Abstract
Geranoaetus melanoleucus is a widely distributed accipitrid across South America. Like other accipitrids, it employs powerful hindlimb muscles to capture and immobilize prey. Whereas previous research has focused predominantly on the grip muscles of diurnal raptorial birds, detailed myological descriptions of the entire hindlimb are lacking. This study offers a comprehensive overview of the hindlimb musculature of G. melanoleucus, comparing it with existing information on other raptors. Several features are shared with other accipitrids, including the absence of m. flexor cruris lateralis, the fusion of m. iliotrochantericus cranialis and medialis, and a vinculum connecting distally m. flexor hallucis longus and m. flexor digitorum longus, among others. However, G. melanoleucus exhibits distinctive characteristics, such as a smaller origin area for m. tibialis cranialis and a distal trifurcated tendon of m. flexor perforatus digiti IV, among others. Similar to other diurnal raptorial birds, digit flexors constitute the majority of the muscle mass, aligning with their primary role in grip force generation. In summary, accipitrids appear to demonstrate a conservative muscular anatomy pattern, but the study of different species is crucial for detecting specific features and taxonomic differences. Moreover, detailed myological descriptions provide essential information for morphofunctional analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds of prey are top predators in many ecosystems and they share a reliance on their hindlimbs, particularly their feet and talons, as their primary weapons for attacking, capturing, grasping, carrying, and often, killing their prey. This characteristic has prompted numerous studies by researchers interested in understanding the morphology and functional performance of the hindlimbs of these birds and how these features are linked to their predatory behavior (e.g., Goslow 1972; Csermely and Gaibani 1998; Ward et al. 2002; Einoder and Richardson 2006, 2007; Fowler et al. 2009; Sustaita and Hertel 2010; Backus et al. 2015; Tsang and McDonald 2019; Tsang et al. 2019; Liang et al. 2022).
Diurnal raptorial birds are typically grouped into two families, Accipitridae and Falconidae, with the former being the larger and most diverse of the two (Sarasola et al. 2018). These two groups employ distinct hunting strategies. Accipitrids utilize ambush tactics for prey capture, engaging in rapid pursuits characterized by sudden tail chases. Ultimately, they extend their legs forward, using their feet to firmly grasp their prey. In contrast, falconids employ high-speed aerial attacks, swooping down from above while keeping their feet open and securely positioned close to their bodies to seize prey (Einoder and Richardson 2007; Sustaita 2008; Sustaita and Hertel 2010). Accipitrids typically dispatch their prey by tightly gripping their toes and talons, leading to suffocation through chest compression or by inflicting fatal injuries to vital organs (Einoder and Richardson 2007; Sustaita 2008; Sustaita and Hertel 2010). Conversely, falconids efficiently immobilize their prey upon striking and disarticulate the neck vertebrae with their beaks, damaging the spinal cord (Goslow 1972; Csermely and Gaibani 1998; Einoder and Richardson 2007; Sustaita 2008; Sustaita and Hertel 2010; Liang et al. 2022). Previous research has explored whether these differences in predatory strategies result in variations in the features of hindlimb musculature. It has been observed that, in accipitrids, the total mass of digital flexor muscles is greater, indicating a higher overall grip force compared to falcons (Sustaita and Hertel 2010; Liang et al. 2022). This aligns with their greater reliance on foot gripping to subdue and dispatch prey. Although some studies have examined or focused on the muscular features of these birds, including the comprehensive work by Hudson (1937) and others specifically on diurnal birds of prey (Hudson 1948; Goslow 1972; Ward et al. 2002; Sustaita 2008; Hertel et al. 2015; Mosto 2017b; Liang et al. 2022), detailed myological descriptions of the hindlimb have primarily been conducted in falconids and accipitrids (Berger 1956; Jollie 1976, 1977; Mosto et al. 2013, 2021; Mosto 2014). However, works that include muscle mass data for accipitrids are scarce (Hertel et al. 2015; Liang et al. 2022), and myological descriptions of Neotropical raptorial birds, such as Geranoaetus, are virtually non-existent.
Geranoaetus melanoleucus, commonly known as black-chested buzzard-eagle or grey eagle-buzzard, is a medium-sized accipitrid widely distributed across South America. Its range spans from the northernmost to the southernmost regions of the continent, both west and east of the Andes, and extends into the lowlands to the east, reaching Brazil, Uruguay, and Argentina (Jiménez and Jaksic 1989, 1990; Saggese and De Lucca 2001; Jaksic et al. 2002; and works cited therein). Previous studies have provided insights into the natural history of this species (Schlatter et al. 1980; Jaksic et al. 1981; Schoonmaker 1984; Jaksic and Jimenez 1986; Pavez 2001; Saggese and De Lucca 2001), revealing a diet that typically consists of small to medium-sized mammals, birds, and small reptiles, depending on the specific habitat in which it is found (Schlatter et al. 1980; Jiménez and Jaksic 1989, 1990; Jaksic et al. 2002).
Despite the existing body of research on the myological characteristics of diurnal raptorial birds, there remains a need for more comprehensive descriptions of hindlimb musculature in these animals. Such descriptions not only serve to enhance our understanding of their anatomy but also enable comparisons with other birds exhibiting similar behaviors. Additionally, they provide essential foundational data for subsequent studies in functional and morphoecological fields. The primary objective of this study is to provide a detailed account of the hindlimb musculature of Geranoaetus melanoleucus and to compare its observed morphology with that of other accipitrids and diurnal birds of prey in a broader context.
Materials and methods
To morphologically characterize the musculature, two specimens of Geranoaetus melanoleucus were dissected. These specimens were retrieved from the field, having been found deceased by natural causes in the Flora and Fauna Reserve of La Florida (San Luis Province, Argentina) and were subsequently deposited at the Universidad Nacional de San Luis. They were stored in a freezer for six months prior to the dissections, and their overall condition indicated a relatively short interval between death and discovery. Dissections were carried out on both hindlimbs of each specimen. The musculature was systematically described from proximal to distal regions and from superficial to the deep muscles. Each muscle was meticulously identified and isolated, with due consideration given to its points of origin and insertion, taking into account the corresponding osteological correlations. Additionally, photographs of the muscles were captured using a Canon EOS Rebel T3i camera. Mass data for dry muscles were obtained from one limb of one dissected specimen. Furthermore, we incorporated muscle mass data from other three specimens: two of which were previously documented in the Ph.D. thesis of Mosto (2014) (with their skeletons housed in the Museo de La Plata, Argentina), and the remaining specimen is currently housed in the Fundación de Historia Natural ‘Félix de Azara,’ Buenos Aires, Argentina. Mass measurements for these three specimens were derived from fresh muscles. The mass for all the specimens was recorded with a digital scale to the nearest 0.01 g (see Supplementary Table 1). For anatomical nomenclature, we followed Vanden Berge and Zweers (1993) for myology and Baumel and Witmer (1993) for osteology. The main actions of muscles (see Table 1) were determined based on Goslow (1967), Jacobson and Hollyday (1982), and Gatesy (1999).
