Abstract
The present study explored the effects of crude leaf acetone, chloroform, hot water, methanol, petroleum ether (60–80°C), and water extracts of Calotropis procera (Ait) R. Br., Canna indica L., Hibiscus rosa-sinensis Linn., Ipomoea carnea Jacq. spp. fistulosa Choisy, and Sarcostemma brevistigma Wight that were selected for investigating larvicidal potential against second and fourth instar larvae of the laboratory-reared mosquito species, Culex quinquefasciatus Say, in which the major lymphatic filariasis was used. All plant extracts showed moderate larvicidal effects after 24 h of exposure at 1,000 ppm; however, the highest larval mortality was found in leaf acetone, chloroform, methanol, and petroleum ether of C. indica (LC50 = 29.62, 59.18, 40.77, and 44.38 ppm; LC90 = 148.55, 267.87, 165.00, and 171.91 ppm) against second instar larvae (LC50 = 121.88, 118.25, 69.76, and 56.31 ppm; LC90 = 624.35, 573.93, 304.27, and 248.24 ppm) and against fourth instar larvae and acetone, hot water, methanol, and petroleum ether extracts of I. carnea (LC50 = 61.17, 41.07, 41.82, and 39.32 ppm; LC90 = 252.91, 142.67, 423.76, and 176.39 ppm) against second instar larvae (LC50 = 145.37, 58.00, 163.81, and 41.75 ppm; LC90 = 573.30, 181.10, 627.38, and 162.63 ppm) and against fourth instar larvae of C. quinquefasciatus, respectively. These results suggest that the acetone, methanol extracts of C. indica and hot water, petroleum ether extracts of I. carnea have the potential to be used as an ideal eco-friendly approach for the control of the major lymphatic filariasis vector, C. quinquefasciatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Culex quinquefasciatus is the most widely distributed mosquito in India, mainly found in urban and suburban areas. The most efficient approach to control the vector is to target the immature stages of the life cycle. Synthetic chemical larvicides continue to be applied for controlling mosquitoes in most parts of the world. But many of these chemicals are toxic to human, plant, and animal life, and resistance can be problematic in maintaining control, especially with organophosphate and pyrethroid larvicides. There has been serious concern about the use of chemical-based mosquito larvicides and repellents in recent past. As a result, researchers are currently investigating natural substances to use as insecticides for controlling larval mosquitoes. Plants may be a source of alternative agents for control of vectors because they are rich in bioactive chemicals, are active against a limited number of species including specific target insects, and are biodegradable. Phytochemical insecticides have received much attention, in this regard, as they are considered to be more environmentally biodegradable and considered safer than synthetic insecticides (Moretti et al. 2002; Cetin et al. 2004). These problems have highlighted the need for the development of new strategies for selective mosquito larval control. Extracts or essential oils from plants may be alternative sources of mosquito larval control agents, since they constitute a rich source of bioactive compounds that are biodegradable into non-toxic products and potentially suitable for use in control of mosquito larvae. In fact, many researchers have reported on the effectiveness of plant extracts or essential oils against mosquito larvae (Rahuman et al. 2008a, b, c; Amer and Mehlhorn 2006a, b).
Calotropis procera, commonly known as ‘Vellai erukku’ in India, is a popular medicinal plant found throughout the tropics of Asia and Africa. Various parts of this plant have been widely used in traditional systems of medicine. The water, ethanol, acetone, and some other organic solvent extracts have insecticidal activity against Sarcophaga haemorrhoidalis (Moursy 1997) as well as anti-bacterial and anti-parasitic (Larhsini et al. 1999) activities. An ethanolic extract of the flower was reported to have anti-microbial, anti-inflammatory, antipyretic, analgesic (Mascolo et al. 1988), and anti-malarial activities (Sharma and Sharma 1999; 2001). The crude latex produced by the green parts of the plant was evaluated for its toxic effects upon egg hatching and larval development (Ramos et al. 2006); the methanol and fresh leaf extract showed larvicidal properties (Singh et al. 2005) and the latex aqueous phase showed activity on third/early fourth instar larvae (Markouk et al. 2000) against Aedes aegypti, Anopheles stephensi, C. quinquefasciatus, and Anopheles labranchiae. A cardiac glycosidal (cardenolide) extract isolated from C. procera was tested for their effects against larvae and adult stages of Hyalomma dromedarii (Al-Rajhy et al. 2003); the latex was used against the third stage larvae of Musca domestica (Morsy et al. 2001); decoction provided significant protection against termites Diorhabda lusca and Piesmopoda obliquifasciella (Bajwa and Rajpar 2001). The leaf extract of Calotropis gigantea used at various dose levels was potent in delaying development and in reducing adult emergence activity against Sitophilus zeamais (Haque et al. 2000); the petroleum ether–acetone extract showed larvicidal and adulticidal activity against C. quinquefasciatus (Neraliya and Srivastava 1996).
