Abstract
The structure of the filtration apparatus of Entoprocta has been mainly investigated by light microscopy. The ultrastructure of the tentacles has been investigated in detail in only one species, Loxosomella elegans, and the ultrastructure of the cells of the vestibular groove has not been described at all. In this paper, the structures of the filtration apparatuses of two species are studied: the colonial species Pedicellina cernua (Pallas, 1774) (Entoprocta, Coloniales) and the solitary species Loxosomella nordgaardi Ryland 1961 (Entoprocta, Solitaria). The ultrastructure of the epidermis of the tentacles, as well as sensitive organs, muscles and nerve fibers, is described in detail. A comparison of the structures of tentacles of different species facilitated distinguishing two possible variants of organization of the tentacle—one with the frontal groove and one without the frontal groove, depending on the size of the calyx and tentacles. Some differences are also noted in the ultrastructure of the abfrontal cells of the tentacles in colonial and solitary species, which possibly determine the differences in the process of contraction of the tentacles. In some cases, the tentacles only bend without changing their length, and in other cases, they also shorten. The ciliary vestibular groove lies at the base of the tentacles. It was noted that the ciliary rows of the tentacles continue as the ciliary cells of the rims and walls of the groove. The ultrastructure of the cells of the rim of the groove is similar to the ultrastructure of the lateral cells of the tentacles. The cells of the walls of the groove are similar to the frontal cells of the tentacles. The cells of the bottom of the groove differ from all types of cells of the tentacles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entoprocta (Kamptozoa) is a small group of sessile invertebrates that feed on small food particles captured during filtration. The structure of the filtration apparatus and feeding mechanism of Entoprocta are well studied, but only for several species, mainly by light microscopy (Atkins 1932a; Brien 1959; Mariscal 1965; Riisgard et al. 2000). The main role in filtration belongs to the ciliated tentacles, which form an unclosed ring along the edge of the calyx. The number of tentacles varies in different species from 6 to 30 or even more (Nielsen 1989, 1996; Borisanova and Potanina 2016; Borisanova et al. 2018). The number of tentacles increases during the growth of the calyx, with new tentacles developing on the aboral side of the body on both sides of the anal cone (Atkins 1932a; Hyman 1951; Brien 1959; Nielsen 1964). The ultrastructure of the tentacles has been described in detail only for Loxosomella elegans (Nielsen and Rostgaard 1976; Nielsen and Jespersen 1997). Additionally, Emschermann (1982) provided a scheme of the fine structure of the tentacle but without a detailed description.
An important role in the process of the transfer of food particles belongs to the ciliary vestibular groove passing along the base of the tentacles (Atkins 1932a; Hyman 1951; Mariscal 1965; Nielsen and Jespersen 1997). The vestibular groove begins on each side of the anal cone, continues along the edge of the calyx, and leads into the mouth. The fine structure of the vestibular groove has never been examined.
Here, the fine structures of the tentacles and vestibular grooves of the colonial species Pedicellina cernua (Pallas, 1774) and the solitary species Loxosomella nordgaardi Ryland 1961 are investigated using transmission electron microscopy. The new data can help clarify the general organization of the tentacle crown in Entoprocta.
Materials and methods
The material was collected in the Velikaja Salma Bay (“Great Salma strait”) of the Kandalaksha Bay in the White Sea by diving at a depth 5–25 m in July–August, 2017–2019. Fragments of colonies of Pedicellina cernua were collected from the shells of the bivalve Mytilus edulis (L., 1758), specimens of Loxosomella nordgaardi were collected from the cystids of the bryozoans Electra sp. and Scrupocellaria sp., living on M. edulis. All specimens were relaxed in a solution of 7% MgCl2 before fixation.
For light and electron microscopy, samples were fixed in a solution of 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (PBS) and postfixed in a solution of 1% OsO4 in PBS. The samples were then dehydrated in an ethanol series of increasing concentration, in 96% ethanol mixed with acetone, and in pure acetone. For scanning electron microscopy (SEM), the dehydrated material was transferred into liquid CO2 and critical-point dried. The dried specimens were sputter-coated with platinum–palladium and examined with JEOL JSM 6380LA and Cambridge Instruments CamScan S2 scanning electron microscopes. For light and transmission electron microscopy (TEM), the dehydrated samples were embedded in epoxy resin and then cut on an ultratome (Leica EM UC6). Histological sections were stained with methylene blue solution and mounted in an epoxy resin. The sections were photographed with the Leica DM2500 microscope. Ultrathin sections were stained for 40 min with saturated uranyl acetate and for 7 min with lead citrate and then examined and photographed with JEOL JEM-1011 and JEM 1400-Flash transmission electron microscopes.
Results
Pedicellina cernua
Tentacles
The number of tentacles in the investigated zooids of P. cernua varies from 10 in young zooids to 16 in large zooids, and usually the calyx carries 14 tentacles (Fig. 1a, b). The tentacles are 200–250 μm in length, which is comparable to the length of the calyx. The bases of the tentacles are connected by a tentacular membrane (Fig. 1b, c).
