Abstract
The study of the lophophore organization is of great importance for the reconstruction of lophophorate phylogeny and for understanding the evolutionary transformation in each phylum of Lophophorata. The innervation of the lophophore in ctenostome bryozoan Flustrellidra hispida was studied using immunocytochemistry and confocal laser scanning microscopy. It has been demonstrated that this species has an outer nerve ring giving rise to the tentacle nerves. The outer nerve ring was earlier described in some ctenostomates and cyclostomates, but not as connected with nerves. The discovered feature of lophophore innervation in F. hispida suggests the evolutionary transformation from a hypothetical phoronida-like ancestor lophophore bearing a prominent outer nerve ring with numerous tentacle nerves emanating from it, to the complex bell-shaped lophophore of F. hispida with a well-pronounced outer nervous ring bearing a few tentacle nerves. The next one in this hypothetical row is the lophophore of the other ctenostomates and some cyclostomates with no ring-nerve connection and cheilostomates lophophore with no outer nerve ring at all.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bryozoa is a distinct phylum of invertebrates with not yet identified position within the bilaterian tree. According to molecular phylogenetics, Bryozoa either stands apart from all other Lophotrochozoa, or, together with Kamptozoa and Cycliophora, form the Polyzoa clade [1, 2]. However, traditionally bryozoans are viewed as a part of a supraphylum Lophophorata, which also includes phoronids and brachiopods besides bryozoans. The Lophophorata monophyly is supported by a comparative anatomy analysis [3, 4] and a new data obtained from molecular genetic analysis [5, 6]. Characteristic feature of all lophophorates is a special tentacle organ called lophophore which probably emerged in a distant common ancestor of the Lophophorata and subsequently evolved differently in phoronids, brachiopods, and bryozoans [4]. Views on the evolutionary transformations of bryozoan lophophore are controversial [3, 7, 8].
Here, we studied the lophophore innervation in the ctenostomate bryozoan Flustrellidra hispida.
We used Flustrellidra hispida (Fabricius, 1780) colonies collected in the proximity of the White Sea Biological Station of Moscow State University (WSBS MSU). Zooids were relaxed in MgCl2 solution and then fixed in 4% paraformaldehyde in 0.1 М phosphate buffer. After washing with phosphate buffer containing 5% Triton-X100, the zooids were incubated in a block solution with 1% bovine serum albumine, 0.1% cold fish skin gelatin, 0.5% Triton-X100, and 0.05% Tween 20 for 24 h. The subsequently samples were incubated at +4°С for 72 h in the solution of primary antibodies against serotonin and tyrosinated α-tubulin. After block solution wash, the zooids were incubated with secondary antibodies, washed again, embedded in the mixture of benzyl alcohol and benzyl benzoate, and studied using a Nikon Eclipse Ti laser confocal microscope (Tokyo, Japan) at WSBS MSU. Z-projections were made using the ImageJ 1.43 software.
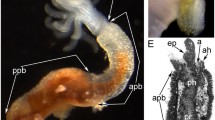
Flustrellidra hispida has a bell-form lophophore with 26 tentacles. Each tentacle is innervated by two longitudinal nerves—frontal and abfrontal (Fig. 1a). The abfrontal nerve is very large, made up of a great number of nerve fibers and connected with ciliated sensory organs located along the abfrontal side of each tentacle. The abfrontal tentacle nerve starts as two large radiuses from long thin intertentacular nerves stretching from the circumoral nerve ring. Each intertentacular nerve has a complex structure: it comprises both the nerve tracts emanating immediately from the cerebral ganglion and a large axon of a serotoninergic sensory cell located between tentacles. F. hispida has 24 such cells located on the inner side of the lophophore with sensory flagellum, however, going between the tentacles on the outer side of the lophophore. The circumoral nerve ring consists of two parts located one above the other (Fig. 1a). The lower part of the circumular nerve ring is formed by intertentacular nerves around the mouth partly merging with each other to form larger nerve tracts and turning into the lateral nerve plexus of the cerebral ganglion. The upper part of the circumoral nerve ring is connected with the cerebral ganglion via thin lateral radiuses; it is a large bundle of nerve fibers in the shape of a semicircle opened at the oral side of the body. Frontal tentacle nerves emanate from the upper part of the circumoral ring.