The effect of dried, coarsely powdered leaf, flower, rhizome, and seed benzene and methanol extracts of Canna indica showed significant central, peripheral analgesic activity in mice and anthelmintic activity against Pheritima posthuma (Nirmal et al. 2007); the water extracts of Ipomoea carnea subsp. fistulosa (aerial parts), Vitex glabrata (branch), Vitex trifolia (aerial part), Vitex negundo (aerial part), C. indica (rhizome), and Justicia gendarussa (aerial part) showed HIV-1 RT inhibition (Woradulayapinij et al. 2005); the active fractions of Punica granatum bark or C. indica root or in combination with other plant-derived molluscicides significantly inhibited the activity of acetylcholinesterase, acid/alkaline phosphatase, Na(+)K(+)ATPase, and lactic dehydrogenase in the nervous tissue of Lymnaea acuminate (Tripathi et al. 2004); the toxic effects of P. granatum and C. indica were tested against L. acuminate (Tripathi and Singh 2000). The molluscicidal activity potency of C. indica studied against Bilinus truncate increased according to its prolonged exposure to sub-lethal concentrations, and it was shown that the plant extracts produce an immediate effect against the fecundity and embryonic survival of the snail (Farag and Khalil 1990); an increase in molluscicide concentration at sub-lethal levels resulted in a significant reduction in embryo growth rate and embryo hatchability (Cheung and Lam 1998).
The herb Hibiscus rosa-sinensis is a glabrous shrub widely cultivated in the tropics as an ornamental plant and has several forms with varying colors of flowers. The larvicidal activity of methanol extract was evaluated against the Aedes(s) albopictus and C. quinquefasciatus (Nath et al. 2006), and the aqueous fraction exhibited a combination of weak spasmogenic and spasmolytic effects (Gilani et al. 2005). Dua et al. (2006) have reported that the aqueous extract from the roots of Hibiscus abelmoschus was evaluated against the larvae of Anopheles culicifacies, A. stephensi, and C. quinquefasciatus.
Alkaloids 3, 4, and 6 isolated from the leaves, flowers, and seeds of I. carnea showed a potent inhibitory activity toward rat lysosomal beta-glucosidase (Haraguchi et al. 2003). Saxena and Sumithra (1985) confirmed that the insecticidal effects of acetone leaf extract of I. fistulosa caused 70% larval mortality; 15% in pupae and 10% in adult emergence against A. stephensi. Poor farmers have developed a novel technique to use the extracts of I. fistulosa for controlling insect pests, such as the “pod-borer” in pigeon pea, and for the control of “boil-worms” in cotton in the Vidharbha region of Maharastra, India (Sinha 1998). The essential oil extracted by steam distillation from leaves of Ipomoea cairica was evaluated for their topical repellency effects against malarial vector A. stephensi (Rajkumar and Jebanesan 2007); it is most highly toxic to the larvae of C. tritaeniorhynchus followed by A. aegypti, A. stephensi, and C. quinquefasciatus (Thomas et al. 2004), and the steroidal alkaloid toxin isolated from Ipomoea species was reported poisonous to animals (Molyneux et al. 2007). Ipomoea aquatica extract was tested for their activity against fibroblast cell lysis after Heterometrus laoticus scorpion venom treatment (Uawonggul et al. 2006); the hot water extract of Ipomoea sepiaria was tested against Dicladispa armiger (Haque 2002). Hebling et al. (2003) reported that the laboratory colonies of the leaf-cutting ants Atta sexdens fed daily with leaves of I. batatas showed ant mortality and a significant decrease in the size of the fungal garden after the second week, with complete depletion of nests after 5 weeks of treatment.
Sarcostemma brevistigma grows throughout India and other tropical regions of the world. It is found to be active as an anti-rheumatic, anti-allergy, anti-emetic, and bronchodilator (Kirtikar and Basu 1993); the effect of chloroform-soluble fraction (F-A) of twigs of S. brevistigma on contractions induced by KCl, histamine, and acetylcholine in the isolated guinea pig ileum and taenia coli smooth muscles has been evaluated (Kumar et al. 2007). Sacidumlignan A (1) isolated from the ethanolic extract of the whole plant of Sarcostemma acidum showed moderate anti-microbial activities against two Gram-positive bacteria in an in vitro study (Gan et al. 2005); stem extract arrests spermatogenesis in male rats (Venma et al. 2002). Sethuraman et al. (2003) have reported decreased activities of GOT and GPT and increased level of ALP in CCl4-intoxicated rats fed with a crude extract of S. brevistigma.