On the transverse sections, the tentacles are irregularly trapezoidal, with the largest width near the frontal surface (Fig. 2a, b). The width of the tentacles in the middle part is approximately 15–20 μm, but on the frontal side, it increases to 25–35 μm. The height of the tentacles from the abfrontal to frontal side is approximately 30 μm. The frontal surface is formed by five rows of ciliated cells—the central row of frontal cells, with one row of laterofrontal cells and one row of lateral cells on each side. The frontal surface of the tentacle usually forms a frontal groove. The depth of the frontal groove gradually increases from the apex of the tentacle (depth 1.5–2 μm) to the base of the tentacle (depth approximately 5 μm). In some cases, the frontal groove is not distinguishable, and the frontal surface is just slightly concave.
Structure of the frontal surface of the tentacles of Pedicellina cernua. a Histological transverse section of the tentacles showing the lengths of the frontal cilia (fr), laterofrontal cilia (lf) and lateral (lat) cilia. b Transverse section of the tentacle. c Protocuticle (pr) of the frontal surface of the tentacle penetrated by cilia (ci) and microvilli (mv). d Cuticle (c) of the lateral surface of the tentacle with capitate swellings (cs) formed by microvilli (mv). e Frontal cell of the tentacle. f Laterofrontal cell of the tentacle. g Myofilaments (fil) of a laterofrontal cell (lfc) in a longitudinal section through the tentacle. h Myofilaments (fil) of a laterofrontal cell (lfc) in a transverse section through the tentacle. i Lateral cell of the tentacle. j Apical part of the lateral cell with rows of kinetosomes (k). amc ameboid cell of body cavity, an abfrontal tentacle nerve, asc abfrontal cell, c cuticle, ci cilia, cs capitate swellings, ds desmosoma, fc frontal cell, fg frontal groove, fil myofilaments of laterofrontal cell, fr cilia of frontal cell, k kinetosoma, lat cilia of lateral cell, lc lateral cell, lf cilia of laterofrontal cell, lfc laterofrontal cell, lfn laterofrontal tentacle nerve, lsc cell of lateral surface of tentacle, m muscle, mt mitochondria, mv microvilli, n nucleus, pr protocuticle, str striated roots, tc tentacle cavity
The frontal surface is covered with a protocuticle, which is a thin (0.15–0.2 μm) layer of a fibrous matrix with thin fibers (Fig. 2c). The fibers of the protocuticle are separated from the apical surface of the frontal cells by a gap of approximately 0.4–0.45 μm. The lateral and frontal surfaces of the tentacles are covered with a denser and thicker (0.3–0.5 μm) cuticle layer (Fig. 2d).
Frontal cells are triangular on transverse sections, with a concave apical surface, which usually forms a frontal groove (Fig. 2b, e). A nucleus lies in the central part of the frontal cell. The nucleus is oval shaped, 4.5 μm in length and 3 μm in width, with peripherical heterochromatin. The apical part of the cells has numerous mitochondria and bears microvilli and cilia that pass through the protocuticle. The length of the cilia is approximately 6 μm. The distance between the kinetosomes of the cilia (on transverse sections) is approximately 0.3–0.35 μm. Striated roots stretch from the kinetosomes towards the nucleus. Laterofrontal cells are elongated on transverse sections and slightly narrowed at the base (Fig. 2f). A nucleus lies closer to the basal part of this cell type. It is oval shaped, 3.5 μm in length and 2 μm in width, with a large nucleolus. The cytoplasm contains a well-developed granular endoplasmic reticulum, with rounded mitochondria located mainly around the nucleus and in the apical part of the cell. The cilia of laterofrontal cells are approximately 12 μm in length and arranged at a distance of 0.25–0.35 μm from each other. Kinetosomes have long striated roots that stretch toward the nucleus and short lateral roots the stretch to the adjacent kinetosomes. Two bundles of myofilaments lie on the sides in the apical part of the laterofrontal cells (Fig. 2e–h). The bundles are oval or round on the transverse section and 1.3–1.5 μm in diameter. The diameter of the filaments is 20–27 nm. Laterofrontal cells are connected to frontal and lateral cells by desmosomes. Lateral cells are oval-prismatic in transverse sections (Fig. 2i). A roundish nucleus of 4 μm in a diameter lies in the basal part of the cell, containing karyoplasma with zones of heterochromatin and a nucleolus. The cytoplasm is dense, with numerous large oval or rounded mitochondria. The cilia are long (20–30 μm) and densely located (the distance between the kinetosomes of the cilia is approximately 0.2 μm). Long striated roots depart from the kinetosomes in the basal direction (Fig. 2i). Lateral roots extend to the kinetosomes of adjacent cilia so that rows of connected cilia are located across the longitudinal axis of the tentacles (Fig. 2j). The rows of basal bodies located one under another along the length axis of the tentacle are not connected by roots.
