 57 E) and reared in the laboratory (29 ± 3 °C, 75 to 85 % RH). The larvae were fed with Brewer’s yeast/dog biscuit (1:3). The larvae at the early fourth instar stage were used for larvicidal assay.
The larvicidal effect of leaf essential oil from M. exotica against A. aegypti, A. stephensi and C. quinquefasciatus was studied with the standard procedures recommended by the WHO (1981). The essential oil was dissolved in 1 ml of acetone and prepared into different concentrations viz. 12.5, 25, 50, 75 and 100 ppm with distilled water. Twenty larvae (in a 100-ml beaker) of the early fourth instar stage were used for larvicidal assay, and five replicates were maintained for each concentration. During this experiment, no food was offered to the larvae. The larval mortality was calculated after 12 and 24 h of exposure period. The lethal concentrations, LC50 and LC90, and their 95 % confidence limit of upper and lower confidence levels were calculated by probit analysis (SPSS, Version 16.0).
Results
Yield and chemical constituents of essential oil
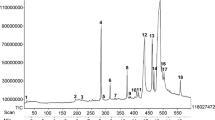
The hydro-distillation of leaf of M. exotica yielded 0.1 % (v/w) of essential oil with pale yellow in colour. Table 1 shows the chemical constituents of the essential oil analysed by GC (Fig. 1) and GC-MS. In total, 27 compounds were identified and account about 99.26 % of the total oil. The major chemical constituents were β-humulene (40.62 %), benzyl benzoate (23.96 %), β-caryophyllene (7.05 %) and α-terpinene (5.66 %).
Larvicidal activity of essential oil
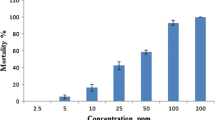
The larvicidal activity of leaf essential oil from M. exotica was investigated. The essential oil had promising larvicidal activities (Table 2) against the early fourth instar larvae activity after 12 h of exposure period which were LC50 = 74.7 and LC90 = 152.7 ppm (A. aegypti), LC50 = 56.3 and LC90 = 107.8 ppm (A. stephensi), and LC50 = 74.4 and LC90 = 136.9 ppm (C. quinquefasciatus), and the larvicidal activities after 24 h of exposure period were LC50 = 35.8 and LC90 = 85.4 ppm (A. aegypti), LC50 = 31.3 and LC90 = 75.1 ppm (A. stephensi), and LC50 = 43.2 and LC90 = 103.2 ppm (C. quinquefasciatus). The essential oil showed 98 % larval mortality against A. stephensi at 100 ppm (after 24 h).
Discussion
Botanical insecticides may serve as suitable alternatives to synthetic insecticides as they are relatively safe, degradable and are readily available in many areas of the world. Although several plants form different families have been reported for mosquitocidal activity, only a few botanicals have moved from the laboratory to field use like neem-based insecticides, which might be due to the light and heat instability of phytochemicals compared to synthetic insecticides (Green and Singer 1981). In the present study, the leaf essential oil from M. exotica showed larvicidal activity against A. aegypti, A. stephensi and C. quinquefasciatus. Similarly, these results are supported by previous reports in the same species of M. exotica leaf extracts having larvicidal activity against C. quinquefasciatus. The LC50 values of M. exotica for III and IV instar larvae and pupae are 135.539 and 154.361 ppm, respectively (Dass and Mariappan 2014), although several studies have reported the larvicidal activity of essential oils against A. aegypti, A. stephensi and C. quinquefasciatus. Govindarajan et al. (2012) evaluated that larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species, A. aegypti, A. stephensi and C. quinquefasciatus. The oil of M. spicata exhibited a significant toxic effect against the larvae of three species under study, with LC50 values of 49.71 ppm for A. stephensi, 56.08 ppm for A. aegypti and 62.62 ppm for C. quinquefasciatus. Senthilkumar and Venkatesalu (2012) reported that larvicidal potential against C. quinquefasciatus in five compounds was derived from essential oils from Acorus calamus leaf including Beta asarone, cis-beta-terpineol, Limonene, Carvone and Amyl isovalerate with an LC50 value of 63.43 ml/L and LC90 value of 145.95 ml/L. Govindarajan (2011) reported the larvicidal and repellent effect against Culex tritaeniorhynchus Giles and Anopheles subpictus in Zingiber officinale with LC50 and LC90 values of 98.83 and 57.98 ppm, and 186.55 and 104.23 ppm. Ravi Kiran et al. (2006) reported the larvicidal effect against A. aegypti and A. stephensi in Chloroxylon swietenia leaves and stem essential oil with LC50 values of 16.5 and 14.9 μg/ml and 20.2 and 19 μg/ml. Senthilkumar et al. (2013) reported the larvicidal activity of essential oil of Feronia limonia leaf against A. stephensi with LC50 38.93 and LC90 108.64 ppm (after 12 h), LC50 15.03 and LC90 36.69 ppm (after 24 h); A. aegypti with LC50 37.60 and LC90 104.69 ppm (after 12 h), LC50 11.59 and LC90 42.95 ppm (after 24 h); and C. quinquefasciatus with LC50 52.08 and LC90 124.33 ppm (after 12 h), LC50 22.49 and LC90 60.90 ppm (after 24 h), respectively. The essential oil of Plectranthus amboinicus leaves possessed larvicidal activity against A. stephensi with LC50 values of 33.54 (after 12 h) and 28.37 ppm (after 24 h) and LC90 values of 70.27 (after 12 h) and 59.38 ppm (after 24 h), respectively (Senthilkumar and Venkatesalu 2010).