Larvicidal activity of crude hexane, ethyl acetate, petroleum ether, acetone, and methanol extracts of Abutilon indicum, Aegle marmelos, Euphorbia thymifolia, Jatropha gossypifolia, and Solanum torvum was assayed (Rahuman et al. 2008a); the extracts of peel and leaf extracts of Citrus sinensis, Ocimum canum, Ocimum sanctum, and Rhinacanthus nasutus (Bagavan et al. 2009); the water, hot water leaf, stem-bark, and flower extracts of Acacia arabica, Cedrus deodara, Hibiscus rosa-sinensis, Mangifera indica, Nerium indicum, Nicotiana tabacum, Pongamia pinnata, and Solanum nigrum (Rahuman et al. 2009); the extracts of the leaf of Centella asiatica, Datura metal, Mukia scabrella, Toddalia asiatica, and extracts of whole plant of Citrullus colocynthis and Sphaeranthus indicus (Rahuman et al. 2008b); the same solvent extracts of C. sinensis, O. canum, O. sanctum, and Rhinacanthus nasutus (Kamaraj et al. 2008a,b); extracts of the leaf and bark of Ficus racemosa (Rahuman et al. 2008d); the leaf extracts of Acalypha indica, Achyranthes aspera, Leucas aspera, Morinda tinctoria, and O. sanctum (Bagavan et al. 2008); and extracts of Citrullus colocynthis, Coccinia indica, Cucumis sativus, Momordica charantia, and Trichosanthes anguina (Rahuman and Venkatesan 2008) were assayed for their toxicity against the early fourth instar larvae of C. quinquefasciatus, A. subpictus, C. tritaeniorhynchus, A. stephensi, and A. aegypti. Chaubal et al. (2005) have reported that the alicyclic polyalcohol, which was found to be d-pinitol (=3-O-methyl-d-chiro-inositol; 1), isolated from the aerial parts of acetone extract of Acacia nilotica showed chronic toxicity against A. aegypti and C. quinquefasciatus fourth instar larvae. Aqueous extracts of Piper retrofractum showed the highest level of larvicidal activity against C. quinquefasciatus and A. aegypti (Chansang et al. 2005); the ethanolic and acetone extracts of Nerium indicum and Thuja orientalis (Sharma et al. 2005), the acetone and petroleum ether extracts of Murraya koenigii, Coriandrum sativum, Ferula asafetida, and Trigonella foenum graceum (Harve and Kamath 2004), and the acetone fraction of the petroleum ether extract of seeds from Argemone mexicana (Sakthivadivel and Thilagavathy 2003) exhibited larvicidal and growth-inhibiting activity A. stephensi, C. quinquefasciatus, and A. aegypti.
The purpose of the present investigation was to explore the larvicidal activity of different solvent extracts from leaves of five plant species, C. procera, C. indica, H. rosa-sinensis, I. carnea spp. fistulosa, and S. brevistigma, against the lymphatic filariasis vector C. quinquefasciatus.
Materials and methods
The leaves of Calotropis gigantean (L.) W.T. Aiton (Asclepiadaceae), Canna indica L. (Cannaceae), Hibiscus rosa-sinensis Linn. (Malvaceae), Sarcostemma brevistigma Wight (Asclepiadaceae), and Ipomoea carnea Jacq. spp. fistulosa Choisy (Convolvulaceae) were collected from the Tamil Nadu Medical Plant Farms & Herbal Medicine Corporation Limited medicinal plant farm, Arumbakkam (13°13′ 4 N, 79° 59′ 7 E; altitude 118 ft), Chennai, Tamil Nadu and the taxonomic identification was made by Dr. B. Annadurai, Department of Plant Biology and Biotechnology, C. Abdul Hakeem College, Melvisharam, India.
Insect rearing
C. quinquefasciatus Say (Diptera: Culicidae) larvae were collected from a stagnant water area of Melvisharam (12° 56′ 23″ N, 79° 14′ 23″ E) and identified in Zonal Entomological Research Centre, Vellore (12° 55′ 48″ N, 79° 7′ 48″ E), Tamil Nadu to start the colony, and larvae were kept in plastic and enamel trays containing tap water. They were maintained and reared in the laboratory as per the method of Rahuman et al. (2008d).
Preparation of plant extracts
The dried leaves (750 g) were powdered mechanically using a commercial electrical stainless steel blender and extracted with acetone (1,000 ml; Qualigens), chloroform (1,200 ml; Fine), methanol (2,000 ml; Qualigens), and petroleum ether (60–80°C, 2,200 ml; Qualigens) in a Soxhlet apparatus separately until exhaustion. The aqueous and hot water extracts were prepared as per the procedure of Chowdhury et al. (2008) and Ross and Brian (1977). The extract was concentrated under a reduced pressure of 22–26 mmHg at 45°C and the residue obtained was stored at 4°C.