The epidermis of the lateral surfaces of the tentacle is formed by nonciliated flattened epithelial cells 1.6–2.3 μm in height (Fig. 3a). A rounded nucleus lies in the expanded part of the cell. The diameter of the nucleus is approximately 3 μm, and it includes karyoplasma with peripheral heterochromatin. The cytoplasm contains vacuoles with light contents and rare mitochondria. The apical surface carries numerous microvilli that pass through the entire cuticular layer and form capitate swellings on the surface of the cuticle (Fig. 2d). The cells of the lateral surfaces of the tentacles are connected to neighboring cells by desmosomes and interdigitations. The epidermis of the abfrontal surface of the tentacle is formed by rows of specialized cells, with a strongly swollen cytoplasmatic outgrowth protruding into the cavity of the tentacle (Fig. 3b). The height of the cells in the zones of contact with the cells of the lateral surface is only 1 μm, while the height of the outgrowth is 7–9 μm. A small round nucleus with a diameter of approximately 2.5 μm lies in the basal part of the cytoplasmatic outgrowth. The cytoplasm contains a well-developed granular endoplasmic reticulum and many vacuoles of different sizes filled with transparent contents. Microvilli extend from the apical surface, pass through the cuticle layer and form capitate swellings. The abfrontal cells are connected to each other by desmosomes and interdigitations. The abfrontal surface of each tentacle carries a row of sensitive organs located at a distance of approximately 30–40 μm from each other (Figs. 1c, 3c, d). The cells are bulbous, and they protrude into the body cavity but form a single layer with epidermal cells and are separated from the body cavity by a basal lamina (Fig. 3e). The apical surface of the sensitive cells forms an invagination (Figs. 3e, f) and carries microvilli and seven to eight cilia (Fig. 3d). The cytoplasm of a sensitive cell is denser than that of neighboring epidermal cells and contains a well-developed endoplasmic reticulum and mitochondria in the apical part of the cell. Sensitive cells are connected to adjacent epidermal cells by desmosomes (Fig. 3f).
Structure of the lateral and abfrontal surfaces (a, b), sensitive organs (c–f), and body cavity (g–k) of the tentacles of Pedicellina cernua. a Nonciliated cell of the lateral surface of the tentacle. b Nonciliated cell of the abfrontal surface of the tentacle, or abfrontal cell. c Fronto-lateral view of the tentacle with easily visible cilia of sensitive organs (so) on the abfrontal surface. d Cilia of the sensitive organ. e Ultrastructure of the cell of the sensitive organ. f Apical part of the sensitive cell with cilia (ci), microvilli (mv) and cell contacts (ds). g Ameboid cell of the tentacle cavity with a well-developed granular endoplasmic reticulum (epr). h Muscle fibers (m) of the tentacle surrounded by the basal lamina (bl). i Part of the muscle cell with a nucleus (n), mitochondria (mt) and periferical myofilaments (mf). j Abfrontal tentacle nerve (an) in the tentacle cavity. k Laterofrontal tentacle nerve (lfn) near the lateral cell (lc). amc ameboid cell of body cavity, an abfrontal tentacle nerve, asc abfrontal cell, bl basal lamina, c cuticle, ci cilia, ds desmosoma, epr endoplasmic reticulum, lc lateral cell, lfn laterofrontal tentacle nerve, lsc cell of lateral surface of tentacle, m muscle, mf myofilaments of muscle fiber, mt mitochondria, mv microvilli, n nucleus, nf nerve fiber, so sensitive organ, tc tentacle cavity, va vacuole
The tentacle cavity is filled with a loose fibrous extracellular matrix (Fig. 2b). The diameter of the body cavity inside the tentacles is 10–15 µm. Ameboid cells are found in the body cavity (Fig. 3g). A round nucleus lies in the central part of the cytoplasm. It is 2–2.7 μm in a diameter, with clumps of peripheral heterochromatin. The cytoplasm contains many vacuoles of different sizes and a developed granular endoplasmic reticulum. Two bundles of muscle fibers pass along the lateral sides of the tentacle close to the frontal surface (Figs. 2b, 3a). Each muscle bundle consists of one or several muscle fibers with a diameter of 0.5–2 μm. The bundle is surrounded by a basal lamina of up to 60 nm thick. The cytoplasm is filled with myofilaments (Fig. 3h). The diameter of the filaments is 22–32 nm. At the base of the tentacles, the profile of muscle cells was found, which can be part of the tentacle muscle fiber. This profile contains a nucleus and mitochondria in the central part and myofilaments in one of the peripheral zones (Fig. 3i). In the central part of the body cavity of the tentacle is located the abfrontal tentacle nerve. It includes several (3–5) nerve processes surrounded by a basal lamina (Figs. 2b, 3j). Paired laterofrontal tentacle nerves lie near the basal part of the lateral cells (Figs. 2b, 3k). These nerves are intraepithelial, and on transverse sections, processes are separated from the body cavity by the same basal lamina that separates the lateral cells. The nerve processes are round or slightly elongated, with a 0.5–0.7 μm diameter. Microtubules and membrane vesicles are visible on the transverse sections of the nerve processes.
Vestibular groove
The ciliary vestibular groove lies at the base of the tentacles (Fig. 4a). The two branches of the vestibular groove begin on the sides of the anal cone, pass along the edge of the calyx and pass into the mouth opening. The ciliary cells of the inner rim of the vestibular groove are a continuation of the proximal rows of the lateral cells of two aboral tentacles located on the sides of the anal cone (Fig. 4a). The ciliary cells of the outer rim of the groove are connected to the lateral ciliary rows of each tentacle. The whole ciliary structure of the rims of the vestibular groove is shaped like an almost-closed horseshoe. At the base of the tentacles, the ciliary rows of neighboring tentacles form small basal crescent-shaped ciliary ridges (Fig. 4a, b). The frontal ciliary surfaces of the tentacles descend to the bottom of the vestibular groove.
