Abstract
Anisakis morphotype I is the principal etiologic agent of human anisakiasis, with differences in pathogenicity found between the Anisakis simplex s.s. and A. pegreffii species; however, the role of morphotype II larvae in this illness is not well understood. The purpose of this study is to verify the ability of morphotype II larvae to invade tissues via the experimental infection of Wistar rats, an animal model which simulates infection in humans. In the in vivo assay, 7.1 % (4/56 L3 morphotype II) showed pathogenic potential, defined as the capacity of the larvae to cause lesions, attach to the gastrointestinal wall or penetrate it. Two of these larvae, one of A. physeteris and one of A. paggiae, penetrated the stomach wall and were found within the abdominal cavity, with the first one producing a small lesion with blood vessel breakage. The majority of the L3 larvae of morphotype II were found in the intestine (51.8 %; 29/56) with the caecum being the least frequent location (8.9 %; 5/56). In contrast, 44.0 % (11/25) of the morphotype I larvae demonstrated pathogenic potential. Isoenzyme electrophoresis, PCR-RFLP of ITS1-5.8 s-ITS2 and PCR-sequencing of the cox2 mitochondrial gene were used to identify these larvae as A. physeteris (42.9 %), A. paggiae (30.3 %) and A. brevispiculata (1.8 %). Although the morphotype II larvae of A. physeteris and A. paggiae have lower pathogenic potential than morphotype I larvae of A. simplex s.s. (93 and 91 % lower, respectively), they may still be implicated in human anisakiasis, as they are capable of attaching to and penetrating the gastrointestinal wall of animals, demonstrating a similar pathogenicity to that of A. pegreffii. The techniques used for the identification of species reveal a great genetic heterogeneity of A. paggiae and A. physeteris, suggesting the existence of sibling species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anisakiasis is a parasitic disease occurring in certain countries in which insufficiently cooked or raw fish is regularly eaten. The majority of the reported cases are caused by Anisakis L3 larvae of morphotype I which are capable of producing gastrointestinal illnesses and/or allergic reactions in humans (Daschner et al. 2000; Repiso Ortega et al. 2003; Umehara et al. 2007). Recently, it was found that A. simplex s.s. and A. pegreffii larvae of morphotype I have different abilities of penetrating the muscular tissue of fish and surviving in artificial gastric fluids, also presenting pathogenecity differences in Wistar rats, an animal model used to simulate the infection occurring in humans (Suzuki et al. 2010; Quiazon et al. 2011; Romero et al. 2013; Zuloaga et al. 2013). However, little is known regarding the ability of morphotype II larvae to infect humans, as only a few cases of infections have been reported (Clavel et al. 1993; Arizono et al. 2012). In the Iberian Peninsula, A. physeteris is the most frequent morphotype II anisakid, found mainly in the Mediterranean region. However, it is not very prevalent (Adroher et al. 1996; Mattiucci et al. 2007; Valero et al. 2000, 2006a, b). Only a few larvae of A. paggiae have been reported in several hake (Merluccius merluccius) specimens from the Galician coasts (northeastern Spain), and A. brevispiculata has not been found in this region (Mattiucci et al. 2007). The limited morphological differences found in the species of each of these morphotypes, particularly in the L3 larval stage, suggest the need for species identification by biochemical or molecular techniques (D’Amelio et al. 2000; Perteguer et al. 2004; Martín-Sánchez et al. 2005; Umehara et al. 2007, 2008; Murata et al. 2011). Techniques such as isoenzyme analysis, PCR-RFLP of the ITS1-5,8 s-ITS2 or PCR-sequencing of the cox2 mitochondrial gene have been shown to be usefulness in the genetic characterization and specific identification of these parasites, even in cases of human infection (D’Amelio et al. 1999; Perteguer et al. 2004; Umehara et al. 2007; Fumarola et al. 2009, Mattiucci et al. 2007, 2011, 2013). Given the limited information on the pathogenicity of the Anisakis species of morphotype II in humans, this study was designed to determine its behavior in an animal model that simulates a human infection, for comparison with morphotype I, whose pathogenicity is better known. The use of molecular tools allowed for proper species identification.