Results
The following muscles were absent in the dissected specimens: m. flexor cruris lateralis, m. plantaris, m. adductor digiti IV, and m. lumbricalis.
Muscles on the pelvis and femur
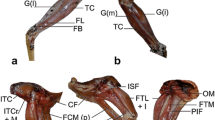
M. iliotibialis cranialis (IC). This is the most superficial muscle of the anterolateral side of the thigh (Fig. 1). It has a strap-shaped belly and its posterior border is partially covered by the m. iliotibialis lateralis.
Superficial muscles of the left hindlimb of Geranoaetus melanoleucus in lateral a and medial b view. Limits between muscles are indicated with dash lines. See Table 1 for abbreviations of muscles. Scale bar = 1 cm
Origin: fleshy from the anterior end of the fossa iliaca dorsalis (Fig. 2). The origin of this muscle leaves a well-marked scar with a crescent-like form. The proximal part covers the anteroproximal portion of the m. iliotrochantericus caudalis.
Sketch of the left ilium of Geranoaetus melanoleucus in lateral view, showing the origin zones of muscles. See Table 1 for abbreviations of muscles. Scale bar = 1 cm
Insertion: through a small tendon on the medial surface of the crista cnemialis cranialis (Fig. 3). It has an additional fleshy insertion on the ligamentum patellaris.
Sketches of the left femur a–d and tibiotarsus and fibula e–h of Geranoaetus melanoleucus in anterior a, e, lateral b, f, posterior c, g, and medial d, h view, showing the origins (unfilled areas) and insertions (filled areas) of muscles. See Table 1 for abbreviations of muscles. Scale bars = 1 cm
M. iliotibialis lateralis (IL). This muscle is the most superficial of the thigh musculature on the lateral side and presents a subtriangular shape (Fig. 1a). The proximal portion is fleshy but the distal portion is aponeurotic.
Origin: fleshy, along the entire extension of the crista iliaca dorsalis of the ilium, from the anterior end to the vortex dorsally located to the acetabulum (Fig. 2).
Insertion: through an aponeurosis on the patella.
M. iliofibularis (IF). It is a well-developed muscle with a triangular shape, posteriorly located to the m. iliotibialis lateralis. It is not covered by the IL and is completely visible in lateral view (Fig. 1a).
Origin: fleshy along the posterior part of the crista dorsolateralis ilii, posterodorsal to the acetabulum (Fig. 2).
Insertion: this muscle tapers distally and continues in a well-developed tendon that inserts on the tuberculum m. iliofibularis, located on the lateral side of the fibula (Fig. 3f and 3g), after passing through the ansa iliofibularis at the level of the distal and lateral part of the femur.
M. iliofemoralis externus (IFE). It is a short and triangular muscle, covered by the IL (Fig. 4).
Deep muscles of the left hindlimb of Geranoaetus melanoleucus that originate from the pelvis and pygostilum in lateral a, b and posterolateral c, d view. Limits between muscles are indicated with dash lines. See Table 1 for abbreviations of muscles. Scale bars = 1 cm
Origin: fleshy, along the middle and anterior parts of the crista dorsolateralis ilii (Fig. 2).
Insertion: by a tendon on the lateral surface of the trochanter femoris (Fig. 3b).
M. iliofemoralis internus (IFI). This is one of the smallest muscles that links the ilium and the femur.
Origin: fleshy, on the ventral border of the ilium, anterior to the acetabulum, and posteriorly located to the origin of the m. iliotrochantericus cranialis et medialis (Fig. 2).
Insertion: by a tendon on the posteromedial surface of the proximal part of the femur (Fig. 3c, d).
M. iliotrochantericus caudalis (ITC). It is a wide muscle that occupies most of the preacetabular part of the ilium (Fig. 4a, b). It covers the anterior part of the m. iliofemoralis externus.
Origin: fleshy, along the ventral surface of the crista iliaca dorsalis and most of the fossa iliaca dorsalis (Fig. 2).
Insertion: by a wide tendon on the trochanter femoris on the proximolateral surface of the femur (Fig. 3b). Its insertion leaves a well-marked scar, which forms part of the impressiones iliotrocantericae.
M. iliotrochantericus cranialis (ITCr) and M. iliotrochantericus medialis (ITM). These two muscles are partially separated in their origins. The anterior one (ITCr) has a fleshy aspect and is strap-shaped, whereas the ITM has an aponeurotic lateral portion that covers a fleshier belly and has a triangular shape (Fig. 4a, b).
Origin: both muscles have a fleshy origin in the anteroventral border of the ilium (Fig. 2).
Insertion: both muscles fuse distally and insert by a tendon on the proximolateral surface of the femur (Fig. 3b).
M. ambiens (A). This is a long and narrow muscle.
Origin: by a thin tendon on the tuberculum preacetabulare, which is ventrally located to the acetabulum (Fig. 2).
Insertion: this muscle presents a fusiform belly which continues distally in a long tendon. This tendon, after crossing the patellar ligament, runs medially to the tendon of the m. iliofibularis and is fused distally to the surface of the m. flexor perforatus digiti II.
M. flexor cruris medialis (FCM). It is an elongated and strap-like muscle located on the posteromedial region of the thigh and contacting the posterior border of the m. puboischiofemoralis (Fig. 1 and Fig. 4a and 4c). The proximal portion is partially covered by the m. caudofemoralis.
Origin: fleshy, in the posteroventral part of the ala ischii (Fig. 2).
Insertion: by a thick well-developed tendon, on the proximomedial part of the tibiotarsus (Fig. 3h).
M. ischiofemoralis (ISF). Origin: the origin of this muscle is laterally covered by the m. caudofemoralis (Fig. 4a, c). It is fleshy, in most of the ala ischii and extended to the lamina infracristalis ilii (Fig. 2).
Insertion: by a tendon, on the proximolateral surface of the femur, between the insertions of the ITC and the ITCr + ITM (Fig. 3b).
M. puboischiofemoralis (PIF). This is a muscular complex formed by a lateral and a medial belly, both intimately related. It has a wide aspect in lateral view (Fig. 4c).
Origin: fleshy, along the ventral surface of the ala ischii, anterior to the origin of the m. flexor cruris medialis (Fig. 2).
Insertion: both bellies have a fleshy insertion along most of the posterior surface of the femoral diaphysis (Fig. 3c and 3d). The insertion area widens distally and reaches the zone proximally located to the fossa poplitea. The medial portion also has an insertion close to the origin of the m. gastrocnemius pars intermedia.