Vestibular groove of Pedicellina cernua. a Top view of an atrial cavity of a calyx with a vestibular groove (vg) on the periphery. b Lateral view of the vestibular groove (vg). c Histological oblique-frontal section of the calyx, where the vestibular groove (vg) is shallow. d Histological oblique-frontal section of the calyx, where the vestibular groove (vg) is deep. e Ultrastructure of the cells of the vestibular groove (vg). f Cell of the rim of the vestibular groove. g Cell of the inner wall of the vestibular groove. h Cell of the bottom of the vestibular groove. ac anal cone, bc cell of bottom of vestibular groove, ci cilia, cr ciliary ridge, ds desmosoma, es esophagus, fg frontal groove, ir inner rim of vestibular groove, iw inner wall of vestibular groove, mo mouth, mv microvilli, mt mitochondria, n nucleus, or outer rim of vestibular groove, ow outer wall of the vestibular groove, pr protocuticle, prx proximal row of lateral cells of aboral tentacle, str striated roots, t tentacle, va vacuole, vg vestibular groove
The vestibular groove is triangular and V-shaped in the longitudinal sections through the calyx (Fig. 4c–e). The depth of the groove increases in the direction from the aboral side of the calyx to the oral side. The groove is absent opposite to the anal cone, between the aboral tentacles. It is very shallow on the sides of the anal cone (3–4 µm), but the depth gradually increases as it approaches the mouth opening and reaches 20 µm (Fig. 4c, d). The width of the groove is approximately 20 µm in the upper (expanded) part and approximately 3 µm near the bottom. The epidermis of the vestibular groove is covered with a protocuticle penetrated by microvilli and cilia (Fig. 4f–h). The epidermis of the groove is represented by prismatic cells of 8–10 μm in height, with large nuclei in the basal part of the cells. The nuclei contain heterochromatin and large nucleoli (Fig. 4e–h). The cells of the upper border of the groove contain many mitochondria in the apical part of the cytoplasm and closely located cilia (the distance between neighboring kinetosomes is approximately 250 nm). Striated roots extend from the kinetosomes deep into the cell in the lateral direction. The cells of the vestibular groove contain mitochondria and vacuoles in the apical zone of the cells, with a well-developed endoplasmic reticulum. The cilia are located at a distance of 350–400 nm from each other. The ciliary cells of the bottom of the groove are lower than the cells of the walls of the groove, at only 4–5 μm in height (Fig. 4e, h). The nuclei are round, at approximately 4 μm in diameter, with heterochromatin. The cytoplasm contains a well-developed endoplasmic reticulum and many mitochondria in the apical zone. The cilia are located at a distance of 500–600 nm from each other. The cells of the groove are connected to each other by desmosomes.
Loxosomella nordgaardi
Tentacles
The calyx of L. nordgaardi always bears eight tentacles. The length of the expanded tentacle is 100–120 μm (with the length of the calyx 150–200 μm) (Fig. 5a), while the length of the contracted tentacle can be from 80 to 40 μm (Fig. 5b–d).
Light photographs (a, b) and scanning electron micrographs (c, d) of Loxosomella nordgaardi. a Abfrontal view of an alive specimen with expanded tentacles. b Lateral view of an alive specimen with partly contracted tentacles. c Laterofrontal view of a specimen with partly contracted tentacles. d Top view of a tentacle crown. b bud, ca calyx, es esophagus, lat cilia of the lateral cell, st stalk, t tentacle
The tentacles are oval-triangular in the cross section, 10–12 μm in width near the abfrontal surface and 16–20 μm in width near the frontal surface (Fig. 6a, b). The frontal surface is straight or slightly concave and does not form a frontal groove (Fig. 6a–c). The frontal surface of the tentacles is covered with a protocuticle, and the lateral and abfrontal surfaces of the tentacles are covered with a fibrous cuticle (Fig. 6d, e). The thickness of the protocuticle is 30–35 nm. The cuticle thickness on the lateral sides is approximately 350 nm and that on the abfrontal surface is 700–800 nm. The frontal cells are triangular, without the frontal groove (Fig. 6f). A nucleus is large and elongated along the apical–basal axis of the cell, at approximately 5.5 μm in length, with clumps of heterochromatin. The cytoplasm of the cell is transparent and contains mitochondria in the apical part of the cell. The apical surface of the cell carries microvilli and cilia that penetrate the protocuticle. Long striated roots extend from the kinetosomes of the cilia deep into the cell, and short roots extend to the neighboring kinetosomes. The distance between the kinetosomes of adjacent cilia is 0.32–0.33 μm. The laterofrontal cells are elongated and slightly narrowed at the base and at the apical part (Fig. 6b, c). A large oval nucleus lies closer to the base of the cell, and it is approximately 3.5–4.5 μm in length and contains heterochromatin. Long striated roots extend from the kinetosomes to the nucleus and to adjacent cilia. The distance between the cilia is approximately 0.27 μm. In the apical zone, two thin, weakly expressed bundles (200–250 nm in diameter) of myofilaments are visible (Fig. 6f, g). The lateral cells are elongated and slightly expanded in the apical part (Fig. 6b, c). An oval nucleus lies in the basal part of the cell. It is 4–5 μm in length, containing karyoplasma with heterochromatin. The cytoplasm contains many vacuoles of different sizes, with large mitochondria in the apical zone and all over the cell. The cilia are located close to each other, with a distance between the kinetosomes of approximately 0.22 μm (Fig. 6h, i). The length of the cilia is 11–14 μm (Fig. 5b–d). Long striated roots stretch toward the basal part of the cell, and each cilium is connected by short lateral roots to the cilium on the left, on the right and under it so that transverse double rows are formed (Fig. 6i). The lateral cells are connected to the laterofrontal cells and to the cells of the lateral surface of the tentacles by desmosomes (Fig. 6g, h).