Materials and methods
Parasite
Anisakis spp. L3 larvae were obtained from blue whiting (Micromesistius poutassou) caught at different points along the Atlantic and Mediterranean coasts of the Iberian Peninsula (Fig. 1). Collected larvae were identified morphologically using a stereo microscope, and based on their characteristics, they were subsequently assigned to morphotypes I or II. For this study, those larvae with the greatest degree of mobility were selected (56 morphotype II L3 larvae and 25 morphotype I L3 larvae).
Experimental infection
81 female Wistar rats weighing approximately 150 g were infested with one L3 of morphotype II or morphotype I Anisakis via gastric probe. Regulated necropsy of the rats was performed at 4 h post-infestation (Zúñiga et al. 2011), recording the morphotype and locations of the larvae, whether they were alive or dead and the presence of any gastrointestinal lesions. Later, larvae that were recovered in good condition were introduced individually into Eppendorf tubes and frozen at −80 ° C prior to their genetic identification.
Isoenzyme analysis
Each individual larva was cut into two fragments, reserving the smaller piece for subsequent molecular identification. The larger piece was subjected to physical and mechanical disruption by freezing/thawing in liquid nitrogen and the use of a pistil. After the addition of Triton (5 %) to encourage cell lysis, the samples were centrifuged at 3,000 rpm for 5 min. In order to prevent enzyme degradation, the entire manipulation process was carried out under cold conditions. The enzymes studied in order to identify the different species of morphotype II were: Isocitrate dehydrogenase (ICD, EC 1.1.1.42) and 6-phosphogluconate dehydrogenase (6 PGD, EC 1.1.1.44) whose alleles are considered to be of diagnostic value for the identification of A. brevispiculata and A. physeteris at a 99 % level (Mattiucci et al. 2001). L3 larvae of A. simplex s.s. were used as references. For electrophoresis and the subsequent enzymatic activity processing, the buffers and solutions listed in Table 1 were used. For the result interpretation and allele identification, different values were assigned so as to indicate their mobility, with 100 being the most common allele value (Martín-Sánchez et al. 2004). Subsequently, attempts were made to find a match of these alleles with those described by Mattiucci et al. (2001).
DNA extraction and PCR-RFLP
The DNA of each larva was extracted individually using the RealPure kit for genomic DNA extraction by REAL (Ref RBMEG01), having previously ruptured the tissue of the fragment parasite by mechanical means using a pistil and subjecting it to processes of freezing/thawing in liquid N2. The precipitated pellet was resuspended in 20 μl of bidistilled water and maintained at −20 ° C until use.
PCR amplification of the ITS1-5,8 s-ITS2 of the rDNA was carried out using the primers NC5 (Forward), 5′ GTA GGT GAA CCT GCG GAA GGA TCA TT 3′ and NC2 (Reverse), 5′ TTA GTT TCT TTT CCT CCG CT 3′ reported by Zhu et al. (1998). Digestion with Taq I (Bioron international), Hinf I (Bioron international), and Cfo (Roche) enzymes was carried out following the manufacturer’s recommendations.
The digestion product was subjected to 3 % agarose gel electrophoresis for species identification according to the generated band pattern, using the genetic markers identified by D’Amelio et al. 2000; Martín-Sánchez et al. 2005; Farjallah et al. 2008; Ceballos-Mendiola et al. 2010; Cavallero et al. 2011 and Murata et al. 2011 as a reference.
PCR-Sequencing and comparative sequence analysis
For further confirmation of the taxonomical identity of the PCR-RFLP-identified morphotype II Anisakis species, the mtDNA cox2 region of 38 specimens was amplified and sequenced. The mitochondrial cox2 region was amplified using the forward primer 211 (5′-TTT TCT AGT TAT ATA GAT TGR TTY AT-3′) and the reverse primer 210 (5′-CAC CAA CTC TTA AAA TTA TC-3′); (Nadler and Hudspeth 2000). The PCR products were purified using the Real Clean Spin Kit (Real; Ref. RBMCS01) and then were directly sequenced in both directions using the primers used for DNA amplification. The obtained sequences were aligned using the Clustal X 1.81 program and were adjusted when necessary so as to identify the different haplotypes. They were compared with those published in the GenBank using the BLASTn and Megablast tools.
