Abstract
A characteristical feature of a raptors’ hindlimb is their strengthen musculature that aids gripping prey with their sharp talons. To trace specific anatomical modifications it is necessary to study closely related species, with this aim, the myology of the hindlimb of the three subfamilies of Falconidae is explored. For this, a description of a Herpetotherinae member (Micrastur ruficollis) was made for the first time. The hindlimb muscle mass of Polyborinae, Falconinae and Herpetotherinae was compared according to their main function (flexion and extension) on their joints (femur, tibiotarsus, tarsometatarsus and digits). The pattern of Micrastur ruficollis resembles that of the Falconidae except for a few differences towards the development of certain muscles. As it is noteworthy the presence of the second belly of the musculus flexor cruris medialis (unique among birds), its identity will be discussed. Also, Micrastur ruficollis had the highest values of the hindlimb mass. Polyborinae and Falconinae had several differences between each other. The muscles mass of the hip and knee, both flexion and extension, were higher in the Polyborinae, this is in accordance to their more terrestrial habit. Instead, Falconinae had a higher mass in the m. flexor digitorum longus, m. flexor hallucis longus and m. tibialis cranialis, the most important muscles for gripping prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation in raptor birds can be considered as a distinct habit in the clade Aves (Goslow 1967). This habit is reflected on their morphology with a hocked beak, sharp talons, a strengthen hindlimb, among others (Hudson 1937, 1948; Goslow 1967; White et al. 1994; Mosto et al. 2013; Mosto and Tambussi 2014). It should be taken into account that, in this case, the design of the raptor’s hindlimb is a compromise resultant of also other biological needs and phylogeny (Goslow 1967; Stoessel et al. 2013). For this condition, a feasible approach to trace specific anatomical modifications, for a particular action of a particular form-function complex, is the comparison of closely related species (Engles 1940; Berger 1952; Spring 1965; Goslow 1967). Thus, the family Falconidae Leach, 1820 is an excellent group of study which has a relatively low number of species (64) grouped in 11 genera (Dickinson 2003) within three well recognized subfamilies: Herpetotherinae, Polyborinae Bonaparte, 1837 and Falconinae Leach, 1820 (Fuchs et al. 2015). Herpetotherinae and Polyborinae are endemic of the Neotropical region, whereas Falconinae is cosmopolitan (Fuchs et al. 2012). Moreover, Herpetotherinae has a more restricted distribution on the lowland and mid-elevation humid forest of Central and South America than the Polyborinae (GRIN 2016). The Falconidae are predators that differ in food preference and how to obtain it and locomotion (White et al. 1994; Bó et al. 2007). Falcons uses their hindlimb to attack and grip prey on the air, Micrastur ruficollis hunts from perches and runs on the ground after small prey (White et al. 1994; GRIN 2016) and Caracaras (Polyborinae) scavenge carrion or walk on the ground searching for food (Brown and Amadon 1968).This different use of the hindlimbs may be reflected on their myology (e.g. Hartman 1961; Picasso 2015). Detailed myological descriptions for the family are those of Hudson (1937, 1948) just for the genus Falco. Berger (1956) described the hindlimb muscles of Polihierax semitorquatus Smith, 1836; another Falconinae. Even though the work of Jollie (1976a, b, c, 1977a, b, c) regarding this family was exhaustive (e.g. osseous, myological, feathers), specific details have not been given. Polyborinae members were not thoroughly described until recent (Mosto et al. 2013, 2016); and to date, there is no description on the hindlimb myology of any Herpetotherinae, Herpetotheres or Micrastur. This unbalanced amount of information within the Falconidae is probably due to the different geographical distribution of the three subfamilies. Or possibly due to the less impressive hunting strategies of Polyborinae or the secretive behavior of Herpetotherinae (GRIN 2016). All things considered, studies comparing the myological morphology within the family are scarce.