Structure of the frontal surface of the tentacles of Loxosomella nordgaardi. a Transverse section near the base of the tentacle. b Transverse section in the middle part of the tentacle. c Frontal surface of the tentacle. d Protocuticle (pr) of the frontal surface of the tentacle penetrated by cilia (ci) and microvilli (mv). e Cuticle (c) of the lateral surface of the tentacle with capitate swellings (cs) on the surface. f Frontal cell of the tentacle. g Apical part of the laterofrontal cell of the tentacle with bundles of myofilaments (fil). h Lateral cell of the tentacle. i Apical part of the lateral cell with prominent double rows (dr) of cilia. am atrial muscle ring, asc abfrontal cell, c cuticle, ci cilia, cs capitate swellings, dr double row of cilia, ds desmosoma, fc frontal cell, fil myofilaments of laterofrontal cell, k kinetosoma, lfc laterofrontal cell, lc lateral cell, lr lateral roots, lsc cell of lateral surface of tentacle, m muscle, mt mitochondria, mv microvilli, n nucleus, pr protocuticle, str striated roots, tc tentacle cavity
The cells of the lateral surface of the tentacle are low, 2.5–3 μm in height, with a central part protruding into the cavity of the tentacle. A nucleus is located in the protrusion. It is 4 μm in diameter, with noticeable heterochromatin zones in the center and on the periphery (Figs. 6a, 7a). The cytoplasm contains vacuoles of different sizes. The cells of the abfrontal surface are 6–7 μm in height (Fig. 6a, b). A rounded nucleus lies closer to the basal part of the cell. It contains noticeable heterochromatin zones (Fig. 7b). The cytoplasm is transparent, with vacuoles filled with fine-fibrous material. The abfrontal cells contact the adjacent cells through desmosomes (Fig. 7b). Sensitive organs are located only at the base and the lower part of the tentacles (Fig. 7c). Sensitive cells lie between the abfrontal cells of the tentacle. Each sensitive cell has a concave apical part (Fig. 7d, e) with a bundle of three to five cilia (Fig. 7c). The central cavity of the tentacles is small and sandwiched between the basal parts of the cells of the frontal and abfrontal surfaces (Fig. 6a–c). The cavity is filled with a large number of fine fibers and contains the profiles of the cells of the body cavity (Fig. 7f). Muscle fibers lie in the body cavity near the frontal surface of the tentacles between the lateral cells and the cells of the lateral surface of the tentacle (Fig. 6a, b). The diameter of the muscle fibers is approximately 1–2 μm. Numerous myofilaments with a diameter of approximately 25 nm fill almost the entire space of the cytoplasm of the muscle fibers (Fig. 7g). A basal plate (26–30 nm in thickness) separates the muscle fibers from the body cavity. Nerve fibers lie on two sides of the center of the tentacle, near the bases of lateral and laterofrontal cells (Fig. 7h, i). In some sections, it can be seen that one nerve fiber lies near the lateral cell, while the other lies under the frontal cell closer to the abfrontal side. In one of the sections, a bundle of two processes was found on the abfrontal surface of the tentacle between the abfrontal cells (Fig. 7j, k). Most likely, these were the nerve fibers of the abfrontal nerve associated with the sensitive cells that lie on the abfrontal side.
Structures of the lateral and abfrontal surfaces and body cavity of the tentacles of Loxosomella nordgaardi. a Nonciliated cell of the lateral surface of the tentacle. b Nonciliated cell of the abfrontal surface of the tentacle, or abfrontal cell. c Base of the tentacle with two sensitive organs. d Abfrontal surface of the tentacle with a sensitive organ (so). e Sensitive cell. f Tentacle body cavity with many fibers of the extracellular matrix (exm). g Muscle fibers (m) of the tentacle surrounded by the basal lamina (bl). h Laterofrontal tentacle nerves (lfn) near the lateral cells (lc). i Laterofrontal tentacle nerve. j Abfrontal tentacle nerve (an) between the abfrontal cells of the tentacles. k Abfrontal tentacle nerve. an abfrontal tentacle nerve, asc abfrontal cell, bl basal lamina, c cuticle, ci cilia, cs capitate swellings, ds desmosoma, exm extracellular matrix, fc frontal cell, lc lateral cell, lfc laterofrontal cell, lfn laterofrontal tentacle nerve, lsc cell of lateral surface of tentacle, m muscle, mt mitochondria, mv microvilli, n nucleus, nf nerve fiber, so sensitive organ, t tentacle, tc tentacle cavity, va vacuole
Vestibular groove
The vestibular groove is poorly expressed (Fig. 8a). The inner rim of the vestibular groove is a continuation of the lateral proximal ciliary rows of aboral tentacles and bears long cilia of up to 13 µm. The aboral tentacles contact closely at the base so that the inner rim of the groove looks annular. The outer rim of the groove is a continuation of the distal lateral ciliary rows of the aboral tentacles and the lateral ciliary rows of all other tentacles. Ciliary ridges are absent. The tentacle crown is directed disto-frontally, so the aboral side of the calyx is located higher than the oral side (Fig. 5b, c). This is why the vestibular groove is inclined, and closer to the aboral side of the calyx the inner wall is almost horizontal (Fig. 8b). The vestibular groove is flat, U-shaped, and shallow along almost its entire length, at only 6–6.5 µm in depth, and only near the mouth does it get a little deeper, at up to 10–12 µm (Fig. 8c, d). The width of the vestibular groove near the mouth is 20–24 µm in the upper part and 3–4 µm near the bottom (Fig. 8d). The epidermis of the vestibular groove bears cilia and microvilli (Fig. 8e). Differences between the cells of the inner wall, outer wall and bottom of the groove were not detected. The cells are 7–8 μm in height, with large nuclei, and 5–5.5 μm in length, containing heterochromatin and nucleoli. The cytoplasm contains vacuoles, cisterns of the endoplasmic reticulum, and many mitochondria (Fig. 8e). The cilia are located at a distance of 170–200 nm from each other. Striated roots reach deep down into the cell.