Once the sequences were compared, phylogenetic analysis of the same was undertaken using the PHYLIP 3.65 software package (http://evolution.genetics.washington.edu/phylip). A published sequence of the A. simplex s.s. (DQ116426; Valentini et al. 2006) corresponding to our capture area was used as an outgroup. Phylogenetic analysis was carried out using maximum parsimony (MP) and analysis based on distance matrices (Neighbor Joining and UPGMA). We used the F84 model of nucleotide substitution (the default method) with both NJ and UPGMA methods of clustering. The F84 model incorporates different rates of transition and transversion, and different frequencies of the four nucleotides. We used the bootstrap as a measure of support or stability of the clades. In order to be considered sufficiently robust, the clades had to have a bootstrap percentage greater than or equal to 50 %. Estimation of genetic distance (p-distance) and number of nucleotide base differences between and within the Anisakis species was carried out using the MEGA 5.05 software (http://www.megasoftware.net/).
For intra-specific analyses, statistical parsimony in TCS (v. 1.21) software was used. TCS is a Java computer program used to estimate genetic genealogies including multifurcations and/or reticulations (i.e. networks) (Clement et al. 2000).
Statistical analysis
Logistic regression analysis was performed using the categorical variable of L3 larvae pathogenic potential (no, yes) –defined as its capacity to cause lesions, attach itself onto the gastric or intestinal wall, or penetrate them to reach the abdominal cavity– as the dependent variable (N = 81). The independent variable consisted of the larval morphotype (morphotype I, morphotype II) or the species (A. simplex s.s., A. pegreffii, A. physeteris, A. paggiae, A. brevispiculata and unknown species). A similar analysis was also conducted using “ability to cause lesions” as the dependent variable.
Statistical analysis was carried out using SPSS 15.0.; p-values of ≤ 0.05 were considered significant.
Results
Experimental infection
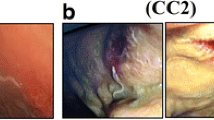
7.1 % of the morphotype II larvae (4/56) displayed pathogenic potential, in accordance with the aforementioned description. Two of these larvae, one of A. physeteris and one of A. paggiae, penetrated the stomach wall and were found within the abdominal cavity, with the first one producing a small lesion with blood vessel breakage (1 mm2) (Fig. 2b). The other two larvae, also of the A. physeteris and A. paggiae species, were found attached to the rodent’s stomach wall (Fig. 2c), with the latter causing a lesion with vascular damage (1 mm2) (Table 2). The majority of the L3 larvae of morphotype II were found in the intestine (51.8 %; 29/56) with the caecum being the least frequent location (8.9 %; 5/56). In contrast, 44.0 % (11/25) of the morphotype I larvae demonstrated pathogenic potential; details of this classification and location of both larvae types in the animal upon necropsy are shown in Table 2.
Isoenzyme analysis
42 of the 56 morphotype II larvae used in the in vivo assay were recovered alive and were available for use in the isoenzymatic study. Table 3 shows the alleles identified in each of the two loci studied (6PGD and ICD), as well as their corresponding allele frequencies.
Identification of species by PCR-RFLP
Of the morphotype II larvae, A. physeteris represented 42.9 % (24/56 L3), 30.3 % (17/56 L3) were identified as A. paggiae and 1.8 % (1/56 L3) as A. brevispiculata. The remaining 25 % could not be identified at a species level. Table 4 shows the band patterns obtained through use of the three restriction enzymes, HinfI, Cfo and TaqI. Of the morphotype I larvae, 48.0 % (12/25) were identified as A. simplex s.s. and 40.0 % (10/25) were identified as A. pegreffii. In this case, the differentiating enzymes were HinfI and TaqI. As for the remainder of the morphotype I larvae, 12.0 % (3/25) revealed a hybrid PCR-RFLP band pattern with the two restriction enzymes, which is the sum of the patterns generated for the two above-mentioned species (Table 2).