In the present study, chemical components were identified from essential oil of M. exotica leaf, and major chemical components were β-humulene (40.62 %), benzyl benzoate (23.96 %), β-caryophyllene (7.05 %) and α-terpinene (5.66 %). Li et al. (2010) reported that M. exotica aerial parts of essential oils are composed of spathulenol, α-pinene, caryophyllene oxide, bicyclogermacrene, 1,2,3,5,6,7,8,8a-octahydro-1-methyl-6-methylene-4-(1-methylethyl)-naphthalene and γ-selinene. This observed differences in the chemical composition may be attributed to occurrence of chemotypes, geographical locations, season at the time of collection, stage of development, culture climate and other culture conditions, which may affect biological activities (Runyoro et al. 2010).
The result of the present study showed that the significant larvicidal activity of the essential oil of M. exotica leaf may be due to the presence of major chemical constituents like β-humulene, benzyl benzoate and β-caryophyllene. Vera et al. (2014) reported that benzyl benzoate is one of the major chemical components from the essential oil of Cananga odorata with an LC50 value of 52.9 ppm. This essential oil had a larvicidal activity against A. aegypti. β-caryophyllene is a major component from the essential oil of Alpinia purpurata and showed larvicidal activity against fourth instar larvae of A. aegypti with IC50 values of 80.7 and 71.5 ppm, respectively (Santos et al. 2012). Senthilkumar and Venkatesalu (2010) reported that α-humulene and caryophyllene oxide are major chemical components from the essential oil of P. amboinicus. This essential oil had strong larvicidal activity against A. stephensi. The essential oil from Blumea densiflora had larvicidal activity against A. anthropophagus. β-caryophyllene is one of the major chemical components of this essential oil (Zhu and Tian 2011).
The vector control is facing a threat due to the emergence of resistance in vector mosquitoes to conventional synthetic insecticides, warranting either counter measures or development of newer insecticides (Chandre et al. 1998). The isolation and identification of the larvicidal components from the leaf essential oil of M. exotica as described here could lead to the development of natural mosquitocidal products to replace the synthetic insecticides. The use of natural plant-based products by individuals and communities would generate local employment, reduce dependence on expensive imported synthetic products and stimulate local efforts to enhance public health (Bowers et al. 1995). For example, essential oil from citronella, lemon and eucalyptus provides the active ingredients of some commercial repellents. Such substances are sold under several brand names (Trongtokit et al. 2005).
The mode of action and site of effect for larvicidal essential oils and isolated compounds has received little attention. In this study, the mode of action of tested materials was not studied. The larvicidal mode of action of essential oils was previously investigated by Corbet et al. (1995); WHO observed that essential oils increase the tendency of tracheal flooding and chemical toxicity in mosquito larvae. Usta et al. (2002) demonstrated that isolated pure compounds interfered with proton transfer in the mitochondria leading to larval mortality.