Studies on muscular mass are useful because provides information of the potential power of a muscle during the activity it performs (Hartman 1961). Greater mass is directly related to higher forces, as it has more fibers and a greater cross-sectional area (Bock 1974), which ultimately enables a better performance of the needed activity.
The objective of this work is to explore the hindlimb myology of Falconidae and describe for the first time that of a Herpetotherinae member (Micratur ruficollis). This information contributes to a better understanding of the ecomorphological relationships and the evolutionary history of this Family.
Materials and methods
A total of seven species were considered in this work (in brackets the number of specimens): F. peregrinus Tunstall, 1771 (1), Falco spaverius Linnaeus, 1758 (5), F. femoralis Temminck, 1822 (2), Caracara plancus Miller, 1777 (5), Milvago chimachima Vieillot, 1816 (3), M. chimango Vieillot, 1816 (13) and Micrastur ruficollis Vieillo, 1817 (1). The specimens of M. chimango and their muscle masses are those of Mosto et al. (2013). The specimens included in this work belong to different museums according to their availability and only if their musculature was in good conditions for its dissection. The specimens were acquired from agreements with different entities: Museo Argentino de Ciencias Naturales, Bernardino Rivadavia (MACN), National Park Nahuel Huapi, Museo de La Plata (MLP).
The description of Micrastur ruficollis was made although only stating the differences within the Family. For this objective, the descriptions of Hudson (1937, 1948), Mosto et al. (2013, 2016) and Mosto (2014) were used for comparison. The anatomical nomenclature follows Baumel et al. (1993).
In each specimen, a dissection was performed on one limb and the other limb was used in case of any doubt. The mass of each muscle and bodymass were obtained with a digital scale (precision 0.01 g). Dunning (2008) was used for bodymass when this data was not available from the specimen. For each muscle, the percentage with respect to the body mass was calculated, and also, the percentage of muscle mass of the hindlimb. Finally, the percentages of the muscles were grouped together according to their main function (flexion and extension) of the femur, tibiotarsus, tarsometatarsus and digits. Muscle action was taken from Goslow (1967), Jacobson and Hollyday (1982) and Gatesy (1999).
Moran’s autocorrelation coefficient was performed to quantify whether the distribution the percentage of muscles according to main action (flexion or extension) and joint (femur, tibiotarsus, tarsometatarsus or digits) among Falconidae is affected or not by their phylogenetic relationships. The Ape package of R 3.3.2 (R Development Core Team, 2016) was used.
Results
Qualitative differences of Micrastur ruficollis hindlimb muscles
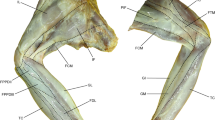
In general, the hindlimb muscles of Micrastur ruficollis resemble that of the Falconidae (Figs. 1, 2). Only a few differences with the Family can be highlighted: (1) the musculus ambiens was even smaller in this species that in the other species, where is already reduced. (2) The m. extensor digitorum longus had a calcified tendon along the anterior surface of the tarsometatarsus unlike the Falconidae. (3) The m. abductor digiti II extends along the surface of the tarsometatarsus. It has a small belly on the proximomedial edge of this bone and a long tendon that inserts on the proximomedial edge of the first phalange of digit II. In the rest of the family, the muscle originates on the distal end of the tarsometatarsus.
Hindlimb muscles of Micrastur ruficollis. Lateral (a) and medial (b) view of the superficial muscles of the hip and knee joints, lateral (c, d) and medial (d) view of the deep muscles of the hip joint. Scale bar 1 cm, the abbreviations are in Table 1
Hindlimb muscles of Micrastur ruficollis. Lateral (a) and medial (b) view of the deep muscles of the tibiotarsus; anterior (c) and posterior (d) view of the deep muscles of the tarsometatarsus. Scale bar 1 cm, the abbreviations are in Table 1
Although not a difference, the m. flexor cruris medialis had the two bellies described by Falco, confirming that this muscle, with two distinct bellies, is present in the whole family (see discussion; Figs. 1c, d, 2a, b).