Comparative analysis of cox 2 sequences
In the 38 morphotype II Anisakis larvae, comparative analysis sequences allowed us to identify 32 haplotypes defined by 137 polymorphic sites (Table 5). 98 sites were found to be parsimony informative, occurring in more than one haplotype. The trees generated by the cox2 sequence confirmed the identification of the larvae by PCR-RFLP of the ITS1-5,8 s-ITS2 fragment as A. physeteris (22 samples, 17 haplotypes), A. paggiae (15 samples, 14 haplotypes) and A. brevispiculata (1 sample, 1 haplotype). The topology of the trees constructed with both distance analysis methods (UPGMA and NJ) and maximum parsimony (MP) analysis was very similar (Fig. 3, NJ and MP trees not shown). In all three cases, 3 large branches strongly supported by the bootstrap analysis (values between 71 and 100 %) were differentiated, formed by the haplotypes of A. physeteris, A. brevispiculata and A. paggiae, respectively (Fig. 3); a closer relationship was found between the first two. The intra-specific study of the A. brevispiculata species reveals a p-distance value of between 0.019 and 0.032 and the existence of 12 to 20 polymorphic sites in their sequences. For A. physeteris, the p-distance ranges from 0.002 to 0.04 and between 2 and 25 polymorphic sites are found in its sequences. Relative to A. paggiae, in all of the trees, two sub-branches are generated having bootstrap values of 100 %. In one sub-branch the 14 identified haplotypes having p-distance of between 0.005 and 0.022 (3–24 polymorphic sites) were found while the other sub-branch contained the sequences deposited in GenBank (KC342895, KC342896, EU560910, DQ116434). Between the haplotypes integrated in these two sub-branches, the p-distance value ranged between 0.040 and 0.061 and 25 to 38 polymorphic sites were found. The number of fixed differences was 18 for A. physeteris, 19 for A. paggiae and 12 for A. brevispiculata.
The cox2 haplotype sequences were subjected to further analysis by statistical parsimony. This algorithm sorted the 32 sequences into 14 independent networks: three for A. brevispiculata, two for A physeteris and nine for A. paggiae (Fig. 4). The program calculates the frequencies of the haplotypes in the sample. These frequencies are used to estimate haplotype outgroup probabilities, which correlate with haplotype age. The oldest ancestral haplotype, from which the rest would have been derived by mutation, appears in each network, enclosed within a rectangle. A certain relationship was found to exist between these networks (Fig. 4) and the subgroups of the phylogenetic tree (Fig. 3). Fixed differences were detected, characterizing some of these networks (Table 5).
Association between experimental pathogenic potential and Anisakis morphotype or species
Within the framework of the method used, the pathogenic potential of the larva was defined as its ability to cause lesions, attach itself to the gastric or intestinal wall or penetrate them to reach the abdominal cavity. 7.1 % (4/56) of the morphotype II larvae and 44.0 % (11/25) of the morphotype I larvae used in the in vivo tests displayed this pathogenic potential.
A logistic regression analysis of the data was conducted in order to detect the potential association between experimental pathogenic potential and morphotype of the larva or Anisakis species. In these univariate models, both independent variables showed associations with the pathogenic role of the larva. In the first of these, using morphotype I as our reference, the OR for the morphotype II was 0.1 (p < 0.001). In the second one, using A. simplex s.s. as our reference, the OR for A. pegreffii was 0.079 (p = 0.036), for A. physeteris it was 0.068 (p = 0.004) and for A. paggiae it was 0.089 (p = 0.011). Statistically significant differences were not detected between the pathogenic potentials of A. pegreffii, A. physeteris and A. paggiae (p ≥ 0.543).
Discussion
A. physeteris is the predominant anisakid species of morphotype II found in the Iberian Peninsula coasts, followed by A. paggiae. Their prevalences are much lower than those of species of morphotype I (Valero et al. 2000; Mattiucci et al. 2007). A. brevispiculata is not well represented here, given that only one larva has been found in a blue whiting from the Atlantic Coast and this was the first time that this species has been cited along the coasts of the Iberian Peninsula. It was also the first time that A. paggiae was found in different fishing points of the Mediterranean, thus broadening the geographic zone and host organisms of this species. Despite the fact that the presence of Anisakis of morphotype II is apparently low, a case of human infection in Spain has been reported and allergic reactions have been attributed to this morphotype (Clavel et al. 1993; Valero et al. 2000, 2003). In our experimental model using Wistar rats (Romero et al. 2013; Zuloaga et al. 2013), 7.1 % (4/56 L3) of the morphotype II larvae penetrated or were found attached to the animal’s gastric wall, and therefore, A. physeteris, like A. paggiae were responsible for lesions with signs of vascular damage (Table 2). This preference for the wall of the stomach was also observed in the Anisakis morphotype I species (Fig. 2a) (Romero et al. 2013).