The C. quinquefasciatus larvicidal activity was assessed by the procedure of WHO (1996) with some modification and as per the method of Rahuman et al. (2000, 2008d). From the stock solution, different concentrations ranging from 4.69 to 1,000 ppm were prepared. Based on the preliminary screening results, different solvent crude leaf extracts prepared from C. indica and I. carnea were subjected to dose–response bioassay for larvicidal activity against C. quinquefasciatus. The numbers of dead larvae were counted after 24 h of exposure, and the percentage mortality was reported from the average of five replicates. However, at the end of 24 h, the selected test samples turned out to be equal in their toxic potential.
Statistical analysis
The average larval mortality data were subjected to probit analysis for calculating LC50, LC90, and other statistics at 95% fiducial limits of upper confidence limit and lower confidence limit, and chi-square values were calculated by using the software developed by Reddy et al. (1992). Results with p < 0.05 were considered to be statistically significant.
Results and discussion
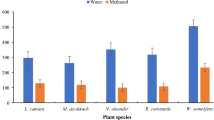
The preliminary screening is a good means of evaluating the potential larvicidal activity of plants popularly used for this purpose. Larvicidal activity of different solvent crude extracts of six plants are noted and presented in Table 1 and Fig. 1a. Among the crude extracts tested, the present results showed the highest larval mortality was found in the leaf acetone, chloroform, methanol, and petroleum ether of C. indica (LC50 = 29.62, 59.18, 40.77, and 44.38 ppm; LC90 = 148.55, 267.87, 165.00, and 171.91 ppm) against second instar larvae (LC50 = 121.88, 118.25, 69.76, and 56.31 ppm; LC90 = 624.35, 573.93, 304.27, and 248.24 ppm) and against fourth instar larvae and acetone, hot water, methanol, and petroleum ether extracts of I. carnea (LC50 = 61.17, 41.07, 41.82, and 39.32 ppm; LC90 = 252.91, 142.67, 423.76, and 176.39 ppm) against second instar larvae (LC50 = 145.37, 58.00, 163.81, and 41.75 ppm; LC90 = 573.30, 181.10, 627.38, and 162.63 ppm) and against fourth instar larvae of C. quinquefasciatus, respectively. Chi-square value was significant at p < 0.05 level (Table 2 and Fig. 1b).
It has been observed earlier that the whole latex of C. procera was shown to cause 100% mortality of third instars within 5 min and, when fractionated into water-soluble dialyzable and non-dialyzable fractions, were partially effective in preventing egg hatching; most of the individuals growing under experimental conditions died before reaching second instars or stayed in first instars, and besides, the fractions were very toxic to third instars causing 100% mortality within 24 h against A. aegypti (Ramos et al. 2006). The aqueous and ethanolic extracts of flowers and leaves of C. procera at 1,000 ppm did not exhibit any activity, and the aqueous phase latex showed activity (with LC50 = 28 ppm) against Anopheles labranchiae (Markouk et al. 2000). Rahuman et al. (2009) have reported that the highest larval mortality was found in stem-bark hot water, acetone, and methanol extracts of C. deodara (LC50 = 133.85, 141.60, and 95.19 ppm; LC90 = 583.14, 624.19, and 639.99 ppm) and leaf hot water, acetone, methanol, and chloroform extracts of N. tabacum (LC50 = 76.27, 163.81, 83.38, and 105.85 ppm; LC90 = 334.72, 627.38, 709.51, and 524.39 ppm) against the larvae of C. quinquefasciatus, respectively. Thomas et al. (2004) reported that the essential oil of I. cairica possessed remarkable larvicidal properties as it could produce 100% mortality in the larvae of Culex tritaeniorhynchus, A. aegypti, A. stephensi, and C. quinquefasciatus mosquitoes at concentrations ranging from 100 to 170 ppm. The median lethal concentration (LC50) values for Khaya senegalensis using acetone, ethanol, hexane, and methanol extracts were 20.12, 5.1, 5.08, and 7.62 mg/l, respectively and the LC50 values for Daucus carota were 236.00, 36.59, 77.19, and 241.8 mg/l, respectively against Culex annulirostris (Shaalan et al. 2006). Komalamisra et al. (2005) have reported that the petroleum ether extract of Rhinacanthus nasutus possessed larvicidal effects with LC50 values