Abstract
Orussidae, a small family of parasitoid wasps targeting woodboring insect larvae, are characterized by having the ovipositor apparatus invaginated in the body and operated by a mechanism unique among Hymenoptera. The first and second valvulae when not in use lie in an invaginated ovipositor sack extending forward throughout the abdomen and thorax. During oviposition, the valvulae are extended and retracted by a combination of small median apodemes on the anterior margin of abdominal sterna 3–7 gripping and releasing the ovipositor, and rocking motions of the sterna. Females representing different genera of Orussidae were examined by dissection and SEM, revealing overall similarity of the ovipositor system and its inferred mode of operation in a larger sample across the family, but also variation between genera in the way the ovipositor is accommodated. This includes differences in the prophragma, profurca and the internal part of the mesonotum where the anterior loop of the ovipositor is stored, and the shape of the loop itself which occurs in two configurations: simple hair pin-like bend or whorled, safety pin-like. The former is putatively the plesiomorphic condition in Orussidae, the latter possibly having evolved more than once to accommodate a longer ovipositor. Selected features in the ovipositor apparatus are mapped and discussed in the context of recent phylogenetic hypotheses for the family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Orussidae is a small family of parasitoid wasps, comprising less than 100 species worldwide (e.g., Vilhelmsen 2003, Vilhelmsen et al. 2013, 2017, and Blank and Vilhelmsen 2016). They have a worldwide distribution (Vilhelmsen 2004), but are rarely collected and biological information is only available for a fraction of the known species. They are associated with wood, most host records being from beetle larvae boring in dead wood (e.g., Buprestidae). The fossil record of Orussidae extends back into the middle Cretaceous (Vilhelmsen 2004; Vilhelmsen and Zimmermann 2014), the radiation of crown group Orussidae possibly having taken place in the mid-Cretaceous.
The Orussidae possess some striking adaptations to locating and reaching potential hosts inside wood. Vilhelmsen et al. (2001) described a potential vibrational sounding system in female orussids involving the modified tips of the antennae (Vilhelmsen et al. 2001, fig. 1), the fore tarsi (Vilhelmsen et al. 2001, fig. 2), which are three-segmented in the female with a much enlarged basitarsus, and an enlarged sensory organ, the subgenual organ (Vilhelmsen et al. 2001, figs. 3–5), inside the swollen and subdivided fore tibia. The external features associated with this mechanism are easily observable and constitute a suite of characters strongly supporting the monophyly of the family (Vilhelmsen 2003).
Vilhelmsen et al. (2001) also provided detailed information on the ovipositor apparatus of the Orussidae, which is also unique, but less obvious as it is mostly concealed. The basal parts of the ovipositor are either extended (second valvifer) or rotated (first valvifer; see Vilhelmsen et al. 2001, fig. 8), inverting the base of the ovipositor proper (first and second valvulae) which is pointing anteriorly. The ovipositor shaft is at least twice the length of the body of the female; when not in use, it is stored in an invaginated membranous sack (Vilhelmsen et al. 2001, figs. 9, 10) extending forward through the abdomen and into the thorax. The ovipositor in Orussus is coiled inside the anterior part of the thorax like a watch spring (Vilhelmsen et al. 2001, fig. 11) before extending posteriorly to exit the abdomen at the posterior end of abdominal sternum 7, which is modified to guide it. The extension and retraction of the ovipositor is facilitated by a series of small apodemes medially on the abdominal sterna 3–7 (Vilhelmsen et al. 2001, figs. 6, 9) which alternately grip and release the ovipositor shaft through the membranous sack and in combination with rocking movements of the abdominal sterna move the ovipositor.
The exploration of the ovipositor apparatus by Vilhelmsen et al. (2001) was mainly done on Orussus spp. In addition to the more detailed investigation of members of this genus, they also made observations from dissected specimens of Guiglia schauinslandi (Ashmead, 1903) and Orussobaius minutus Benson, 1938. In general, the outlay of the ovipositor apparatus is similar to that observed in Orussus. However, Vilhelmsen et al. also noted some differences. In Orussus, the ovipositor is coiled in the thorax when at rest (i.e., the outline of the shaft resembles a safety pin), and the profurca of the female is deeply cleft medially to allow the ovipositor coil to be stored anteriorly of the profurcal bridge (see Fig. 4 in Vilhelmsen 2000; Fig. 4g shows the condition in the male of Orussus, where the profurcal bridge is not cleft and extended posteriorly). In Guiglia and Orussobaius, the ovipositor also extends into the thorax, but it is not coiled, only bent in a simple loop (i.e., like a hairpin), and the profurcal bridge is not cleft medially, but straight, as in the male of Orussus.
Due to the small taxon sample in Vilhelmsen et al. (2001), it was not possible to explore the evolution of the traits associated with the ovipositor apparatus across the genera of Orussidae. Since then, information from additional genera has been obtained, both from dissected specimens but also from pinned specimens that have been damaged, fortuitously revealing some of the internal features associated with the ovipositor apparatus. In this paper, I report these findings and trace the evolution of ovipositor characters on the current phylogeny of Orussidae, as well as present some additional observations of different aspects of the orussid ovipositor apparatus that were treated in less detail by Vilhelmsen et al. (2001).
Materials and methods
Specimens examined
Representatives of seven different genera of Orussidae were examined, see Table 1.