M. obturatorius lateralis (OL). Small muscle mostly covered by the M. ischiofemoralis (Fig. 4c, d).
Origin: fleshy, posterior to the acetabulum and between the foramen ilioischiadicum and the foramen obturatum (Fig. 2).
Insertion: by a tendon on the proximolateral part of the femur, posterior to the insertion of the ITC and leaving the impressiones obturatorie (Fig. 3b, c).
M. obturatorius medialis (OM). This muscle contacts the ventral border of the OL and, in lateral view, is completely covered by the ISF and by the PIF in its proximal portion (Fig. 4c, d).
Origin: fleshy, on the medial surface of the ala ischii.
Insertion: its fibers pass through the foramen obturatum and continue in two tendons, which insert on the proximolateral part of the femur, below the insertion of the OL (Fig. 3b, c).
M. caudofemoralis (CF). Only the pars caudalis was present in the dissected specimens. This is a strap-like muscle that runs obliquely from the pygostyle to the posterior side of the femur and covers the origins of the FCM and ISF and part of the belly of the PIF (Fig. 4a, c).
Origin: by a tendon from the aponeurosis cruciata of the m. depresor caudalis, on the base of the pygostyle.
Insertion: by a proximodistally wide tendon on the posteromedial surface of the femoral shaft, immediately medial to the insertion of the PIF (Fig. 3d).
M. femorotibialis lateralis and M. femorotibialis intermedius (FTL and FTI). Both muscles form a fleshy mass that covers most of the lateral and anterior surfaces of the femoral diaphysis (Fig. 4, c). They are almost entirely fused except for their origins.
Origin: these muscles have an extended fleshy origin along the lateral and anterior surfaces of the femoral diaphysis. Proximally, the origin extends, in part, on the caudal and medial surfaces (Fig. 3a–d). The FTL has also a proximal tendinous origin from the anterior zone located between the trochanter femoris and the caput femoris. The separation between the bellies of both muscles is demarcated proximally by the linea intermuscularis cranialis, which runs on the anteromedial side until the middle of the diaphysis.
Insertion: through a wide aponeurosis on the patella.
M. femorotibialis medialis (FTM). Origin: wide and fleshy, on the distal two-thirds of the medial surface of the femoral shaft (Fig. 3c, d). The origin zone widens distally and it is separated from the insertion surface of the PIF by the linea intermuscularis caudalis, which runs along the posteromedial side of the femoral diaphysis until the condylus medialis.
Insertion: by a tendon, on the medial surface of the crista patellaris of the tibiotarsus (Fig. 3e).
Muscles with origins from the distal femur and proximal tibiotarsus
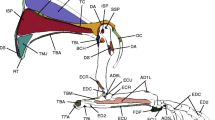
M. fibularis longus (FL). This is a long and fusiform muscle that covers the lateral surface of the fibula (Fig. 5d).
Superficial and deep muscles originated from the distal epiphysis of the femur and tibiotarsus. Limits between muscles are indicated with dashed lines. a left tibiotarsus in posterior view showing m. gastrocnemius pars lateralis and pars medialis separated allowing to see m. flexor hallucis longus. b left tibiotarsus in medial view showing the additional belly of the m. gastrocnemius pars intermedia (indicated as ‘ab’). c right tibiotarsus in posterior view showing flexor muscles of digits. d left tibiotarsus in lateral view showing the insertions of the distal tendons of the m. fibularis longus (indicated as ‘FLt’). e left tibiotarsus in posterior view. f left tibiotarsus in anterior view. g isolated m. flexor hallucis longus and m. flexor digitorum longus showing the distal union between them through a vinculum (indicated as ‘vi’). See Table 1 for abbreviations of muscles. I tendon of FHL corresponding to digit I, II, III, and IV tendons of FDL corresponding to the respective digits. Scale bar = 1 cm
Origin: this muscle has two origins, one by a tendon from the lateral surface of the crista cnemialis lateralis and a fleshy one along the middle part of the lateral surface of the fibular shaft (Fig. 3e, f).
Insertion: it is distally divided into two tendons. One is posteriorly directed and inserts on the tibial cartilage whereas the other continues distally and joins the tendon of insertion of the m. flexor perforatus digiti III at the level of the proximal part of the tarsometatarsus (Fig. 5d).
M. fibularis brevis (FB). This is a long and fusiform muscle that covers the anterodistal part of the fibula (Fig. 5f).
Origin: it is mainly aponeurotic, from the lateral side of the tibial shaft and medial surface of the fibula, around the foramen interosseum distale and occupying part of the distal portions of the fibula and the anterolateral surface of the distal part of the tibiotarsus, until almost being in contact with the condylus medialis (Fig. 3e).
Insertion: by a tendon, on the tuberculum m. fibularis brevis on the proximal posterolateral part of the tarsometatarsus (Fig. 6b).
Sketches of the left tarsometatarsus and digits showing the origins (unfilled areas) and insertions (filled areas) of muscles, in anterior a and posterior b view. See Table 1 for abbreviations of muscles. Scale bar = 1 cm
M. tibialis cranialis (TC). This muscle is well-developed and is the most superficial one on the anterior side of the tibiotarsus (Fig. 1). It is separated into two well-differentiated heads.
Origin: one of the heads, the caput femorale, originates through a strong tendon from the condylus lateralis of the femur (Fig. 3a, b), specifically from the fovea tendineus m. tibialis cranialis, and the second head, the caput tibiale, has a fleshy origin from the proximal part of the tibia, between the crista cnemial cranialis and the crista cnemial lateralis (Fig. 3e). Both heads fuse at the level of the mid-length of the tarsometatarsus forming an extensive belly.
Insertion: the belly extends distally in a tendon that inserts on the tuberositas m. tibialis cranialis located on the anteroproximal part of the tarsometatarsus (Fig. 6a).
M. extensor digitorum longus (EDL). This is a long muscle that runs along the anterior surface of the tibiotarsus and is covered by the TC (Fig. 5f).
Origin: fleshy, from the anteroproximal zone of the tibiotarsus, occupying part of the lateral side of the crista cnemialis cranialis, and it is extended along the proximal two-thirds of the anteromedial surface of the tibial shaft (Fig. 3e).
Insertion: this muscle continues in a long tendon that passes beneath the pons supratendineus of the tibiotarsus and, then, runs along the anterior surface of the tarsometatarsus. More distally, it separates into a lateral and a medial branch. The medial branch is wider and extends towards digit II. The lateral branch divides into two tendons which extend toward digits III and IV. Each of the tendons inserts on the extensor tubercle of the distal phalanges (unguals) of the digits II, III, and IV (Fig. 6a).
M. gastrocnemius (GM, GL, GI). This muscle is large and is the most superficial of the posterior side of the tibiotarsus (Fig. 1 and Fig. 5a, b). It is formed by three well-differentiated fleshy portions, pars lateralis, pars medialis, and pars intermedia.