Quantitative characteristics of the Falconidae hindlimb muscles
The percentage of the total muscle mass of the hindlimb of M. ruficollis (8.23%; Table 1) was the highest between the other species. Caracara plancus (6.75%; Table 1) was the following species after M. ruficollis and the genera Falco and Milvago shared similar values (3.7–5.13%; Table 1).
Considering each muscle isolated, M. ruficollis had mostly all muscles with the highest percentage compared with the other subfamilies (e.g. m. pubo-ischio-femoralis, m. ischiofemoralis, m. femorotibialis medialis, m. tibialis cranialis, m. flexor digitorum longus; Table 1). Caracara plancus had several muscle with the highest percentage (e.g. m. flexor perforatus digiti IV, m. fibularis longus, m. flexor cruris medialis (pars proximalis and distalis), m. iliotrochantericus caudalis; Table 1). It is also interesting to notice the low value of the m. caudofemoralis in C. plancus.
Besides M. ruficollis, the subfamily Polyborinae surpassed Falconinae in the values of some of the muscles (e.g. m. pubo-ischio-femoralis, m. iliotrochantericus caudali, m. flexor cruris medialis (pars proximalis) m. gastrocnemius, m. fibularis longus; Table 1). Both bellies of the FCM had a higher mass in Polyborinae than Falconinae but it is noteworthy that the belly with the more proximal insertion was much larger in Polyborinae. Instead, Falconinae exceeded Polyborinae in the mass of m. tibialis cranialis, m. flexor digitorum longus and m. extensor hallucis longus. Several muscles had a relatively similar mass between these two subfamilies: e.g. m. iliotrochantericus cranialis et medialis and m. femorotibialis lateralis et intermedius; and both, m. fibularis brevis and m. plantaris had a similar mass within the Family Falconidae.
Differences among joint and actions
The flexion and extension of the femur, tibiotarsus and tarsometatarsus have a similar percentage of mass between each other, whereas the difference was noticeable in the digits, with the extension having the lowest percentage of mass and the flexion the highest one (Fig. 3).
Percentage of the muscles of the hindlimb grouped in flexors (fx) and extensors (ex) of the femur, tibiotarsus (Tbt), tarsometatarsus (Tmt) and digits. Table 1 shows which muscles were included in each group
M. ruficollis and C. plancus, as stated above, had the highest values compared with the other species (Fig. 3). In the femur extension and flexion, tibiotarsus and tarsometatarsus extension, Polyborinae had higher values than the Falconinae, whereas the opposite occurred in those muscles that act on the digits, both extension and flexion had higher values in Falconinae than Polyborinae.
Table 2 shows that all eight groupings have a p value lower than 0.05 at the level of species. This implies that all the differences found regarding the muscles are not random. For the extension of both the femur and the tibiotarsus the segregation was found at the level of genus. The remaining groupings, all the flexors of the limb, showed a p value lower than 0.05 at the level of subfamily.
Discussion
This paper had the aim of comparing the hindlimb muscle mass between the three subfamilies of the family Falconidae: Herpetotherinae, Polyborinae and Falconinae. The myology pattern, merely the presence and absence of the muscles, found on Micrastur ruficollis was similar to that of the other members of the family (Hudson 1937, 1948; Jollie 1976, 1977a, b, c; Mosto et al. 2013, 2016). Most of the differences between them reside on the mass of each muscle. It is noteworthy the calcification on the tendon of the m. extensor digitorum longus with respect to the other Falconidae species. The m. flexor digitorum longus and m. flexor hallucis longus were also calcified like in the other members of the family (Harcourt-Brown 2001; Mosto et al. 2013). This characteristic is related to a high force regime of the muscle (Ward et al. 2002) or to avoid stretching of these long tendons during their actions (Bennett and Stafford 1988; Vanden Berge and Storer 1995). Another difference found on M. ruficollis was the development of the m. abductor digiti II. This muscle extends through almost the entire length of the tarsometatarsus in this species, whereas in the other two subfamilies, this muscle originates on the anteromedial facet on the distal fourth of this bone (Hudson 1937; Mosto et al. 2013). This increased development of the muscle, due to a larger tendon, gives a higher inertia moment and force, compared to those species where the muscle originates at the distal end of the tarsometatarsus (Ilyinsky 2008). Added to this longer excursion of the muscle, it should be considered that the tarsometatarsus is as large as that of the Polyborinae (Mosto et al. 2016). This feature may be helpful to M. ruficollis while hunting their main prey item: reptiles (Thorstrom 2000).