between 3.9 and 11.5 mg/l and Derris elliptica showed LC50 values between 11.2 and 18.84 mg/l against A. aegypti, C. quinquefasciatus, Anopheles dirus, and Mansonia uniformis. Larvicidal efficacies of methanol extracts of Momordica charantia, Trichosanthes anguina, Luffa acutangula, Benincasa cerifera, and Citrullus vulgaris tested with LC50 values were 465.85, 567.81, 839.81, 1,189.30, and 1,636.04 ppm, respectively, against the late third larval age group of C. quinquefasciatus (Prabakar and Jebanesan 2004). Sharma et al. (2005) reported that the acetone extract of Nerium indicum and Thuja orientalis have been studied with LC50 values of 200.87, 127.53, 209.00, and 155.97 ppm against third instar larvae of A. stephensi and C. quinquefasciatus. Earlier authors reported that the methanol leaf extracts of Vitex negundo, Vitex trifolia, Vitex peduncularis, and Vitex altissima were used for larvicidal assay with LC50 values of 212.57, 41.41, 76.28, and 128.04 ppm, respectively, against the early fourth instar larvae of C. quinquefasciatus (Kannathasan et al. 2007). The larvicidal activity of petroleum ether, ethanolic, and aqueous extracts of dried leaves and fixed oil from the seeds of Caesalpinia bonduc showed 100% mortality in 1% concentration of petroleum ether and ethanolic extract of leaves, whereas it was 55% in 2.5% concentration of aqueous extract and 92.6% in 2.5% concentration of fixed oil against the fourth instar larvae of C. quinquefasciatus (Saravanan et al. 2007); 100% larval mortality was found at 1,000 ppm in whole plant petroleum ether extract of C. colocynthis against the early fourth instar larvae of C. quinquefasciatus (Rahuman et al. 2008b); the petroleum ether (60–80°C) extracts of the leaves of Vitex negundo were evaluated for larvicidal activity with LC50 and LC90 values of 2.4883 and 5.1883 mg/l against larval stages of C. tritaeniorhynchus, respectively (Karunamoorthi et al. 2008); the compounds 4-gingerol (1), (6)-dehydrogingerdione (2), and (6)-dihydrogingerdione (3) isolated from petroleum ether extract of Zingiber officinale exhibited larvicidal activities against fourth instar larvae of A. aegypti (LC50 = 4.25, 9.80, and 18.20 ppm) and C. quinquefasciatus (LC50 = 5.52, 7.66, and 27.24 ppm), respectively (Rahuman et al. 2008c). The combination of ethanolic water extract (10% concentration) from the seed and leaf parts of Annona squamosa, Artemisia annua, Centella asiatica, Eucalyptus globulus, Myristica fragrans, and Cymbopogan citratus mixed at equal proportions displayed an LC50 of 0.80 ppm, making it the most active of all extracts tested to the larvae followed by E. globules (1.14 ppm), C. asiatica (1.52 ppm), A. annua (1.52 ppm), M. fragrans (2.22 ppm), C. citratus (2.59 ppm), A. squamosa (2.65 ppm), Cassia fistula (3.30 ppm), Justicia gendarussa (4.16 ppm), I. carnea (7.36 ppm), Ricinus communis (8.56 ppm), and finally by Datura stramonium and Prosopis juliflora (9.30 ppm) against third instar larvae of A. stephensi (Senthilkumar et al. 2009). Rahuman et al. (2008e) have reported that the LC50 value of petroleum ether extracts of Jatropha curcas, Pedilanthus tithymaloides, Phyllanthus amarus, Euphorbia hirta, and Euphorbia tirucalli were 11.34, 76.61, 113.40, 424.94, and 5.52 ppm, respectively, against the fourth instar larvae of C. quinquefasciatus. The methanol extracts of Euphorbia tirucalli latex and stem bark were evaluated for larvicidal activity against laboratory-reared larvae of C. quinquefasciatus with LC50 values of 177.14 and 513.387 mg/l, respectively (Yadav et al. 2002). Mullai and Jebanesan (2007) have reported that the ethyl acetate, petroleum ether, and methanol leaf extracts of C. colocynthis and Cucurbita maxima showed LC50 values of 47.58, 66.92, and 118.74 ppm and 75.91, 117.73, and 171.64 ppm, respectively, against C. quinquefasciatus larvae.
In conclusion, an attempt has been made to evaluate the role of plant extract larvicidal bioassay against C. quinquefasciatus activity. The results reported here open the possibility of further investigations of efficacy on their larvicidal properties of natural product extracts. The isolation and purification of crude extract of C. indica and I. carnea are in progress.