The obtained results reveal that pathogenic potential is linked to the morphotype or species is linked to the larva’s morphotype or species. Thus it was found that the morphotype II larva had a 90 % lower risk of penetrating than morphotype I larva (CI 95 %: 64 to 97). In addition, as seen in Table 2 and Fig. 2, morphotype II larvae produced less vascular damage, as found in previous studies (Romero et al. 2012, 2013). In our study, morphotype I larvae were 42 times more likely to produce lesions than the morphotype II larvae (p = 0.001) (Fig. 2).
Romero et al. (2013), using the same experimental model, demonstrated that within the morphotype I, A. simplex s.s. is a more pathogenic species than A. pegreffii, thus justifying its status as an etiological agent in the majority of human cases. In addition, this study finds that the respective risk of A. physeteris and A. paggiae morphotype II larvae penetrating the rodent’s gastric wall is 93 % (CI 95 %: 57 to 99) and 91 % (CI 95 %: 42 to 99) lower than that of A. simplex s.s. The pathogenic potential of these species of morphotype II is similar to that of A. pegreffii.
The cox2 gen sequences were useful in confirming the species identification of the morphotype II larvae, based on the PCR-RFLP technique of the ITS1-5,8 s-ITS2 fragment (D’Amelio et al. 2000; Cavallero et al. 2011; Murata et al. 2011). On the other hand, isoenzyme analysis was not found to be useful. Comparative analysis of the sequences obtained in this study and those taken from the GeneBank allowed us to verify the existence of a wide genetic diversity in morphotype II larvae, particularly within A. paggiae (Figs. 3 and 4). The p-distance values obtained for this species are similar to those obtained by Valentini et al. (2006) for the complex of sibling species, A. simplex. It has been argued that traditional phylogenetic methods are based on certain assumptions which make them inappropriate for intra-specific studies but which, on the other hand, would be well represented by network approaches such as those presented in Fig. 4 (Posada and Crandall 2001; Franco et al. 2010). In natural populations, ancestral haplotypes are expected to persist in the population and to be sampled together with their descendants. Figure 4 reveals that morphotype II larvae identified by PCR-RFLP as A. physeteris form two independent networks while those identified as A. paggiae form nine networks. This, along with the existence of fixed differences (Table 5), supports the hypothesis that each of the two is a complex of sibling species, as previously observed in A. paggiae of the Philippine archipelago (Quiazon et al. 2013).
Ethical standards
All experiments were carried out in accordance with European Parliament and of the Council of 22 September 2010 (2010/63/UE and RD 1201/2005).