Form and function of the muscles
The mass of a muscle is directly related with the strength that it can exert (Lieber 2002). Therefore, with the data herein acquired, an approximation towards the importance and use of the muscles and joints can be inferred.
Micrastur ruficollis has several features which conclude on a relatively large hindlimb muscle mass. First, it has a relatively low bodymass (average 161/196 g Dunning 2008; 167.8/233.2 g; Thorstrom 2000 males and females, respectively) and an enlarged tarsometatarsus (Mosto et al. 2016) which leads to a larger excursion of those tendons that originate on the tibiotarsus and insert on the digits (e.g. m. flexor digitorum longus). Therefore, it can be interpreted that a reason for the relatively higher hindlimb muscle mass is directly connected to these results.
The m. caudofemoralis is the only muscle dissected here that acts on the tail (Fisher 1957; George and Berger 1966; Gatesy and Dial 1993) and it had in M. ruficollis a large mass compared to that of other species. This could be related to the long tail of the forest Falcon that aids maneuvering between branches, a common feature in birds that live in the forest (White et al. 1994; Fuchs et al. 2011). On the other hand, C. plancus had the lowest mass in this particular muscle, being the only muscle where this occurs. A possible explanation is that, between the raptors studied here, C. plancus is the only one that soars on open areas unlike M. ruficollis (Bierregaard 1994) and does not make aerial pursuits like Falco (Cade 1982).
The subfamilies Polyborinae and Falconinae had several differences between each other. The muscles mass of the hip and knee, both flexion and extension, were higher in the former subfamily (e.g. PIF, IC, G, Tables 1, 2; Fig. 3). Instead, the Falconinae had a higher mass in the digits articulation and the tarsometatarsus flexion (FDL, FHL, TC, Tables 1, 2; Fig. 3). This is in accordance to the more terrestrial habit of the Polyborinae, which spend more time on the ground than the Falconinae (White et al. 1994; Biondi et al. 2005; Mosto et al. 2016). The muscles of the hip and knee (e.g. m. iliotibialis lateralis, m. flexor cruris) are more important while walking on the ground (Jacobson and Hollyday 1982; Gatesy 1999). On the other hand, Falconinae rely on their feet to grip prey while hunting (Goslow 1969; Sustaita 2008). From the muscles that are in charge of gripping, only the m. flexor digitorum longus and m. flexor hallucis longus have higher values in Falconinae. On the other hand, the small flexor muscles of the digits (FPPDII; FPPDIII, FPDII, FPDIII and FPDIV) have higher values in Polyborinae than Falconinae. The FDL and FHL insert on the flexor tubercle of the ungueal phalanx (Mosto et al. 2013; Mosto and Tambussi 2014), flexing toes from the most distal end of the digit. The small flexor muscles insert on the proximal phalanges. This difference is in agreement with the results found by Backus et al. (2015) as FDL and FHL are the most important for gripping, whereas small muscles are more important while perching.
As stated before, the m. tibialis cranialis also had a higher mass in Falconinae than Polyborinae. This muscle is the most important one to flex the tarsometatarsus (Jacobson and Hollyday 1982) and it aids, indirectly, while gripping prey when raptors are hunting (Conroy et al. 1997; Ward et al. 2002). It is the second mechanism to close the talons, which may function together with the toe flexors (Ward et al. 2002). This muscle probably exerts a great force to have a stronger closure of the talons.