References
Al-Rajhy DH, Alahmed AM, Hussein HI, Kheir SM (2003) Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae). Pest Manag Sci 59(11):1250–1254
Amer A, Mehlhorn H (2006a) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006b) Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol Res 99:473–477
Bagavan A, Rahuman AA, Kamaraj C, Geetha K (2008) Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 103(1):223–229
Bagavan A, Kamaraj C, Rahuman AA, Elango G, Zahir AA, Pandiyan G (2009) Evaluation of larvicidal and nymphicidal potential of plant extracts against Anopheles subpictus Grassi, Culex tritaeniorhynchus Giles and Aphis gossypii Glover. Parasitol Res (in press) doi:10.1007/s00436-008-1295-7
Bajwa GA, Rajpar MN (2001) Biological activity of extract of different plants against termites, nettle tree leaf beetle and amaltas leaf stitcher. Pak J For 51(2):31–41
Cetin H, Erler F, Yanikoglu A (2004) Larvicidal activity of a botanical natural product, AkseBio2, against Culex pipiens. Fitoterapia 75:724–728
Chansang U, Zahiri NS, Bansiddhi J, Boonruad T, Thongsrirak P, Mingmuang J, Benjapong N, Mulla MS (2005) Mosquito larvicidal activity of aqueous extracts of long pepper (Piper retrofractum vahl) from Thailand. J Vector Ecol 30(2):195–200
Chaubal R, Pawar PV, Hebbalkar GD, Tungikar VB, Puranik VG, Deshpande VH, Deshpande NR (2005) Larvicidal activity of Acacia nilotica extracts and isolation of D-pinitol—a bioactive carbohydrate. Chem Biodivers 2(5):684–688
Cheung CCC, Lam PKS (1998) Effect of cadmium on the embryonic and juveniles of tropical freshwater snail, Physa acuta (Draparnaud, 1805). Water Sci Technol 38(7):263–270
Chowdhury N, Ghosh A, Chandra G (2008) Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement Altern Med 3(8):10
Dua VK, Pandey AC, Alam ME, Dash AP (2006) Larvicidal activity of Hibiscus abelmoschus Linn. (Malvaceae) against mosquitoes. J Am Mosq Control Assoc 22(1):155–157
Farag EA, Khalil MT (1990) Chronic molluscicidal activity of Canna indica leaves on Bulinus truncatus snail, the intermediate host of Schistosoma hematobium. J Egypt Ger Soc Zool 2:25–33
Gan LS, Yang SP, Fan CQ, Yue JM (2005) Lignans and their degraded derivatives from Sarcostemma acidum. J Nat Prod 68(2):221–225
Gilani AH, Bashir S, Janbaz KH, Shah AJ (2005) Presence of cholinergic and calcium channel blocking activities explains the traditional use of Hibiscus rosasinensis in constipation and diarrhoea. J Ethnopharmacol 102(2):289–294
Haque MA (2002) Chemical methods of leaf extraction of Bankalmi, Polygonum hydropiper for controlling rice hispa beetles, Dicladispa armiger (Olivier) (Coleoptera: Chrysomelidae) in Bangladesh. J Biol Sci 2(12):782–784
Haque MA, Nakakita H, Ikenaga H, Sota N (2000) Development-inhibiting activity of some tropical plants against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J Stored Prod Res 36(3):281–287
Haraguchi M, Gorniak SL, Ikeda K, Minami Y, Kato A, Watson AA, Nash RJ, Molyneux RJ, Asano N (2003) Alkaloidal components in the poisonous plant, Ipomoea carnea (Convolvulaceae). J Agric Food Chem 51(17):4995–5000
Harve G, Kamath V (2004) Larvicidal activity of plant extracts used alone and in combination with known synthetic larvicidal agents against Aedes aegypti. Indian J Exp Biol 42(12):1216–1219
Hebling MJA, Bueno OC, Maroti PS, Pagnocca FC, da Silva OA (2003) Effects of leaves of Ipomoea batatas (Convolvulaceae) on nest development and on respiratory metabolism of leaf-cutting ants Atta sexdens L. (Hym., Formicidae). J Appl Entomol 124(5–6):249–252
Kamaraj C, Rahuman AA, Bagavan A (2008a) Screening for antifeedant and larvicidal activity of plant extracts against Helicoverpa armigera (Hübner), Sylepta derogata (F.) and Anopheles stephensi (Liston). Parasitol Res 103(6):1361–1368
Kamaraj C, Rahuman AA, Bagavan A (2008b) Antifeedant and larvicidal effects of plant extracts against Spodoptera litura (F.), Aedes aegypti L. and Culex quinquefasciatus Say. Parasitol Res 103(2):325–331
Kannathasan K, Senthilkumar A, Chandrasekaran M, Venkatesalu V (2007) Differential larvicidal efficacy of four species of Vitex against Culex quinquefasciatus larvae. Parasitol Res 101(6):1721–1723