Mosquito control is vital for many countries and is still in a state of evolution. During the last decades, it depended upon synthetic organic insecticides, many of which have been removed from the arsenal of weapons (Floore 2006) and botanicals are the new weapons of mosquito control under exploration. Natural pesticides derived from plants are a promising tool especially for targeting mosquitoes in the larval stage (Amer and Mehlhorn 2006). Cheng et al. (2004) showed the data and revealed that a-phellandrene, limonene, p-cymene, c-terpinene, terpinolene and ɑ-terpinene examined in this study exhibited great larvicidal performance. Vector control is one of the most powerful weapons in the process of managing vector populations to reduce/interrupt the transmission of disease. As a result, vector control remains considered to be a cornerstone in the vector-borne disease control programme due to a lack of reliable vaccine, drug resistance parasites and insecticide resistance of insect vector disease (Karunamoorthi 2011). Some of our reported LC50 values differ from reported data (Perumalsamy et al. 2009) which may be the results of different methodologies and analysis. Additionally, different species, from different ecological niches, appear to be more susceptible or resistant to specific compounds (Waliwitiya et al. 2009). The use of natural products may be considered as an important alternative insecticide for the control of vector-borne diseases since they constitute a rich source of bioactive compounds that are biodegradable, nontoxic and potentially suitable for use in integrated larvae management programmes. In conclusion, the present study has shown that the leaf essential oil from M. exotica may have a potential in the control of larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. The results could be useful in search of newer, safer and more effective natural compounds as larvicides.
References
Amer A, Mehlhorn H (2006) Larvicidal effects of various essential oils against Aedes, Anopheles and Culex larvae (Dipteria, Culicidae). Parasitol Res 99:466–472
Anonymous (1964) The Wealth of India: raw materials. Publication and Information Directorate, CSIR, New Delhi 6:447–448
Ansari MA, Razdan RK (2000) (F. Leguminosae) oil against mosquitoes. Bioresour Technol 73:207–211
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46(2):446–475
Bernhard L, Bernhard P, Magnussen P (2003) Management of patients with lymphoedema caused by filariasis in North-eastern Tanzania: alternative approaches. Physiother 89:743–749
Borah R, Kalita MC, Kar A, Talukdar AK (2010) Larvicidal efficacy of Toddalia asiatica (Linn.) Lam against two mosquito vectors Aedes aegypti and Culex quinquefasciatus. Afr J Biotechnol 9(16):2527–2530
Bowers WL, Sener B, Evans PH (1995) Activity of Turkish medicinal plants against mosquitoes Aedes aegypti and Anopheles gambiae. Insect Sci Appl 16(3/4):339–342
Chandre F, Darriet F, Darder M, Cuany A, Doannio JMC, Pasteur N (1998) Pyrethroid resistance in Culex quinquefasciatus from West Africa. Med Vet Entomol 12:359–366
Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (2004) Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem 52:4395–4400
Chopra RN (1956) Glossary of Indian medicinal plants. CSIR, New Delhi, p 171
Ciccia G, Coussio J, Mongelli E (2000) Insecticidal, repellent activity against Aedes aegypti larvae of some medicinal South American plants. J Ethnopharmacol Pharm 71:267–274
Conti B, Benelli G, Flamini G, Cioni PL, Profeti R, Ceccarini L, Macchia M, Canale A (2012) Larvicidal and repellent activity of Hyptis suaveolens (Lamiaceae) essential oil against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 110:2013–2021
Corbet SA, Danahar CW, King V, Chalmers CL, Tiley CF (1995) Surfactant-enhanced essential oils as mosquito larvicides. Entomol Exp Appl 75:229–236
Dass K, Mariappan P (2014) Larvicidal activity of Lawsonia inermis and Murraya exotica leaves extract on filarial vector, Culex quinquefasciatus. Int J Mosq Res 1(2):25–27
Floore TG (2006) Mosquito larval control practices: past and present. J Am Mosq Control Assoc 22:527–533
Govindarajan M (2011) Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med 106–111
Govindarajan M, Sivakumar R, Rajeswari M, Yogalakshmi K (2012) Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol Res 110:2023–2032
Green MM, Singer JM (1981) Larvicidal activity of Tagetes minuta (marigold) towards Aedes aegypti. J Am Mosq Control Assoc 7:282–286
Jantan I, Yalvema MF, Ahmad NW, Jamal JA (2005) Insecticidal activities of the leaf oils of eight Cinnamomum species against Aedes aegypti and Aedes albopictus. Pharm Biol 43:526–532