The musculi flexor cruris lateralis (FCL) and medialis (FCM)
In relation to these two muscles, Falconidae was characterized for the absence of the FCL and the presence of FCM with a unique double belly among birds (Hudson 1937, 1948; Berger 1956). This feature was attributed to the whole family even though only members of the Falconinae subfamily were described at that time. Recently, Polyborinae species were studied (Mosto et al. 2013, 2016; Mosto 2014) and it was concluded that the FCL was present and that the FCM had a longitudinal division without being able to distinguish the two different portions previously described (Mosto et al. 2013, 2016).
The status of these muscles is reconsidered in this paper. The muscle that originates on the posterior limit of the processus terminalis ischii of the pelvis and inserts on the proximomedial region of the tibiotarsus (Mosto et al. 2013) is identified as the second belly of the FCM of Falco for Hudson (1937, 1948) and Berger (1956) and as the FCL of Polyborinae for Mosto et al. (2013, 2016). In this paper, this muscle is named m. flexor cruris medialis pars proximalis [FCM (p), Fig. 1 for M. ruficollis].
Besides the terminology of the muscle, Table 1 shows that there is a great difference of mass between the FCM (p) in Polyborinae than Falconinae (almost twice as large) having an important role for the Polyborinae related to the terrestrial locomotion of the subfamily (Mosto et al. 2013, 2016). Given the topography of the belly, this muscle might aid to extend the hip while walking on the ground. This muscle has the same origin and insertion of the FCL described for the Polyborinae (Mosto et al. 2013) and its morphological variability towards different lifestyles within a family has been previously recorded (Mckitrick 1986, 1993). However, as this belly does not pass above the m. caudofemoralis—as it generally does (George and Berger 1966)—it cannot be considered as FCL with total certainty in the Falconidae. But, in this group, the m. caudofemoralis passes above both flexor cruris as it does not have the pars pelvica, being possible to remove both muscles without removing the CF.
Another possible scenario is that the proximal belly of the FCM described by Hudson (1937, 1948) for Falco and by Berger (1956) for Polihierax semitorquatus might be, in fact, the FCL. And the only difference resides in the topography regarding the m. caudofemoralis. A similar condition occurs with the topography of the tendon of insertion between the m. ambiens and the m. iliofibularis in Falconidae (Hudson 1937, 1948; Mosto et al. 2013; Mosto 2014). In most birds, the tendon of insertion of the m. ambiens is medial to that of the m. iliofibularis being unique in Falconidae, where the converse situation occurs (Hudson 1937). Even within the family there are several arrangements of both tendons, for example, in Caracara plancus the tendon of the m. ambiens crosses laterally the tendon of the IF, whereas in Falco femoralis both tendons run parallel to each other (Mosto 2014).
The discussion about the correct identification of this belly is kept open, the methodology used here is not sufficient and a possible solution would be to study its embryogenesis to elucidate this problematic.
Form and function are related (Bock 1965) and the hindlimb of the family Falconidae is another example of this statement. With the results gather in Mosto et al. (2016) and this work, it is conclusive that the morphological pattern of the hindlimb of the three subfamilies seems to be related to their habits. In Polyborinae, the myological features (e.g. predominance of the extensor of the femur) of the hindlimb are according to their terrestrial locomotion rather than an aerial pursuit. Whereas those who need to grip prey, Falconinae and Herpetotherinae, have a greater development of the muscles that flex the digits.