The vestibular groove of Loxosomella nordgaardi. a Top view of an atrial cavity of a calyx with the vestibular groove (vg) on the periphery. b Parasagittal section through the calyx showing the inclined position of the vestibular groove (vg). c Ultrastructure of the vestibular groove near the anal cone on frontal section through the calyx. d Ultrastructure of the vestibular groove near the mouth on parasagittal section through the calyx. e Cell of the outer wall of the vestibular groove. ac anal cone, ci cilia, epr endoplasmic reticulum, ir inner rim of vestibular groove, iw inner wall of the vestibular groove, mo mouth, mt mitochondria, n nucleus, ow outer wall of the vestibular groove, prx proximal row of lateral cells of aboral tentacle, t tentacle, va vacuole, vg vestibular groove
Discussion
Tentacles of different species of Entoprocta
The structures of the tentacles of Pedicellina cernua and Loxosomella nordgaardi generally correspond to the structures of the tentacles of previously studied species (Atkins 1932a; Mariscal 1965; Nielsen and Rostgaard 1976; Emschermann 1982; Nielsen and Jespersen 1997). The tentacles of all studied species have a nonciliary abfrontal surface and a ciliary frontal surface formed by five rows of cells: the frontal row, two laterofrontal rows and two lateral rows. Frontal cells carry short cilia, laterofrontal cells carry cilia of medium length, and lateral cells carry the longest cilia. Lateral cilia form single transverse rows in P. cernua and double rows in L. nordgaardi and Loxosomella elegans (Nielsen 1964; Nielsen and Rostgaard 1976). All studied species have myofilaments in the laterofrontal cells, which are involved in the contraction of the tentacles together with muscle fibers lying in the tentacle cavities. It should be noted that the laterofrontal cells differ from the lateral and frontal cells in their better-developed endoplasmic reticulum, which may be due to the need for the synthesis of myofilaments.
The frontal cells in the tentacles of P. cernua form the frontal groove. The same frontal groove was described in another colonial species, Barentsia discreta (Busk, 1886) (Borisanova 2018). In all earlier studies, the frontal groove was noted neither in solitary nor in colonial species, including P. cernua (Atkins 1932a: Fig. 3d) and B. discreta (Franzén 1973: Fig. 1g). However, on the figures of the tentacles of both these species, as well as the colonial species Barentsia gracilis (Sars, 1835) (Mariscal 1965: Fig. 4) and solitary Loxosomella crassicauda (Salensky, 1877) (Atkins 1932a: Fig. 3a), the frontal surface is drawn concave. Nickerson (1901) drew a pronounced frontal groove on the transverse section through the base of the tentacle in the species Loxosoma davenporti Nickerson, 1898 (Nickerson 1901: Fig. 10), but did not indicate it. It should be noted that the frontal groove is not shown in the figure of the transverse section of the middle part of the tentacle of L. davenporti (Nickerson 1901: Fig. 9). Most studied solitary species, for example, Loxosomella obesa (Atkins) (Atkins 1932a: Fig. 3b, c), L. elegans (Nielsen and Rostgaard 1976), L. nordgaardi (our data), Loxosomella unicornis Borisanova, Krylova 2014, Loxosomella angusta Borisanova, and Emschermannia ramificata Borisanova (our unpublished data), have straight or even slightly convex frontal surfaces of their tentacles. The frontal groove may develop in the large tentacles of species with a large calyx and wide atrial cavity. Colonial entoprocts are usually larger than those of solitary species, so they form a frontal groove more often. However, the frontal groove can also be formed in large solitary species, such as L. davenporti, with a calyx of up to 1.02 mm in length and bearing 16–25 tentacles (Nielsen 1966). In most solitary species, the calyxes are small, so the frontal groove is not pronounced. For example, the calyx of L. elegans is approximately 292 µm in length and bears 10–13 slender tentacles (Nielsen 1964); that of L. unicornis is 130–215 μm in length, with 8 tentacles (Borisanova and Krylova 2014); the calyx of L. angusta is 174–213 µm and bears 8 tentacles (Borisanova 2016a); and the calyx of E. ramificata is 160–200 μm in length, with 8 tentacles (Borisanova 2016b). L. obesa is a large species (up to 2.4 mm), but its calyx bears only eight (very rarely 9 or 10) short, small tentacles (Atkins 1932b). In species with medium-sized calyxes and tentacles, the frontal surface can be concave but lack the frontal groove. This would explain the absence of the frontal groove in tentacles of Barentsia and Pedicellina studied previously, if we assume that the zooids were young. Some tentacles of P. cernua investigated in this research also had only concavity, but not a groove. It can be assumed that the frontal groove can help transfer small food particles to the bases of the tentacles, which can be easily lost when moving over the surface of a relatively large tentacle.