The innervation of tentacles in bryozoan Flustrellidra hispida. (a) A scheme of the lophophore nerve tracts based on immunocytochemistry staining using antibodies against serotonin and tyrosinated α-tubulin. The number of tentacles is reduced. (b) The maximum Z-projection of four slides of the stack from the oral side of the body. Stained by antibodies against. (c) The maximum Z-projection of four slides of the stack from the oral side of the body. Stained by antibodies against tyrosinated α-tubulin. Designations: (afn) abfrontal tentacle nerve, (cg) cerebral ganglion, (fn) frontal tentacle nerve, (itn) intertentacular nerve, (lco) lower region of the circumoral nerve ring, (ogp) groups of outer tentacle perikarya, (on) outer nerve ring, (otn) outer nerves, (uco) upper region of the circumoral nerve ring.
The outer nerve ring was discovered in F. hispida; it stretches along the outer perimeter of the lophophore (Fig. 1a). The outer nerve ring is formed by two or three nerve fibers exhibiting both serotoninergic (Fig. 1b) and tubulinergic immunoreactivities (Fig. 1c). The outer nerve ring is connected by thin serotoninergic neurits running on the outer side of the tentacle lamella to the groups of outer serotoninergic cells and further to one of the radiuses of the abfrontal tentacle nerve (Fig. 1b). These neurits are a part of abfrontal nerves and stretch along the whole tentacle.
The innervation of the Bryozoa lophophore has been studied in details in the representatives of the main groups: Phylactolaemata, Cyclostomata, and Gymnolaemata, including Ctenostomata and Cheilostomata [3, 7, 10, 11]. All studied species are characterized by a circumoral nerve ring connected with large intertentacular serotoninergic perikarya where the intertentacular nerves emanate from. The number of tenctacles may vary from two to six in the representatives of different groups. The most complex patterns of innervation are observed in phylactolaemata and cyclostomata bryozoans which have six nerve tracts in each tentacle: mediofrontal, medioabfrontal, two lateroabfrontal, and two laterofrontal.
Most gymnostomate bryozoans have four nerve tracts in each tentacle: mediofrontal, medioabfrontal, and two laterofrontal ones. Of all gymnostomate bryozoans two nerve tracts stretching along the tentacle (mediofrontal and medioabfrontal) are only described in ctenostomate Amathia gracilis. As shown in the present study, another ctenostomate bryozoan, F. hispida, also has only two nerve tracts in each tentacle. It is possible that two nerve tracts in tentacles is a common feature of tentacle innervation in Ctenostomata. Other lophophorates, such as phoronids and brachiopods, typically have six tentacle nerves. If the concept of the Lophophorata monophyly is correct, the reduction of tentacle nerve fibers in the Bryozoa should be viewed as a secondary phenomenon.
The peculiarity of the tentacle innervation in F. hispida is a pronounced outer nerve ring giving rise to serotoninergic neurits of the abfrontal tentacle nerves. The outer nerve ring was first described in A. gracilis [3] and then discovered in several representatives of cyclostomate bryozoans, namely, Crisia eburnea [9] and Cinctipora elegans [10]. It should be noted that in all mentioned bryozoan species the outer nerve ring does not take part in the tentacle innervation and is not connected with any tentacle nerves. Moreover, it does not give rise to any nerves at all. In this context, the outer nerve ring in F. hispida can be viewed as a much more complex structure than previously thought for other bryozoan species.