References
Backus SB, Sustaita D, Odhner LU, Dollar AM (2015) Mechanical analysis of avian feet: multiarticular muscles in grasping and perching. R Soc Open Sci 2:140350. doi:10.1098/rsos.140350
Baumel JJ, King SA, Breazile JE, Evans HE, Vanden Berge JC (1993) Handbook of avian anatomy. Publication of the Nuttal Ornitological Club N° 23, Cambridge
Bennett MB, Stafford JA (1988) Tensile properties of calcified and uncalcified avian tendons. J Zool 214:343–351
Berger AJ (1952) The comparative functional morphology of the pelvic appendage in three genera of Cuculidae. Am Midl Nat J 47:513–605
Berger AJ (1956) The appendicular myology of the Pygmy Falcon (Polihierax semitorquatus). Am Midl Nat J 55:326–333
Bierregaard RO (1994) Crested Caracara. P. 250 in del Hoyo J, Elliott A, Sargatal J (eds) Handbook of birds of the world. Vol. 2. New World vultures to guineafowl. Lynx Edicions, Barcelona
Biondi LM, Bó MS, Favero M (2005) Dieta del chimango (Milvago Chimango) durante el periodo reproductivo en el sudeste de la provincia de Buenos Aires, Argentina. Ornitología Neotropical 16:31–42
Bó MS, Baladrón A, Biondi LM (2007) Ecología trófica de Falconiformes y Estrigiformes: Tiempo de síntesis. Hornero 22:97–115.
Bock WJ (1965) The role of adaptive mechanisms in the evolution of higher levels of organization. Syst Zool 14:272–287
Bock WJ (1974) Philosophical foundations of classical evolutionary classification. Syst Zool 22:375–392
Brown LH, Amadon D (1968) Eagles, hawks, and falcons of the world. Country Life Books, London
Cade TJ (1982) Falcons of the world. Cornell University Press, Ithaca, NY
Conroy RM, Weigl PD, Clark JC, Ward AB. 1997. Functional morphology of owl hindlimbs: implications for prey selection and resource partitioning. Am Zool 37:37 A
Dickinson EC (2003) The Howard and Moore Complete Checklist of the Birds of the World. Third ed [M]. Princeton University Press, London, p 606
Dunning JB (2008) Handbook of avian body masses. CRC, Boca Raton
Engles WL (1940) Structural adaptations in the Thrashers. University of California publications. Zoology 42(7):341–400
Fisher HI (1957) The function of m. depressor caudae and m. caudofemoralis in pigeons. Auk 7:479–486
Fuchs J, Chen S, Johnson JA, Mindell DP (2011) Pliocene diversification within the South American forest-falcons (Falconidae: Micrastur). Molecular Phylogenetic. Evol Int J org Evol 60:398–407
Fuchs J, Johnson JA, Mindell DP (2012) Molecular systematic of the caracaras and allies (Falconidae: Polyborinae) inferred from mitochondrial and nuclear sequence data. Ibis 154:520–532
Fuchs J, Johnson JA, Mindell DP (2015) Rapid diversification of falcons (Aves: Falconidae) due to expansion of open habitats in the Late Miocene. Mol Phylogenet Evol 82:166–182
Goslow GE (1967) The functional analysis of the striking mechanisms of raptorial birds. Ph.D. University of California, dissertation
Gatesy SM (1999) Guineafowl hindlimb function. II. Electromyographic analysis and motor pattern evolution. J Morphol 240:127–142
Gatesy SM, Dial KP (1993) Tail muscle activity patterns in walking and flying pigeons (Columba livia). J Exp Biol 176:55–76
George JC, Berger AJ (1966) Avian myology. Academic, New York, p 500
GRIN (Global Raptor Information Network) (2016) Species account. http://www.globalraptors.org/grin/indexAlt.asp
Harcourt-Brown N (2001) Radiographicmorphology of the pelvic limb of Falconiformes and its taxonomic implications. Netherlands. J Zool 51:155–178
Hartman FA (1961) Locomotor mechanisms of birds. Smithsonian Misc Collect 143:1–91
Hudson GE (1937) Studies on the muscles of the pelvic appendages in birds. Am Midl Nat J 18:1–108
Hudson GE (1948) Studies on the muscles of the pelvic appendage in birds II: The heterogeneous order Falconiformes. Am Midl Nat J 39:102–127
Ilyinsky VA (2008) Locomotor adaptations in the hindlimbs of owls: the Burrowing Owl (Athene cunicularia), compared to the Little Owl (Athene noctua). Oryctos 7:271–276.