References
Adroher FJ, Valero A, Ruíz-Valero J, Iglesias L (1996) Larval anisakids (Nematoda: Ascaridoidea) in horse mackerel (Trachurus trachurus) from the fish market in Granada (Spain). Parasitol Res 82:253–256
Arizono N, Yamada M, Tegoshi T, Yoshikawa M (2012) Anisakis simplex sensu stricto and Anisakis pegreffii: biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog Dis 9:517–521
Cavallero S, Nadler SA, Paggi L, Barros NB, D’Amelio S (2011) Molecular characterization and phylogeny of anisakid nematodes from cetaceans from southeastern Atlantic coasts of USA, gulf of Mexico, and Caribbean Sea. Parasitol Res 108:781–792
Ceballos-Mendiola G, Valero A, Polo-Vico R, Tejada M, Abattouy N, Karl H, De las Heras C, Martín-Sánchez J (2010) Genetic variability of Anisakis simplex s.s. parasitizing European hake (Merluccius merluccius) in the little sole bank area in the northeast atlantic. Parasitol Res 107:1399–1404
Clavel A, Delgado B, Sánchez-Acedo C, Carbonell E, Castillo J, Ramírez J, Quílez J, Gómez-Lus R, Kagei N (1993) A live Anisakis physeteris larvae found in the abdominal cavity of a woman in Zaragoza, Spain. Jpn J Parasitol 42:445–448
Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
D’Amelio S, Mathiopoulos KD, Brandonisio O, Lucarelli G, Doronzo F, Paggi L (1999) Diagnosis of a case of gastric anisakidosis by PCR-based restriction fragment length polymorphism analysis. Parassitol 41:591–593
D’Amelio S, Mathiopoulos KD, Santos CP, Pugachev ON, Webb SC, Picanço M, Paggi L (2000) Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int J Parasitol 30:223–226
Daschner A, Alonso-Gómez A, Cábanas R, Suárez-de-Parga JM, López-Serrano MC (2000) Gastroallergic anisakiasis: borderline between food allergy and parasitic disease-clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol 105(1 Pt 1):176–181
Farjallah S, Slimane BB, Busi M, Paggi L, Amor N, Blel H, Said K, D’Amelio S (2008) Occurrence and molecular identification of Anisakis spp. from the North African coasts of Mediterranean Sea. Parasitol Res 102:371–379
Franco FAL, Morillas-Márquez F, Barón SD, Morales-Yuste M, Gálvez R, Díaz V, Pesson B, Alves-Pires C, Depaque J, Molina R, Afonso MO, Gállego M, Guernaoui S, Bounamous A, Martín-Sánchez J (2010) Genetic structure of Phlebotomus (Larroussius) ariasi populations, the vector of Leishmania infantum in the western Méditerranéen, epidemiological implications. Int J Parasitol 40:1335–1346
Fumarola L, Monno R, Lerardi E, Rizzo G, Giannelli G, Lalle M, Pozio E (2009) Anisakis pegreffi etiological agent of gastric infections in two Italian women. Foodborne Pathog Dis 6:1157–1159
Martín-Sánchez J, Artacho-Reinoso ME, Díaz-Gavilán M, Valero-López A (2005) Structure of Anisakis simplex s.l. populations in a region sympatric for A. pegreffii and A. simplex s.s.: Absence of reproductive isolation between both species. Mol Biochem Parasitol 141:155–162
Martín-Sánchez J, Gramiccia M, Di Muccio T, Ludovisi A, Morillas-Márquez F (2004) Isoenzymatic polymorphism of Leishmania infantum in southern Spain. Trans R Soc Trop Med Hyg 98:228–232
Mattiucci S, Paggi L, Nascetti G, Abollo E, Webb SC, Pascual S, Cianchi R, Bullini L (2001) Genetic divergence and reproductive isolation between Anisakis brevispiculata and Anisakis physeteris (nematoda: Anisakidae)s. Int J Parasitol 31:9–14
Mattiucci S, Abaunza P, Damiano S, Garcia A, Santos MN, Nascetti G (2007) Distribution of Anisakis larvae, identified by genetic markers, and their use for stock characterization of demersal and pelagic fish from european waters: an update. J Helminthol 81:117–127
Mattiucci S, Paoletti M, Borrini F, Palumbo M, Palmieri RM, Gomes V, Casati A, Nascetti G (2011) First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect Dis 11:82
Mattiucci S, Fazii P, De Rosa A, Paoletti M, Megna AS, Glielmo A, De Angelis M, Costa A, Meucci C, Calvaruso V, Sorrentini I, Palma G, Bruschi F, Nascetti G (2013) Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg Infect Dis 19:496–499