The abfrontal surfaces of the tentacles in colonial and solitary species are different. In P. cernua, as well as in B. discreta (Borisanova 2018: Fig. 6.16), the cells of the abfrontal surface are swollen and highly vacuolated. In L. nordgaardi, the cytoplasm of the abfrontal cells also contains many vacuoles, but not as many as in colonial species. In L. elegans (Nielsen and Rostgaard 1976; Nielsen and Jespersen 1997), the cells of the abfrontal surface are not vacuolated and do not differ from the cells of the lateral surface. The differences in the organization of the abfrontal surface may lead to the differences in the contraction of the tentacles between colonial and solitary species. When irritated, the tentacles of Entoprocta bend and curl up in the atrial cavity (Atkins 1932a; Mariscal 1965; Emschermann 1982). Our observations show that the length of the tentacles in P. cernua does not change during this process, while in L. nordgaardi, the tentacles not only bend but also shorten. Perhaps, the vacuolated cells of the abfrontal surface in colonial species prevent the shortening of the tentacle during the contraction of the muscles lying near the frontal surface. It can also be assumed that the vacuolated abfrontal cells of colonial species help straighten tentacles after muscle relaxation due to elastic force.
The sensitive organs of the tentacles are organized in the same way in different species (Nielsen and Rostgaard 1976; Emschermann 1982; Borisanova et al. 2019; our data). These are unicellular organs lying between the cells of the abfrontal surface of the tentacle. The sensitive cell has a concave apical surface with cilia and microvilli. The sensitive cell is not covered with a cuticle.
According to previous studies (Nielsen and Jespersen 1997; Fuchs et al. 2006; Borisanova et al. 2019), there are three nerves in the tentacles: the unpaired abfrontal tentacle nerve and two laterofrontal tentacle nerves. Our data confirm the presence of three nerves in the tentacles of P. cernua. However, in L. nordgaardi, in most transverse sections, only two nerves can be distinguished in the tentacle cavity. This is probably due to the location of the sensitive organs. In L. nordgaardi, sensitive cells lie only in the lower parts of the tentacles. Since the abfrontal tentacle nerve is supposed to be connected to the sensitive organs, it is absent on the sections above the sensitive organs.
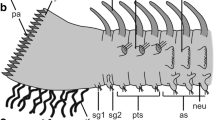
Taking into account all the data, two generalized schemes for organizing tentacles are offered—those with the frontal groove in the example of colonial species with swollen abfrontal cells and those without the frontal groove in the example of solitary species without swollen abfrontal cells (Fig. 9).
Schemes of the two types of structures of the tentacles. a Scheme of the tentacle with the frontal groove of the colonial species. b Scheme of the tentacle without the frontal groove of the solitary species. amc ameboid cell of body cavity, an abfrontal tentacle nerve, asc abfrontal cell, fc frontal cell, lc lateral cell, lfc laterofrontal cell, lfn laterofrontal tentacle nerve, lsc cell of lateral surface of tentacle, m muscle, tc tentacle cavity
Structure of the vestibular groove of Entoprocta
Food particles are captured by tentacles and transported along the frontal surface to the vestibular groove. The cilia of the vestibular groove carry particles to the mouth. The vestibular grooves of Pedicellina cernua and Loxosomella nordgaardi have some differences in structure. The groove of L. nordgaardi is shallower, flatter, has no clear differences between the cells of the walls and the bottom of the groove, and lacks the ciliary ridges described in P. cernua in zones of contact between the frontal surfaces of neighboring tentacles. However, the general organization of the vestibular groove is similar in different species. It is a horseshoe-shaped ciliary groove at the base of the tentacles which is connected to the ciliary rows of the tentacles (Atkins 1932a; Brien 1959; Nielsen and Rostgaard 1976; Emschermann 1982; Borisanova 2018) (Fig. 10). Nielsen and Jespersen (1997) noted that the cilia of the outer rim of the vestibular groove are formed by the continuation of the frontal cilia of tentacles, and the long cilia of the inner rim are a continuation of the lateral cilia of the aboral pair of tentacles located on both sides of the anal cone. Our ultrastructural data on P. cernua confirm that the cells of the inner rim of the groove are close in ultrastructure to the lateral cells of the tentacles, as well as the cells of the outer rim. These cells are similar in shape and have closely located cilia, and the cytoplasm contains many mitochondria all over the cell. The cells of the walls of the groove located below the border are more similar to the frontal cells in terms of the ultrastructure. Cilia are less common, and the cytoplasm contains mitochondria in the apical part. The cells of the bottom of the groove, apparently, are not directly connected to the ciliary cells of the tentacles.