Karunamoorthi K, Ramanujam S, Rathinasamy R (2008) Evaluation of leaf extracts of Vitex negundo L. (Family: Verbenaceae) against larvae of Culex tritaeniorhynchus and repellent activity on adult vector mosquitoes. Parasitol Res 103(3):545–550
Kirtikar KR, Basu BD (1993) Indian Medicinal Plants, International Book Publisher, Dehradun 3:1621–1622
Komalamisra N, Trongtokit Y, Rongsriyam Y, Apiwathnasorn C (2005) Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J Trop Med Public Health 36(6):1412–1422
Kumar PS, Soni K, Jadhav SR, Doshi NS, Saraf MN (2007) Mechanism of spasmolytic activity of a fraction of Sarcostemma brevistigma Wight. Indian J Exp Biol 45(5):419–424
Larhsini M, Oumoulid L, Lazrek HB, Wataleb S, Bousaid M, Bekkouche M, Markouk M, Jana M (1999) Screening of anti bacterial and anti parasitic activity of six Moroccan medicinal plants. Therapie 54(6):763–765
Markouk M, Bekkouche K, Larhsini M, Bousaid M, Lazrek HB, Jana M (2000) Evaluation of some Moroccan medicinal plant extracts for larvicidal activity. J Ethnopharmacol 73(1–2):293–297
Mascolo N, Sharma R, Jain SC, Calpasso F (1988) Ethnopharmacology of Calotropis procera flowers. J Ethnopharmacol 22(2):211–221
Molyneux RJ, Lee ST, Gardner DR, Panter KE, James LF (2007) Phytochemicals: the good, the bad and the ugly. Phytochemistry 68(22–24):2973–2985
Moretti MD, Sanna-Passino G, Demontis S, Bazzoni E (2002) Essential oil formulations useful as a new tool for insect pest control. AAPS Pharm Sci Tech 3:E13
Morsy TA, Rahem MA, Allam KA (2001) Control of Musca domestica third instar larvae by the latex of Calotropis procera (Family: Asclepiadaceae). J Egypt Soc Parasitol 31(1):107–110
Moursy LE (1997) Insecticidal activity of Calotropis procera extract on the flesh fly, Sarcophaga haemorrhoidalis Fallen. J. Egypt Soc Parasitol 2:505–514
Mullai K, Jebanesan A (2007) Larvicidal, ovicidal and repellent activities of the leaf extract of two cucurbitaceous plants against filarial vector Culex quinquefasciatus (Say) (Diptera: Culicidae). Trop Biomed 24(1):1–6
Nath DR, Bhuyan M, Goswami S (2006) Botanicals as mosquito larvicides. Def Sci J 56(4):507–511
Neraliya S, Srivastava US (1996) Effect of plant extracts on post-embryonic development of the mosquito Culex quinquefasciatus. J Adv Zool 17:54–58
Nirmal SA, Shelke SM, Gagare PB, Jadhav PR, Dethe PM (2007) Antinociceptive and anthelmintic activity of Canna indica. Nat Prod Res 21(12):1042–1047
Prabakar K, Jebanesan A (2004) Larvicidal efficacy of some cucurbitaceous plant leaf extracts against Culex quinquefasciatus (Say). Bioresour Technol 95(1):113–114
Rahuman AA, Venkatesan P (2008) Larvicidal efficacy of five cucurbitaceous plant leaf extracts against mosquito species. Parasitol Res 103(1):133–139
Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B (2000) Effect of Feronia limonia on mosquito larvae. Fitoterapia 71(5):553–555
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008a) Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res 102(5):981–988
Rahuman AA, Venkatesan P, Gopalakrishnan G (2008b) Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis (Linn.) Schrad. Parasitol Res 103(6):1383–1390
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K, Bagavan A (2008c) Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiber officinale. Phytother Res 22(8):1035–1039
Rahuman AA, Venkatesan P, Geetha K, Gopalakrishnan G, Bagavan A, Kamaraj C (2008d) Mosquito larvicidal activity of gluanol acetate, a tetracyclic triterpenes derived from Ficus racemosa Linn. Parasitol Res 103(2):333–339
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008e) Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 102:867–873
Rahuman AA, Bagavan A, Kamaraj C, Vadivelu M, Zahir AA, Elango G, Pandiyan G (2009) Evaluation of indigenous plant extracts against larvae of Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 104:637–643
Rajkumar S, Jebanesan A (2007) Repellent activity of selected plant essential oils against the malarial fever mosquito Anopheles stephensi. Trop Biomed 24(2):71–75
Ramos MV, Bandeira Gde P, de Freitas CD, Nogueira NA, Alencar NM, de Sousa PA, Carvalho AF (2006) Latex constituents from Calotropis procera (R. Br.) display toxicity upon egg hatching and larvae of Aedes aegypti (Linn.). Mem Inst Oswaldo Cruz 101(5):503–510