Origin: the pars lateralis originates through a small tendon from the lateral surface of the distal part of the femur, above the epicondylus lateralis (Fig. 3b, c). It leaves a small circular fossa in this zone. The pars intermedia is the smallest portion and presents a fleshy origin from the distal and medial part of the femur, specifically from the epicondylus medialis (Fig. 3c, d). In one of the dissected specimens, this portion appears to present an additional thin belly in the proximal part (Fig. 5b), which originates from the posteromedial surface of the tibiotarsus, posterior to the insertion zone of FCM, and fuses with the main belly at its middle part. This additional belly was only observed in the left hindlimb of a single specimen. The third head, the pars medialis, has an aponeurotic origin from the anteroventral border of the crista cnemialis cranialis and a fleshy origin from the anterodorsal border and part of the medial surface of this crest (Fig. 3h).
Insertion: the three heads converge into a common distal tendon that inserts on the tibial cartilage.
M. flexor perforans et perforatus digiti II (FPPDII). Like the other flexor muscles of the digits, it is formed by a proximal fleshy fusiform belly and a long distal tendon.
Origin: is has a small fleshy origin from the distal and lateral part of the femur, immediately distal to the epicondylus lateralis (Fig. 3b).
Insertion: about half of the tibiotarsus the fleshy portion continues in a tendon, which passes through the tibial cartilage and runs along the posterior surface of the tarsometatarsus together with the tendons of other flexor muscles and superficially to the tendons of the FDL and FHL. It finally inserts on the lateral and ventral part of the second phalanx of digit II (Fig. 6b).
M. flexor perforans et perforatus digiti III (FPPDIII). Origin: the fleshy origin is mainly from the incisura tibialis, although also from the lateral and proximal surface of the crista cnemialis lateralis and the anterior surface of the caput fibulae of the tibiotarsus (Fig. 3e, f).
Insertion: the belly of this muscle becomes a tendon at the middle of the tibiotarsus, which inserts on the distal end of the third phalanx of the digit III (Fig. 6b).
M. flexor perforatus digiti II (FPDII). This is a small and thin muscle (Fig. 5c). Its proximal part is fused with the tendon of the M. ambiens.
Origin: fleshy, from the intercondylar zone of the femur (Fig. 3c).
Insertion: about the distal half of the tibiotarsus the belly becomes a tendon, which runs along the posterior surface of the tarsometatarsus and inserts on the proximolateral surface of the first phalanx of the digit II (Fig. 6b). Before the insertion, it is perforated by the tendon of the m. flexor perforans et perforatus digiti II.
M. flexor perforatus digiti III (FPDIII). Origin: it has two heads, both originating through a tendon. One of the heads originates from the intercondylar zone of the femur and the second one originates from the crista tibiofibularis (Fig. 3c).
Insertion: both heads form a slender belly that continues in a tendon, which divides distally into two parts that insert on the proximal ventral surface of the first phalanx of the digit III (Fig. 6b). Before the insertion, it is perforated by the tendon of the m. flexor perforans et perforatus digiti III.
M. flexor perforatus digiti IV (FPDIV). Origin: this muscle has two heads, one originates through a tendon immediately distal to the epicondylus lateralis, close to the origin of the FPPDII, and the second head has a fleshy origin from the intercondylar zone of the femur (Fig. 3b, c). Both heads fuse at the level of the mid-length of the tibiatarsus forming a slender belly (Fig. 5c).
Insertion: at the level of the distal part of the tibiatarsus the belly continues in a tendon which distally separates into three branches when it reaches digit IV. Each of these branches inserts on the second, third, and fourth phalanx of digit IV, respectively (Fig. 6b).
M. flexor hallucis longus (FHL). It is a large and well-developed muscle (Fig. 5a).
Origin: it presents two heads. One head is lateral and originates from the base of the condylus lateralis and extends proximally to the fossa poplitea (Fig. 3c). This head has a tendon and a fleshy part, which originate medially and laterally, respectively. The second head is medial and originates through a tendon from the fossa poplitea. Both heads fuse at the level of the proximal two-thirds of the tibiotarsus and form a wide fleshy belly.
Insertion: at the distal part of the tibiotarsus, the belly becomes a strong tendon that runs along the posterior surface of the tibiotarsus, covered by the tendons of the flexor muscles of digits II, III, and IV and above the tendon of the FDL. It finally inserts on the flexor tubercle of the ungual phalanx of digit I (Fig. 6b). Before the insertion, it joins to the branch of the FDL that inserts on digit II through a vinculum (Fig. 5g).
M. flexor digitorum longus (FDL). This is the deepest of the flexor muscles of the digits (Fig. 5e).
Origin: it has two origins. One origin is lateral and fleshy from the caput fibulae, extending along its posterior part (Fig. 3f, g). The second origin is also fleshy, distally located to the edge of the facies articularis lateralis and medialis of the tibia, extending on the posterior surface of the shaft of the tibia and fibula and, also, part of the medial surface of the tibia, occupying the proximal two-thirds of both bones (Fig. 3g, h). The fibers of this muscle partially cover the attachments of the m. popliteus.
Insertion: the extensive belly of this muscle becomes a tendon at the level of the distal part of the tibiotarsus, which is wide on its proximal portion. It separates distally into three branches, each of which runs along the ventral surface of digits II, III, and IV, and inserts on the flexor tubercles of the ungual phalanges of these digits (Fig. 6b).
M. popliteus (Po). This is a small and short muscle, deeply located and covered by the proximal part of the FDL.
Origin: fleshy, from the caudal surface of the proximal part of the tibiotarsus (Fig. 3g).
Insertion: fleshy, on the tuberositas popliteus of the fibula (Fig. 3g).
Muscles from the tarsometatarsus
M. extensor hallucis longus (EHL). Origin: it is formed by a lateral and a medial portion. The lateral, in turn, is formed by two bellies, the lateral one being slightly wider (Fig. 7a, b). These two bellies have fleshy origins from the anterior border of the cotyla lateralis and the anteromedial border of the cotyla medialis of the tarsometatarsus (Fig. 6a). The medial head has the form of a larger belly and originates from the anteromedial end of the tarsometatarsus, below the border of the cotyla medialis (Fig. 6a).
Muscles originated from the left tarsometatarsus in anterior a–d and posterior e view. Limits between muscles are indicated with dash lines. b close-up of the proximal portion of the tarsometatarsus showing the different bellies of EHL. c deep muscles after the removal of EHL. d distal portion of the tarsometatarsus showing the fleshy attachment (indicated as ‘fa’) of the tendon of AbDII (indicated as ‘AbDIIt’). See Table 1 for abbreviations of muscles. Scale bars = 1 cm
Insertion: the two lateral heads fuse into a tendon which, in turn, fuses with the tendon of the medial head at the middle of the tarsometatarsus (Fig. 7a, b). The common tendon inserts on the extensor tubercle of the ungual phalanx of digit I (Fig. 6a).