Scheme of the structure of the vestibular groove of a Pedicellina cernua and b Loxosomella nordgaardi. ac anal cone, am atrial muscle ring, bc cell of bottom of vestibular groove, c cuticle, cr ciliary ridge, fg frontal groove, ir inner rim of vestibular groove, iw inner wall of the vestibular groove, mo mouth, or outer rim of vestibular groove, ow outer wall of the vestibular groove, pr protocuticle, prx proximal row of lateral cells of aboral tentacle, tm tentacle membrane, vg vestibular groove
References
Atkins D (1932a) The ciliary feeding mechanism of the Entoproct Polyzoa, and a comparison with that of the Ectoproct Polyzoa. Q J Microsc Sci 75:393–423
Atkins D (1932b) The loxosomatidae of the plymouth area including L. obesum sp. nov. Q J Microsc Sci 75:321–391
Borisanova AO (2016a) A new species of solitary Entoprocta, Loxosomella angusta sp.n., from the White Sea. Invertebr Zool 13:43–50
Borisanova AO (2016b) Emschermannia ramificata—a new genus and species of solitary entoproct from the Kara Sea, Russia. Zootaxa 4084:135–142
Borisanova AO (2018) Entoprocta (Kamptozoa). In: Schmidt-Rhaesa A (ed) Handbook of zoology. Miscellaneous invertebrates. De Gruyter, Berlin, pp 111–162
Borisanova AO, Krylova EM (2014) A new species of Loxosomatidae (Entoprocta, Solitaria) from the White Sea: Loxosomella unicornis sp. nov. Zootaxa 3861:290–296
Borisanova AO, Potanina DM (2016) A new species of Coriella, Coriella chernyshevi n. sp. (Entoprocta, Barentsiidae), with comments on the genera Coriella and Pedicellinopsis. Zootaxa 4184:376–382
Borisanova AO, Malakhov VV, Temereva EN (2019) The neuroanatomy of Barentsia discreta (Entoprocta, Coloniales) reveals significant differences between bryozoan and entoproct nervous systems. Front Zool 16:9
Borisanova AO, Chernyshev AV, Ekimova IA (2018) Deep-sea Entoprocta from the Sea of Okhotsk and the adjacent open Pacific abyssal area: new species and new taxa of host animals. Deep Sea Res Part II 154:87–98
Brien P (1959) Classe des Endoproctes ou Kamptozoaries. In: Pierre-P G (ed) Traité de Zoologie, vol 5. Masson et Cie, Paris, pp 927–1007
Emschermann P (1982) Les Kamptozoaires. État actuel de nos connaissances sur leur anatomie leur development, leur biologie et leur position phylogénétique. Soc Zool Fr 107:317–344
Franzén Å (1973) Some Antarctic Entoprocta with notes on morphology and taxonomy in the Entoprocta in general. Zool Scr 2:183–195
Fuchs J, Bright M, Funch P, Wanninger A (2006) Immunocytochemistry of the neuromuscular systems of Loxosomella vivipara and L. parguerensis (Entoprocta: Loxosomatidae). J Morphol 267:866–883
Hyman LH (1951) The invertebrates. Acanthocephala, Aschelminthes and Entoprocta. The pseudocoelomate Bilateralia, vol 3. McGraw-Hill, London
Mariscal RN (1965) The adult and larval morphology and life history of the Entoproct Barentsia gracilis (M. Sars, 1835). J Morphol 116:311–338
Nickerson WS (1901) On Loxosoma davenporti sp.nov. an entoproct from the New England coast. J Morphol 17:351–381
Nielsen C (1964) Studies on Danish Entoprocta. Ophelia 1:1–76
Nielsen C (1966) Some Loxosomatidae (Entoprocta) from the Atlantic coast of the United States. Ophelia 3:249–275
Nielsen C (1989) Entoprocts: keys and notes for the identification of the species. Synop Br Fauna (New Ser) 41:1–131
Nielsen C (1996) Three new species of Loxosoma (Entoprocta) from Phuket, Thailand, with a review of the genus. Zool Scr 25:61–75
Nielsen C, Jespersen Å (1997) Entoprocta. In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 13. Wiley-Liss, New York, pp 13–43
Nielsen C, Rostgaard J (1976) Structure and function of an entoproct tentacle with a discussion of ciliary feeding types. Ophelia 15:115–140
Riisgard HU, Nielsen C, Larsen PS (2000) Downstream collecting in ciliary suspension feeders: the catch-up principle. Mar Ecol Prog Ser 207:33–51
Acknowledgements
The author is grateful to the SCUBA-Diving Team of White Sea Biological Station for collecting the material. The author is thankful to Alexander Semenov for the photo of a living colony of Pedicellina cernua. The research was supported by the Russian Science Foundation (project no. 18-14-00082).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she does not have competing interests.
Ethics approval
The use of entoprocts in the laboratory does not raise any ethical issues, and therefore approval from regional and local research ethics committees is not required. The field sampling did not involve endangered or protected species. In accordance with local guidelines, permission for collection of material was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borisanova, A.O. Two types of the tentacle structure of Entoprocta and the fine structure of the vestibular groove. Zoomorphology 139, 433–445 (2020). https://doi.org/10.1007/s00435-020-00497-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-020-00497-y