Jacobson RD, Hollyday M (1982) A behavioral and electromyographic study of locomotion in the chick. J Neurophysiol 48:238–256
Jollie M (1976) A contribution to the morphology and phylogeny of the Falconiformes I. Evol Theory 1:285–298
Jollie M (1977a) A contribution to the morphology and phylogeny of the Falconiformes. Pt. II. Evol Theory 2:115–208
Jollie M (1977b) A contribution to the morphology and phylogeny of the Falconiformes. Pt. III. Evol Theory 2:209–300
Jollie M (1977c) A contribution to the morphology and phylogeny of the Falconiformes. Pt. IV. Evol Theory 3:1–142
Lieber RL (2002) Skeletal muscle structure, function, and plasticity: the physiological basis of rehabilitation. Lippincott Williams & Wilkins, Philadelphia, p 369
Mckitrick MC (1986) Individual variation in the flexor cruris lateralis muscle of the Tyrannidae (Aves: Passeriformes) and its possible significance. J Zool 209:251–270
Mckricit MC (1993) Trends in the evolution of hindlimb musculature in aerial-foraging birds. Auk 110(2):189–206
Mosto MC (2014) Estructura y función del complejo apendicular posterior en rapaces diurnas (Falconidae y Accipitridae). PhD Thesis. La Plata: Facultad de Ciencias Naturales y Museo. II volumes 240 p. http://naturalis.fcnym.unlp.edu.ar/id/20140603001348
Mosto MC, Tambussi CP (2014) Qualitative and quantitative analysis of talons of diurnal bird of prey. Anat Histol Embryol 43:6–15
Mosto MC, Carril J, Picasso MBJ (2013) The hindlimb myology of Milvago chimango (Polyborinae, Falconidae). J Morphol 274:1191–1201
Mosto MC, Picasso MBJ, Biondi LM (2016) Long-legged Caracaras: terrestrial habitat and hindlimb morphology. J Zool 298:274–284
Picasso MBJ (2015) Ontogenetic Scaling of the Hindlimb Muscles of the Greater Rhea (Rhea americana) Anatomia, Histologia. Embryololgia 44:452–459
Spring L (1965) Climbing and pecking adaptation in some North American woodpeckers. Condor 67(6):457–488
Stoessel A, Kilbourne BM, Fischer MS (2013) Morphological integration versus ecological plasticity in the avian pelvic limb skeleton. J Morphol 274:483–495
Sustaita D (2008) Musculoskeletal underpinnings to differences in killing behavior between North American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). J Morphol 269:283–301
Thorstrom R (2000) The food habits of sympatric forest-falcons during the breeding season in northeastern Guatemala. Raptor Res 34(3):196–202
Vanden Berge J, Storer W (1995) Intratendinuos Ossification in birds: a review. J Morphol 226:47–77
Ward AB, Weigl PD, Conroy RM (2002) Functional morphology of raptor hindlimbs: implications for resource partitioning. Auk 119:1052–1063
White CM, Olsen PD, Kiff LE (1994) Family Falconidae (Falcons and Caracaras). In: del Hoyo J, Elliott A, Sargatal J, eds. Handbook of the birds of the World, Vol. 2: New World Vultures to Guinea fowl. Lynx Editions, Barcelona, pp 216–277
Acknowledgements
This paper was possible thanks to the access of materials by Yolanda Davies (Museo Bernadino Rivadavia, MACN), GP Guillermo Gil and Tec. Marcelo Cavicchia (Parque Nacional Nahuel Huapi PN° 1380). Thanks to Mariana Picasso for always giving a detailed reading and suggestions for the improvement of this paper, to Erica San Martín for the improvement of the English and Guillermo Cassini for his guidance on some of the contents of this MS and for his (I assume) great patience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mosto, M.C. Comparative hindlimb myology within the family Falconidae. Zoomorphology 136, 241–250 (2017). https://doi.org/10.1007/s00435-017-0343-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-017-0343-1