Karunamoorthi K (2011) Vector control: a cornerstone in the malaria elimination campaign. Clin Microbiol and Infect 17:1608–1616
Li Q, Zhu LF, But PPH, Kong YC, Chang HT, Waterman PG (1988) Monoterpene and sesquiterpene rich oils from the leaves of Murraya species: chemotaxonomic significance. Biochem Syst Ecol 16:491–494
Li WQ, Jiang CH, Chu SS, Zuo MX, Liu ZL (2010) Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 15:5831–5839. doi:10.3390/molecules15085831
Pancharoen C, Kulwichit W, Tantawichien T, Thisyakorn U, Thisyakorn C (2002) Dengue infection: a global concern. J Med Assoc Thail 85:25–33
Perumalsamy H, Kim NJ, Ahn YJ (2009) Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti and Ochlerotatus togoi (Diptera: Culicidae). J Med Entomol 46:1420–1423
Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G (2009) Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 104:1365–1372
Ravi Kiran S, Bhavani K, Sita Devi P, Rajeswara Rao BR, Janardhan Reddy K (2006) Composition and larvicidal activity of leaves and stem essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensi. Bioresour Technol 97:2481–2484
Runyoro D, Ngassapa O, Vagionas K, Aligiannis N, Graikou K, Chinou I (2010) Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chem 119:311–316
Santos GKN, Dutra KA, Barros RA, da Camara CAG, Lira DD, Gusmao NB, Navarro DMAF (2012) Essential oils from Alpinia purpurata (Zingiberaceae): chemical composition, oviposition deterrence, larvicidal and antibacterial activity. Ind Crop Prod 40:254–260
Senthilkumar A, Venkatesalu V (2010) Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi; a malarial vector mosquito. Parasitol Res 107:1275–1278. doi:10.1007/s00436-010-1996-6
Senthilkumar A, Venkatesalu V (2012) Larvicidal potential of Acorus calamus L. essential oil against filarial vector mosquito Culex quinquefasciatus (Diptera: Culicidae). Asian Pac J Trop Dis 324–326
Senthilkumar A, Jayaraman M, Venkatesalu V (2013) Chemical constituents and larvicidal potential of Feronia limonia leaf essential oil against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Parasitol Res 0932-0113. doi:10.1007/s00436-012-3188-z
Thomas TG, Rao S, Lal S (2004) Mosquito larvicidal properties of essential oil of an indigenous plant, Ipomoea cairica Linn. Jpn J Infect Dis 57:176–177
Trongtokit Y, Rongsriyam Y, Komalamiosra N, Apiwathnasnrn C (2005) Comparative repellency of 38 essential oils against mosquito bites. Phytother Res 19:303–309
Usta J, Kreydiyyeh S, Bakajian K, Nakkash-Chmaisse H (2002) In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food Chem Toxicol 40:935–940
Vera SS, Zambrano DF, Rodríguez-Sanabria F, Duque Luna JE (2014) Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae). Parasitol Res 113:2647–2654. doi:10.1007/s00436-014-3917-6
Waliwitiya R, Kennedy CJ, Lowenberger CA (2009) Larvicidal and oviposition altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag Sci 65:241–248
Wang LY, Lu YQ, Luo YP (2009) Antifeeding activities of 45 south herbs extracts against Spodptera litura Fabriciu. Hubei Agric Sci 48:628–630
World Health Organization (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO, Geneva, pp 1–6
World Health Organization (2002) Dengue and dengue haemorrhagic fever. World Health Organization, Geneva, p 117
World Health Organization (2010) Malaria. Factsheet No.94. Geneva
World Health Organization (2014) A global brief on vector-borne diseases. WHO/DCO/WHD/2014.1
Zhu L, Tian Y (2011) Chemical composition and larvicidal activity of Blumea densiflora essential oils against Anopheles anthropophagus: a malarial vector mosquito. Parasitol Res 109:1417–1422. doi:10.1007/s00436-011-2388-2
Acknowledgments
The authors are thankful to Dr. K. Arumugam, Professor and Head, Department of Botany, Annamalai University, for providing laboratory facilities for the study. We are thankful to The Director, Centre for Research in Medical Entomology (ICMR-Government of India), Madurai, for mosquito egg supply. S. Krishnamoorthy acknowledges the University Grants Commission, India, for the financial support by awarding Basic Scientific Research and Special Assistance Programme (UGC BSR-SAP) for this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnamoorthy, S., Chandrasekaran, M., Raj, G.A. et al. Identification of chemical constituents and larvicidal activity of essential oil from Murraya exotica L. (Rutaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 114, 1839–1845 (2015). https://doi.org/10.1007/s00436-015-4370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4370-x