Reddy PJ, Krishna D, Murthy US, Jamil K (1992) A microcomputer FORTRAN program for rapid determination of lethal concentration of biocides in mosquito control. CABIOS 8:209–213
Ross MSF, Brian KR (1977) An introduction to pharmacy. Pitman, Bath
Sakthivadivel M, Thilagavathy D (2003) Larvicidal and chemosterilant activity of the acetone fraction of petroleum ether extract from Argemone mexicana L seed. Bioresour Technol 89(2):213–216
Saravanan KS, Periyanayagam K, Ismail M (2007) Mosquito larvicidal properties of various extract of leaves and fixed oil from the seeds of Caesalpinia bonduc (L) Roxb. J Commun Dis 39(3):153–157
Saxena SC, Sumithra L (1985) Laboratory evaluation of leaf extract of a new plant (Ipomoea fistulosa) to suppress the population of malaria vector Anopheles stephensi. Curr Sci 54(4):201–202
Senthilkumar N, Varma P, Gurusubramanian G (2009) Larvicidal and adulticidal activities of some medicinal plants against the Malarial Vector, Anopheles stephensi (Liston). Parasitol Res 104:237–244
Sethuraman MG, Lalitha KG, Rajkapoor B (2003) Hepatoprotective activity of Sarcostemma brevistigma again carbon tetrachloride-induced hepatic damage in rats. Current Science 84(9):1186–1187
Shaalan EA, Canyon DV, Younes MW, Abdel-Wahab H, Mansour AH (2006) Efficacy of eight larvicidal botanical extracts from Khaya senegalensis and Daucus carota against Culex annulirostris. J Am Mosq Control Assoc 22(3):433–436
Sharma P, Sharma JD (1999) Evaluation of in vitro schizontocidal activity of plant parts of Calotropis procera—an ethnobotanical approach. J Ethnopharmacol 68(1–3):83–95
Sharma P, Sharma JD (2001) In vitro hydrolysis of erythrocytes—by plant extracts with anti plasmodial activity. J Ethnopharmacol 74(3):239–243
Sharma P, Mohan L, Srivastava CN (2005) Larvicidal potential of Nerium indicum and Thuja orientalis extracts against malaria and Japanese encephalitis vector. J Environ Biol 26(4):657–660
Singh RK, Mittal PK, Dhiman RC (2005) Laboratory study on larvicidal properties of leaf extract of Calotropis procera (Family-Asclepiadaceae) against mosquito larvae. J Commun Dis 37(2):109–113
Sinha KR (1998) Embarking on the second green revolution for sustainable agriculture in India: a judicious mix of traditional wisdom and modern knowledge in ecological farming. J Agric Environ Ethics 10:183–197
Thomas TG, Rao S, Lal S (2004) Mosquito larvicidal properties of essential oil of an indigenous plant, Ipomoea cairica Linn. Jap J Infect Dis 57(4):176–177
Tripathi SM, Singh DK (2000) Molluscicidal activity of Punica granatum bark and Canna indica root. Braz J Med Biol Res 33(11):1351–1355
Tripathi SM, Singh VK, Singh S, Singh DK (2004) Enzyme inhibition by the molluscicidal agent Punica granatum Linn. bark and Canna indica Linn. root. Phytother Res 18(7):501–506
Uawonggul N, Chaveerach A, Thammasirirak S, Arkaravichien T, Chuachan C, Daduang S (2006) Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J Ethnopharmacol 103(2):201–207
Venma PK, Sharma A, Mathur A, Sharma P, Gupta RS, Joshi SC, Dixit VP (2002) Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J Androl 4(1):43–47
WHO (1996) Report of the WHO informal consultation on the evaluation on the testing of insecticides CTD/WHO PES/IC/96.1:69
Woradulayapinij W, Soonthornchareonnon N, Wiwat C (2005) In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J Ethnopharmacol 101(1–3):84–89
Yadav R, Srivastava VK, Chandra R, Singh A (2002) Larvicidal activity of latex and stem bark of Euphorbia tirucalli plant on the mosquito Culex quinquefasciatus. J Commun Dis 34(4):264–269
Acknowledgements
The authors are grateful to C. Abdul Hakeem College Management, Dr. S. Mohammed Yousuff, Principal, Dr. Ahmed Najib, HOD of Zoology Department, and Dr. Sait Sahul Hameed, Reader in Zoology, for their help and suggestion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahuman, A.A., Bagavan, A., Kamaraj, C. et al. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 104, 1365–1372 (2009). https://doi.org/10.1007/s00436-009-1337-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1337-9