M. flexor hallucis brevis (FHB). Origin: fleshy, from the fossa parahypotarsalis medialis located on the posteromedial surface of the tarsometatarsus (Figs. 6b and 7e).
Insertion: the fleshy belly becomes a tendon at the level of the middle part of the tarsometatarsus, which inserts on the proximal end of the first phalanx of the hallux (Fig. 6b).
M. abductor digiti II (AbDII). Origin: fleshy, from the cotyla medialis and along the extensor sulcus, medially to the tuberositas m. tibialis cranialis (Fig. 6a and 7c).
Insertion: the short belly of this muscle becomes a long tendon that runs along the anteromedial surface of the tarsometatarsus and inserts on the medial border of the first phalanx of digit II (Fig. 7a). Before the insertion, short fleshy fibers attach the tendon to the medial distal surface of the tarsometatarsus (Fig. 7d).
M. adductor digiti II (AdDII). This is a small muscle located laterally with respect to the FHB (Fig. 7e).
Origin: fleshy, from the crista medialis plantares and below the crista medialis hypotarsi (Fig. 6b).
Insertion: by a tendon, which runs close to the tendon of the FHB and inserts on the lateral surface of the first phalanx of the digit II (Fig. 6a, b).
M. extensor brevis digiti IV (EBDIV). This is a small muscle, with a short fleshy belly (Fig. 7c).
Origin: fleshy, from the laterodorsal surface of the tarsometatarsus, immediately distal to the anterior border of the cotyla lateralis (Fig. 6a).
Insertion: by a tendon, which runs along the anterolateral border of the tarsometatarsus, and inserts on the medial side of the first phalanx of the digit IV (Fig. 6b).
M. abductor digiti IV (AbDIV). Origin: fleshy, from the posterolateral surface of the tarsometatarsus, below the crista lateralis hypotarsi (Fig. 6b and 7e).
Insertion: the fleshy belly of this muscle becomes a tendon at the middle of the tarsometatarsus and inserts on the lateral surface of the base of the first phalanx of digit IV (Fig. 6b).
M. extensor proprius digiti III (EPRDIII). This is a small muscle located distally on the tarsometatarsus (Fig. 7a).
Origin: it has a fleshy small fusiform portion that originates from the anterodistal surface of the tarsometatarsus (Fig. 6a).
Insertion: the fleshy portion becomes a tendon above the trochlea metatarsi III, which inserts on the proximodorsal end of the first phalanx of digit III (Fig. 6a).
Muscle mass
A total of 38 muscles were dissected. The average muscle mass of the hindlimb was 221.74 g, considering only the values from fresh muscles of three specimens (n = 3, SD = 45.85) (Table 1). Considering the mass values of the four specimens measured (fresh muscles from three specimens and dry muscles from one specimen), the highest percentages were found in six muscles (FHL, TC, FTL + FTI, G, PIF, and FDL), with values between 7.66 and 12.81% (Supplementary Table 1). These six muscles account for 57% of the total hindlimb mass and two of them (FHL and FDL) are directly involved in gripping function whereas one (TC) aids them secondarily. The following seven muscles with intermediate percentages of hindlimb mass had values between 2.35 and 5.29%: IF, ITC, IL, IC, FCM, EDL, and ISF (all muscles of the hip and femur, except for EDL). The rest of the muscles (23) had a percentage that ranged from 0.03 to 1.77% (Supplementary Table 1).
Regarding the functional groups, the digit flexors had the highest values (27.08%, Fig. 8) and the highest quantity of muscles, eight (Table 1). Hip flexors and extensors had percentages of 10 and 15%, respectively; knee and ankle flexors and extensors had values between 5 and 10%, and finally, the inward and outward rotators, abductors, and adductors had the lowest values (Fig. 8).
Discussion
The hindlimb musculature of Geranoaetus melanoleucus shares several features with that of other accipitrids. Some of the most informative works about the musculature of diurnal raptorial birds are those of Hudson (1937, 1948), in which the following species of accipitrids were studied: Ictinia mississippiensis, Accipiter cooperi, Buteo jamaicensis, B. swainsoni, B. borealis, Aquila chrysaetos, Circus hudsonius, and C. cyaneus. Therefore, when we refer to generalizations of the musculature of accipitrids citing these authors, they are based on that species. The similarities between Geranoaetus melanoleucus and other accipitrids include the absence of the m. flexor cruris lateralis, the pars iliaca of the m. caudofemoralis, the m. plantaris, and the rudimentary m. lumbricalis (Hudson 1937, 1948; Mosto et al. 2013). These absences also are observed in falconids, except the m. plantaris, which is well-developed in this family (Hudson 1937, 1948; Berger 1956; Mosto et al. 2013). Additionally, as in other accipitrids, the m. iliotrochantericus cranialis is partially fused to the m. iliotrochantericus medialis, although this fusion is also observed in falconids and strigiforms (Hudson 1937, 1948; Volkov 2004; Mosto et al. 2013; Mosto 2017a). The end of the m. ambiens tendon upon fusing with the proximal part of the FPDII is a shared characteristic with Buteo swainsoni (Hudson 1937). Furthermore, the tendon of the m. ambiens runs medially to the tendon of the m. iliofibularis, a common arrangement in Accipitridae and birds in general, although in Falco it passes laterally to the tendon of the IF (Hudson 1937, 1948). The m. iliotibialis lateralis originates exclusively from the preacetabular and acetabular ilium, leaving the m. iliofibularis completely exposed in lateral view of the thigh, a feature documented also in other accipitrids (Hudson 1937, 1948), although also in falconids (Hudson 1937, 1948; Berger 1956; Mosto et al. 2013) and strigiforms (Volkov 2004; Mosto 2017a). The shared origin of the mm. flexores perforatus of digits II, III, and IV from the intercondylar region of the femur, as observed in other accipitrids (Hudson 1948), is also present in G. melanoleucus. The m. flexor digitorum longus exhibits a well-developed belly, visible on the lateral side of the shank, consistent with observations in other accipitrids (and strigiforms, Volkov 2004). Another shared characteristic with other accipitrids, such as Buteo jamaicencis and Accipiter nisus (Hudson 1937, 1948; Goslow 1972; Liang et al. 2022), is the presence of a vinculum connecting the tendon of the m. flexor hallucis longus and the branch of the FDL corresponding to digit II, with the latter ramus being wider than those corresponding to digits III and IV, a feature also observed in falconids (Hudson 1937, 1948; Berger 1956; Liang et al. 2022). The m. flexor hallucis longus is notably well-developed, ranking among the largest muscles of the hindlimb in accipitrids, such as Haliaeetus albicilla (Mosto et al. 2021), and falconids (Berger 1956; Mosto et al. 2013) (see below for comparisons about muscle masses). Muscles originating from the tarsometatarsus in G. melanoleucus also exhibit features commonly observed in other accipitrids (Hudson 1937, 1948). These include the m. extensor hallucis longus, which has a well-developed belly and originates from the anteroproximal surface of the tarsometatarsus with a subdivided medial head; the m. extensor propius digiti III, which is well-developed, short, and confined to the distal portion of the tarsometatarsus; the m. extensor brevis digiti IV, with its belly restricted to the proximal portion of the tarsometatarsus; the origin of the m. abductor digiti II from the proximal two-thirds of the TMT without a connection to metatarsal I; the origin and belly of the m. flexor hallucis brevis limited to the proximal portion of the TMT; and the origin of the m. adductor digiti II and the m. abductor digiti IV from the proximal part of the TMT, a feature also observed in falconids, such as Falco peregrinus and F. sparverius (Hudson 1937, 1948) (however, in the accipitrid Aquila chrysaetos the origin of the AbDIV is from almost the entire length of the tarsometatarsus, Hudson 1948).