Murata R, Suzuki J, Sadamasu K, Kai A (2011) Morphological and molecular characterization of Anisakis larvae (Nematoda: Anisakidae) in Berix splendens from Japanese waters. Parasitol Int 60:193–198
Nadler SA, Hudspeth DS (2000) Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J Parasitol 86:380–393
Perteguer MJ, Ortiz G, García E, Flores M, Rodríguez E, Ubeira FM, Gárate T (2004) Application of the PCR-RFLP technique for the species-specific identification of nematodes involved in human anisakiasis. Med Clin 122:686–689
Posada P, Crandall KA (2001) Intraspecific gene genealogies: trees grafting into networks. Trends Ecol Evol 16:37–45
Quiazon KM, Yoshinaga T, Ogawa K (2011) Experimental challenge of Anisakis simplex sensu stricto and Anisakis pegreffii (Nematoda: Anisakidae) in rainbow trout and olive flounder. Parasitol Int 60:126–131
Quiazon KM, Santos MD, Yoshinaga T (2013) Anisakis species (Nematoda: Anisakidae) of Dwarf Sperm Whale Kogia sima (Owen, 1866) stranded off the Pacific coast of southern Philippine archipelago. Vet Parasitol 197:221–230
Repiso Ortega A, Alcántara Torres M, González de Frutos C, de Artaza VT, Rodríguez Merlo R, Valle Muñoz J, Martínez Potenciano JL (2003) Anisakiasis gastrointestinal. Estudio de una serie de 25 pacientes. Gastroenterol Hepatol 26:341–346
Romero MC, Valero A, Martín-Sánchez J, Navarro-Moll MC (2012) Activity of Matricaria chamomilla essential oil against anisakiasis. Phytomedicine 19:20–523
Romero MC, Valero A, Navarro-Moll MC, Martín-Sánchez J (2013) Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat. Trop Med Int Health 18:979–984
Suzuki J, Murata R, Hosaka M, Araki J (2010) Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int J Food Microbiol 137:88–93
Umehara A, Kawakami Y, Araki J, Uchida A (2007) Molecular identification of the etiological agent of the human anisakiasis in Japan. Parasitol Int 56:211–215
Umehara A, Kawakami Y, Araki J, Uchida A (2008) Multiplex PCR for the identification of Anisakis simplex sensu stricto, Anisakis pegreffii and the other anisakid nematodes. Parasitol Int 57:49–53
Valentini A, Mattiucci S, Bondanelli P, Webb SC, Mignucci-Giannone AA, Colom-Llavina MM, Nascetti G (2006) Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences, and comparison with allozyme data. J Parasitol 92:156–166
Valero A, Martín-Sánchez J, Reyes-Muelas E, Adroher FJ (2000) Larval anisakids parasitizing the blue whiting, Micromesistius poutassou, from Motril bay in the Mediterranean region of southern Spain. J Helminthol 74:361–364
Valero A, Terrados S, Díaz V, Reguera V, Lozano J (2003) Determination of IgE in the serum of patients with allergic reactions to four species of fish-parasite anisakids. J Investig Allergol Clin Immunol 13:94–98
Valero A, Del Mar López-Cuello M, Benítez R, Adroher FJ (2006a) Anisakis spp. in european hake, Merluccius merluccius (L.) from the atlantic off north-west africa and the mediterranean off southern Spain. Acta Parasitol 51:209–212
Valero A, Paniagua M, Hierro I, Díaz V, Valderrama M, Benítez R, Adroher F (2006b) Anisakid parasites of two forkbeards (Phycis blennoides and Phycis phycis) from the mediterranean coasts of Andalucía (southern Spain). Parasitol Int 55:1–5
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterization of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911–1921
Zuloaga J, Rodríguez-Bobada C, Corcuera MT, Gómez-Aguado F, Gonzalez P, Rodríguez-Pérez R, Árias-Díaz J, Caballero ML (2013) A rat model of intragastric infection with Anisakis spp. live larvae: histopathological study. Parasitol Res 112:2409–2411
Zúñiga JM, Orellana JM, Tur JA (2011) Ciencia y Tecnología del Animal de Laboratorio. Universidad de Alcalá de Henares y. SECAL, Madrid
Acknowledgments
We are grateful to the Junta de Andalucía (Regional Autonomous Government of Andalusia) for project P07-CVI-03249.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, M., Valero, A., Navarro, M. et al. Experimental demonstration of pathogenic potential of Anisakis physeteris and Anisakis paggiae in Wistar rats. Parasitol Res 113, 4377–4386 (2014). https://doi.org/10.1007/s00436-014-4113-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4113-4