According to comparative anatomical analysis, the outer nerve ring in the Bryozoa can be viewed as homologous to the tentacle nerve ring of phoronids and lower brachial nerve of brachiopods [4, 12]. In both phoronids and brachiopods, these nerves participate in the tentacle innervation and give rise to abfrontal and lateroabfrontal tentacle nerves. If the concept of the Lophophorata monophyly is correct, then the presence of the outer nerve ring participating in the tentacle innervation should probably be viewed as ancestral state, whereas the reduction of the outer nerve ring and connected tentacle nerves is a secondary phenomenon possibly resulting from a decrease in the size of the body and the lophophore. In this case, evolutionary transformations of the bryozoan lophophore can be presented as the following hypothetical chain: (i) an ancestral, relatively complex lophophore with a large number of tentacles and two nerve rings, each giving rise to large tentacle nerves; (ii) the structure described in the present study for F. hispida: two nerve rings with the outer ring only giving rise to the thin neurits in tentacles and the inner ring responsible for the main tentacle innervation; (iii) the structure described for A. gracilis, C. eburnea, and C. elegans: the outer nerve ring is in no way connected with the tentacle innervation; (iv) the structure specific to most cheilostomates: complete lack of the outer nerve ring with the tentacles innervated only from the circumoral nerve ring.
On the other hand, the presence of only two nerve tracts in F. hispida tentacles suggests an originally small lophophore, whereas an increased number of tentacles is probably a secondary phenomenon which resulted in the formation of additional nerve tracts in this species. At the same time, the presence of the outer nerve ring in bryozoans with extremely small lophophore (A. gracilis, C. eburnea, and C. elegans) calls this assumption into question.
REFERENCES
Hejnol, A., Obst, M., Stamatakis, A., Ott, M., Rouse, G.W., Edgecombe, G.D., Martinez, P., Baguna, J., Bailly, X., Jondelius, U., and Wiens, M., Proc. Roy. Soc. B Biol. Sci., 2009, vol. 276, no. 1677, pp. 4261–4270.
Kocot, K.M., Struck, T.H., Merkel, J., Waits, D.S., Todt, C., Brannock, P.M., Weese, D.A., Cannon, J.T., Moroz, L.L., Lieb, B., and Halanych, K.M., Syst. Biol., 2017, vol. 66, no. 2, pp. 256–282.
Temereva, E.N. and Kosevich, I.A., BMC Evol. Biol., 2016, vol. 16, no. 1, pp. 1–24.
Temereva, E.N., Sci. Rep., 2017, vol. 7, pp. 1–16.
Marletaz, F., Peijnenburg, K., Goto, T., Satoh, N., and Rokhsar, D., Curr. Biol., 2019, vol. 29, no. 2. https://doi.org/10.1016/j.cub.2018.11.042
Zverkov, O.A., Mikhailov, K.V., Isaev, S.V., Rusin, L.Y., Popova, O.V., Logacheva, M.D., Penin, A.A., Moroz, L.L., Panchin, Y.V., Lyubetsky, V.A., and Aleoshin, V.V., Front. Genet., 2019, vol. 10, no. 443.
Schwaha, T.F. and Wanninger, A., BMC Evol. Biol., 2015, vol. 15, no. 223. https://doi.org/10.1186/s12862-015-0508-9
Schwaha, T.F., Ostrovsky, A.N., and Wanninger, A., Biol. Rev., 2020, vol. 95, pp. 696–729.
Temereva, E.N. and Kosevich, I.A., Front. Zool., 2018, vol. 15, no. 1, p. 48.
Schwaha, T.F., Handschuh, S., Ostrovsky, A.N., and Wanninger, A., BMC Evol. Biol., 2018, vol. 18, no. 1, p. 92.
Worsaae, K., Frykman, T., and Nielsen, C., Acta Zool., 2018, vol. 101, no. 2, pp. 133–146.
Temereva, E.N. and Kosevich, I.A., Invertebr. Zool., 2018, vol. 15, pp. 366–372.
Funding
The study was supported by the Russian Science Foundation, project no. 18-14-00082.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Deryabina
Rights and permissions
About this article
Cite this article
Isaeva, M.A., Kosevich, I.A. & Temereva, E.N. Peculiarities of Tentacle Innervation of Flustrellidra hispida and Evolution of Lophophore in Bryozoa. Dokl Biol Sci 496, 30–33 (2021). https://doi.org/10.1134/S0012496621010038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012496621010038