Geranoaetus melanoleucus exhibits several features that distinguish it from the morphology observed in other accipitrids and, more broadly, in diurnal raptors. For instance, in accipitrids, the origin of the m. femorotibialis medialis typically extends more proximally than the insertion point of the m. iliofemoralis internus on the femur (Hudson 1937, 1948). However, in G. melanoleucus, the origin of the FTM is less proximally extended and is separated from the insertion of the IFI. Notably, the additional portion of the m. gastrocnemius pars intermedia observed in one of the specimens is a feature not mentioned in other accipitrids nor in falconids (Hudson 1948; Berger 1956; Mosto et al. 2013). Hudson (1948) does note the presence of a second part of this muscle in Cathartidae, originating through a narrow tendon from the intercondylar zone of the femur. A fourth small portion of the m. gastrocnemius was also observed in Rhea americana, but it originates proximally to the medial femoral condyle and joins the pars medialis, not the pars intermedia as in G. melanoleucus (Picasso 2010). Nevertheless, due to the presence of this feature only on one side of one specimen, we consider it to be an intraspecific variation. Another significant difference is the smaller origin area of the m. tibialis cranialis in G. melanoleucus, which is limited to the anteroproximal zone of the tibia. In contrast, in other accipitrids and falconids, the origin of this muscle is more extensive, extending longitudinally along the medial surface of the tibia (Hudson 1937, 1948; Goslow 1972). Also, in accipitrids and falconids (and in strigiforms, Volkov 2004; Mosto 2017a), this muscle also inserts through two tendons, one of them positioned more distally (in the falconid Falco, there is even a third smaller tendon) (Hudson 1937, 1948). In contrast, in G. melanoleucus, the two heads of this muscle fuse and insert through a single tendon on the tarsometatarsus. The m. flexor perforatus digiti IV of G. melanoleucus presents differences compared to other accipitrids. In the accipitrids studied by Hudson (1948), the second head of origin of this muscle is attached to the caput fibulae, whereas in G. melanoleucus, this head arises from the laterodistal surface of the femur. Additionally, in G. melanoleucus, the tendon of this muscle trifurcates distally and each branch inserts on the second, third, and fourth phalanx of digit IV, respectively. In contrast, in Buteo swainsoni, Buteo borealis and other accipitrids, it does not branch before its insertion, as also occurs in the falconid Falco (Hudson 1937, 1948). A similar condition to that of G. melanoleucus is observed in Sagittarius and the Cathartidae (Hudson 1937, 1948).
With regard to mass, the values obtained in this study for G. melanoleucus are consistent with those reported for other diurnal raptorial birds, with FHL showing the greatest mass among all hindlimb muscles, as in some accipitrids, such as Accipiter nisus, and falconids, such as Falco tinnunculus, F. peregrinus, and Micrastur ruficollis (Mosto 2017b; Liang et al. 2022). In other falconids, such as the polyborines Milvago chimango and Caracara plancus, the FHL has a notably high mass although is surpassed by other muscles, e.g., the m. puboischiofemoralis and the m. gastrocnemius (Mosto et al. 2013; Mosto 2017b). The mass values of the digit flexors underscore their significance in G. melanoleucus, as is the case in other raptorial birds, such as accipitrids (e.g., Haliaeetus albicilla) and falconids (e.g., Micrastur ruficollis and Falco femoralis) (Goslow 1972; Hertel et al. 2015; Mosto 2017b; Mosto et al. 2021). Previous researches into the hindlimb musculature of these birds, particularly in accipitrids such as Buteo lineatus, B. jamaicensis, Accipiter nisus, A. striatus, and Haliaeetus albicilla, have consistently revealed that FHL is the muscle with the largest mass of the shank (and sometimes in the entire hindlimb), and that it exerts the greater digital flexor force-generation, followed by FDL (Ward et al. 2002; Sustaita 2008; Sustaita and Hertel 2010; Mosto et al. 2021; Liang et al. 2022). This is closely associated with the typical foraging behavior observed in most accipitrids, wherein they kill prey by applying a powerful grip of the pedal digits, exerting thoracic compression and kneading (Csermely and Gaibani 1998; Einoder and Richardson 2007; Sustaita 2008; Sustaita and Hertel 2010; Liang et al. 2022; and references therein). Geranoaetus melanoleucus also employs a similar killing mode to that of other accipitrids (Jiménez and Jaksic 1990). Furthermore, in diurnal raptorial birds, the function of the hallux is of paramount importance in capturing and restraining prey due to its posterior orientation, opposed to the fore digits (Ward et al. 2002; Einoder and Richardson 2007). This importance is evidenced by the presence of a powerful flexor muscle (FHL) capable of generating substantial force. Additionally, in G. melanoleucus, the masses of the FHB and EHL are relatively large compared to the mass of the other intrinsic muscles of the tarsometatarsus that are involved in digit motion. This observation aligns with prior studies indicating that FHB (and FHL) can generate a considerable force, especially when compared with other intrinsic digit flexor muscles of the tarsometatarsus (Mosto et al. 2021). Furthermore, the vinculum that connects the FHL to the insertion tendon of FDL on digit II suggests that FHL may also exert control over digit II, thus assisting in the grasping action of this digit (Goslow 1972; Liang et al. 2022). This likely enhances the efficiency of both the hallux and digit II, considering that these two digits form the primary opposable unit and contribute significantly to grip force production (Einoder and Richardson 2007). Indeed, the talons of the hallux and digit II in G. melanoleucus are longer than those of the remaining foot digits, resembling the condition observed in other accipitrids, such as Aquila audax, Accipiter cirrhocephalus, Accipiter gentilis, Buteo jamaicensis, and Haliaeetus albicilla (Einoder and Richardson 2007; Fowler et al. 2009; Mosto et al. 2021).
Previous studies have provided data supporting the idea that raptorial birds relying on their feet to grasp and carry objects, i.e., falconids, accipitrids, and strigiforms, tend to exhibit flexor muscles that insert distally more developed (Backus et al. 2015). In line with this studies, it is noteworthy that among the mm. flexores perforans and perforans et perforatus of G. melanoleucus, those more distally inserted on digits II and III, i.e., FPPDII and FPPDIII, have a greater mass than their more proximally inserted counterparts, FPDII and FPDIII.
Another muscle of significant importance during prey subduing is the m. tibialis cranialis. Besides its primary function of ankle flexion when contracting, it also secondarily contributes to digit flexion (Conroy et al. 1997; Ward et al. 2002; Mosto et al. 2021; Liang et al. 2022). The mass of this muscle reflects its importance, ranking as one of the largest muscles of the shank, following FHL. The m. iliofibularis is another muscle with substantial mass, serving as one of the main shank flexors. The considerable force generated by this muscle, along with that of the TC, is crucial when prey is held in close proximity to the body of the predator (Goslow 1972). Therefore, the combined contraction of the m. iliofibularis, along with the indirect action of talons closure facilitated by TC, contributes to the effectiveness of the foraging behavior (Ward et al. 2002; Liang et al. 2022).
Conclusion
This study has provided a detailed examination of the hindlimb musculature of Geranoaetus melanoleucus. Several similarities were identified with other accipitrids (and also falconids), including the absence of certain muscles such as the m. flexor cruris lateralis; the fusion of ITCr and ITM; the common origin of the mm. flexores perforatus of digits II, III, and IV from the intercondylar region of the femur; the presence of a visible FDL on the lateral side of the shank; a distal connection between FHL and FDL through a vinculum; a well-developed and short EPRDIII restricted to the distal portion of the tarsometatarsus; and an origin and belly of the FHB confined to the proximal TMT; among others. However, there are notable differences when compared to other accipitrids and falconids, including a less proximally extended origin of FTM and separated from the insertion of IFI; a smaller origin area of TC bounded to the anteroproximal zone of the tibia; a second origin of FPDIV from the laterodistal surface of the femur rather than the caput fibulae; and a trifurcated tendon of FPDIV inserted on the second, third, and fourth phalanx of digit IV.
The masses of different muscles are similar to those observed in other diurnal raptorial birds. Digit flexors constitute the largest proportion of the total mass of hindlimb muscles, with FHL and FDL having the greatest values. Similar to other diurnal raptorial birds, there is a suite of muscles collectively implied in the grasping function of the pes. This is pivotal in the primary foraging mode of most accipitrids, including G. melanoleucus, which relies on a powerful grip of the digits to subdue prey. FHL and FDL are the primary contributors to grip force, and the higher mass of FHL in G. melanoleucus aligns with the increased force exerted by the hallux, a common finding in diurnal raptorial birds, particularly accipitrids. In these birds, given the opposing position of the hallux compared to the fore digits, it plays a central role in capturing and restraining prey. FHL produces indirect flexion of the digit II due to the vinculum connecting FHL with FDL. Digit II in conjunction with the hallux produces most of the grip force. TC and IF are two additional muscles indirectly involved in overpowering prey, with TC contributing to digit flexion and, in conjunction with IF, aiding in keeping prey close to the body of the predator when the shank is flexed.
Accipitrids (and diurnal raptorial birds in general) appear to share a consistent pattern of muscular anatomy, as evidenced by the numerous similarities observed among them. Nevertheless, the muscles described in G. melanoleucus exhibit notable differences from those in other accipitrids, underscoring the importance of detailed anatomical studies in uncovering species-level peculiarities. The study of detailed anatomy at the species-level is also relevant to elucidate if phylogenetically closely related species present common characters and to recognize possible patterns along phylogeny of accipitrids. Furthermore, such studies furnish essential data for morphofunctional analyses and subsequent ecomorphological inferences, particularly in species where the hindlimb plays a pivotal role in hunting behavior.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Backus SB, Sustaita D, Odhner LU, Dollar AM (2015) Mechanical analysis of avian feet: multiarticular muscles in grasping and perching. R Soc Open Sci 2:140350. https://doi.org/10.1098/rsos.140350
Baumel JJ, Witmer LM (1993) Osteologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of Avian Anatomy: Nomina Anatomica Avium. Massachusetts, Cambridge, pp 45–132
Berger AJ (1956) The appendicular myology of the pygmy falcon (Polihierax semitorquatus). Am Midl Nat 55:326–333. https://doi.org/10.2307/2422594
Conroy R, Weigl P, Clark J, Ward A (1997) Functional morphology of owl hindlimbs: Implications for prey selection and resource partitioning. Am Zool 37:37A
Csermely D, Gaibani G (1998) Is foot squeezing pressure by two raptor species sufficient to subdue their prey? The Condor 100:757–763. https://doi.org/10.2307/1369762
Einoder LD, Richardson A (2006) An ecomorphological study of the raptorial digital tendon locking mechanism: The raptorial digital tendon locking mechanism. Ibis 148:515–525. https://doi.org/10.1111/j.1474-919X.2006.00541.x
Einoder LD, Richardson AMM (2007) Aspects of the hindlimb morphology of some Australian birds of prey: a comparative and quantitative study. Auk 124:773–788
Fowler DW, Freedman EA, Scannella JB (2009) Predatory functional morphology in raptors: interdigital variation in talon size is related to prey restraint and immobilisation technique. PLoS ONE 4:e7999. https://doi.org/10.1371/journal.pone.0007999
Gatesy SM (1999) Guineafowl hind limb function. I: Cineradiographic analysis and speed effects. J Morphol 240:115–125. https://doi.org/10.1002/(SICI)1097-4687(199905)240:2%3c115::AID-JMOR3%3e3.0.CO;2-Y
Goslow GE (1967) The functional analysis of the striking mechanisms of raptorial birds. PhD Dissertation, University of California
Goslow GE (1972) Adaptive mechanisms of the raptor pelvic limb. Auk 89:47–64. https://doi.org/10.2307/4084059
Hertel F, Maldonado JE, Sustaita D (2015) Wing and hindlimb myology of vultures and raptors (Accipitriformes) in relation to locomotion and foraging. Acta Zool 96:283–295. https://doi.org/10.1111/azo.12074
Hudson GE (1937) Studies on the muscles of the pelvic appendage in birds. Am Midl Nat 18:1–108. https://doi.org/10.2307/2420619
Hudson GE (1948) Studies on the muscles of the pelvic appendage in birds II: The heterogeneous order Falconiformes. Am Midl Nat 39:102–127. https://doi.org/10.2307/2421432
Jacobson RD, Hollyday M (1982) A behavioral and electromyographic study of walking in the chick. J Neurophysiol 48:238–256. https://doi.org/10.1152/jn.1982.48.1.238
Jaksic FM, Greene HW, Yáñez JL (1981) The guild structure of a community of predatory vertebrates in central Chile. Oecologia 49:21–28. https://doi.org/10.1007/BF00376893
Jaksic FM, Iriarte JA, Jiménez JE (2002) The raptors of Torres del Paine National Park, Chile: biodiversity and conservation. Rev Chil Hist Nat 75:. https://doi.org/10.4067/S0716-078X2002000200014
Jaksic FM, Jimenez JE (1986) The conservation status of raptors in Chile. Birds of Prey Bulletin 3:95–104
Jiménez JE, Jaksic FM (1989) Behavioral ecology of grey eagle-buzzards, Geranoaetus melanoleucus, in Central Chile. The Condor 91:913. https://doi.org/10.2307/1368076
Jiménez JE, Jaksic FM (1990) Historia natural del águila Geranoaetus melanoleucus: una revisión. El Hornero 13:97–110. https://doi.org/10.56178/eh.v13i2.1092
Jollie M (1976) A contribution to the morphology and phylogeny of the Falconiformes I. Evol Theory 1:285–298
Jollie M (1977) A contribution to the morphology and phylogeny of the Falconiformes. Pt IV Evol Theory 3:1–142
Liang X, Liu M, Ying C, Zhang Z (2022) Myological variation in the hindlimb of three raptorial birds in relation to foraging behavior. Avian Res 13:100053. https://doi.org/10.1016/j.avrs.2022.100053
Mosto MC (2017a) The hindlimb myology of Tyto alba (Tytonidae, Strigiformes, Aves). Anat Histol Embryol 46:25–32. https://doi.org/10.1111/ahe.12227
Mosto MC (2017b) Comparative hindlimb myology within the family Falconidae. Zoomorphology 136:241–250. https://doi.org/10.1007/s00435-017-0343-1
Mosto MC (2014) Estructura y función del complejo apendicular posterior en rapaces diurnas (Falconidae y Accipitridae). PhD Dissertation, Universidad Nacional de La Plata
Mosto MC, Carril J, Picasso MBJ (2013) The hindlimb myology of Milvago chimango (Polyborinae, Falconidae). J Morphol 274:1191–1201. https://doi.org/10.1002/jmor.20172
Mosto MC, Cassini GH, Picasso MBJ, Krone O (2021) Grasping behavior in the white-tailed sea eagle (Accipitridae, Aves) explained by muscle architecture. J Zool 314:234–244. https://doi.org/10.1111/jzo.12876
Pavez EF (2001) Biología reproductiva del águila Geranoaetus melanoleucus (Aves: Accipitridae) en Chile central. Rev Chil Hist Nat 74:687–697. https://doi.org/10.4067/S0716-078X2001000300014
Picasso MBJ (2010) The Hindlimb Muscles of Rhea americana (Aves, Palaeognathae, Rheidae): Pelvic Limb Muscles of Rhea. Anat Histol Embryol 39:462–472. https://doi.org/10.1111/j.1439-0264.2010.01017.x
Saggese MD, De Lucca ER (2001) Biología reproductiva del águila mora (Geranoaetus melanoleucus) en la Patagonia sur, Argentina. El Hornero 16:77–84. https://doi.org/10.56178/eh.v16i2.898
Sarasola JH, Grande JM, Negro JJ (2018) Birds of prey: biology and conservation in the XXI century. Springer, Berlin
Schlatter RP, Yáñez JL, Jaksic FM (1980) Food-niche relationships between Chilean Eagles and Red-backed Buzzards in Central Chile. Auk 97:897–898
Schoonmaker P (1984) Observations on the nesting of the black-chested buzzard-eagle (Geranoaetus melanoleucus) in Peru. The Condor 86:221. https://doi.org/10.2307/1367052
Sustaita D (2008) Musculoskeletal underpinnings to differences in killing behavior between North American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). J Morphol 269:283–301. https://doi.org/10.1002/jmor.10577
Sustaita D, Hertel F (2010) In vivo bite and grip forces, morphology and prey-killing behavior of North American accipiters (Accipitridae) and falcons (Falconidae). J Exp Biol 213:2617–2628. https://doi.org/10.1242/jeb.041731
Tsang LR, McDonald PG (2019) A comparative study of avian pes morphotypes, and the functional implications of Australian raptor pedal flexibility. Emu—Austral Ornithology 119:14–23. https://doi.org/10.1080/01584197.2018.1483203
Tsang LR, Wilson LAB, Ledogar J et al (2019) Raptor talon shape and biomechanical performance are controlled by relative prey size but not by allometry. Sci Rep 9:7076. https://doi.org/10.1038/s41598-019-43654-0
Vanden Berge JC, Zweers GA (1993) Myologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC (eds) Handbook of Avian Anatomy: Nomina Anatomica Avium. Massachusetts, Cambridge, pp 189–250
Volkov SV (2004) The hindlimb musculature of the true owls (Strigidae: Strigiformes): morphological peculiarities and general adaptations. Ornithologia 31:154–174
Ward AB, Weigl PD, Conroy RM (2002) Functional morphology of raptor hindlimbs: implications for resource partitioning. Auk 119:1052–1063
Acknowledgements
Thanks to the Flora and Fauna Reserve of La Florida (San Luis Province, Argentina) for kindly donating the specimens used in this work and to Andrea Arcucci and Mirta Ortíz for transporting them to the Universidad Nacional de San Luis (UNSL). Many thanks to Anabel Di Carlantonio (Zoology Area, UNSL) for the help offered during the dissection of specimens, and Sergio Bogan and Juan Meluso for allowing access to the collection of the Fundación de Historia Natural ‘Félix de Azara’ (Buenos Aires, Argentina) to study the materials there housed. The comments and suggestions of two anonymous reviewers have substantially improved the quality of this work. Finally, we want to thank the Universidad Nacional de San Luis for supporting this research (grant PROICO 2-0618).
Author information
Authors and Affiliations
Contributions
F.A.G. wrote the main manuscript text and prepared figures 1-7. L.C. wrote the main manuscript text. M.C.M. wrote the main manuscript text and prepared figure 8 and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethics approval
No approval from research ethics committees was necessary to achieve the objectives of this study, as no experimental activities were carried out. The specimens were found deceased in the field within a natural reserve (Reserva de Flora y Fauna de La Florida, San Luis Province, Argentina) by the personnel of the reserve. They had perished due to natural causes and were subsequently deposited in a scientific collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gianechini, F.A., Colli, L. & Mosto, M.C. The hindlimb myology of the South American eagle Geranoaetus melanoleucus (Accipitridae, Aves). Zoomorphology 143, 215–230 (2024). https://doi.org/10.1007/s00435-023-00629-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00629-0