Dissection
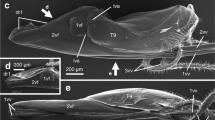
Specimens were dissected in 70% ethanol under a Leica M205C dissection microscope with steel scalpels and forceps. Initially, the head, propectus and wings were removed. After cutting along the membraneous parts anteriorly and laterally of the mesonotum, metanotum and abdominal terga 1–8, these were peeled off to expose the reproductive organs and ovipositor apparatus (Fig. 1). After imaging, further dissection was undertaken to separate the ovipositor apparatus (Fig. 2). Specimens were stored in glycerol after dissection. Measurements were taken with an ocular scale, usually at 10× magnification. The ovipositor base length was measured from the anteriormost point of the second valvifer to the tip of abdominal tergum 10 (see Fig. 2d, e). The length of the ovipositor proper was measured/estimated along suitable segments, the lengths of which were subsequently added together.
Ovipositor apparatus of Orussidae inside body, anterior to the right. Head, propectus and upper parts of meso- and metathorax, and abdomen removed. a, bOphrynopus fulvostigma, lateral and dorsal view; cPseudoryssus henschii, dorsal view. Red arrows = remnants of ovipositor sac; ovipositor; blue arrows = first and second valvulae inside body; green arrow = tip of first and second valvulae. N1 pronotum, pl2 mesopleuron, T9 abdominal tergum 9
Ovipositor apparatus detached, anterior to the right. a, eOrussus occidentalis; bPseudoryssus henschii; c, dOphrynopus fulvostigma. White bar = measurement of ovipositor base; red arrows = remnants of ovipositor sac; blue arrows = processus articularis; green arrows = tip of first valvula; yellow arrows = tip of second valvula; orange arrows = longitudinal carina on T9. 2vf second valvifer, T9 abdominal tergum 9
Imaging
Specimens were imaged in glycerol and stabilized by placing them on a drop of Alcogel during imaging. Digital images were produced with a Visionary Digital imaging setup with flash lightning and P-51 Camlift Driver ver. 2.6.1 to control the camera. A cylinder of semitransparent plastic was placed around the specimen to disperse the light. Images were stored in Adobe Lightroom 2 and composite images were compiled from stacks with the software Zerene Stacker ver.1.04 by implementing the Pyramidal stacking method (PMax).
Scanning electron microscopy
Dissected specimens were treated with 10% KOH at room temperature overnight to dissolve soft tissues and afterwards rinsed with demineralized water, and cleaned with forceps and needles. Specimens were transferred from 70 to 99.9% ethanol through intermediate stages of 80%, 90% and 96% ethanol; the specimens were left for approx. 1 h at each stage. The specimens were critical point dried with an Autosamdri-815cpd unit from Tousimis Research Corporation, Rockville, Maryland. The dried specimens were mounted on SEM stubs with double adhesive tape and sputter coated either with gold in a Balzers Union SCD 040 coater or platinum in a JEOL JFC-2300HR High-Resolution Fine Coater. The preparations were observed and imaged with a JEOL JSM-6335F Field Emission Scanning Electron Microscope.
Results
Descriptions are based primarily on Ophrynopus fulvostigma and Pseudoryssus henschii; these two taxa are similar unless otherwise noted. Additional information from other taxa provided were relevant. A summary of some of the ovipositor observations is presented in Table 2. Terminology follows Vilhelmsen et al. (2001).
Prophragma, mesoscutum and profurca
The ovipositor proper when at rest extends forward to the prophragma (the anterior attachment point of the longitudinal flight muscles situated at the anterior margin of the mesonotum). In P. henschii, the prophragma is deeply cleft medially all the way to the attachment line of the pronotum (Fig. 3b) to allow the ovipositor loop to protrude anteriorly; the mesoscutum does not have a groove ventrally (Fig. 3d). A similar configuration is observed in Orussobaius minutus, Orussus spp., and Pedicrista hyalina (Fig. 3e). In `, the cleft is much shallower, terminating well below the attachment line for the pronotum (Fig. 3a), and the prophragma in anterior view is continuous medially except for the ventralmost part, where two low lobes are situated submedially; posteriorly, a pair of vertical septa flanks a narrow median groove (Fig. 3c) which accommodates the ovipositor loop when at rest. The mesoscutum has a shallow median groove ventrally (i.e., internally) for accommodating the dorsal part of the ovipositor loop. The shallow cleft and posterior groove on the prophragma are also observed in Guiglia schauinslandi and Ophrella amazonica, but only the former has the median groove on the mesoscutum as well. The profurcal arms diverge from the prosternum situated ventrally; they are connected dorsally by a straight profurcal bridge. The bridge is straight in dorsal view, high laterally in posterior view (Fig. 4a, c), medially with a distinct groove accommodating the ovipositor and ovipositor sack. The profurcal bridge is also straight in Guiglia schauinslandi, Ophrella amazonica and Orussobaius minutus, the latter having only a weakly developed median groove. In contrast, Orussus spp. (Fig. 4b, d) and Pedicrista hyalina (Fig. 3e, f) have the profurcal bridge distinctly bent posteriorly, allowing the ovipositor sack with the loop inside it to descend into the propectus anterior to the bridge.

Images e and f are adapted from https://www.waspweb.org/Orussoidea/Orussidae/Pedicrista/Pedicrista_hyalina.htm, © Simon van Noort (Iziko Museums of South Africa). Reproduced with permission
Prophragma, anterior (a, b) and ventral (c, d) view. a, cOphrynopus fulvostigma; b, dPseudoryssus henschii. e, fPedicrista hyalina anterior part of body, head and prothorax detached. Yellow arrows = attachment lines of pronotum; orange arrows = groove(s) for ovipositor; green arrows = prophragma; red arrows = profurcal bridge; blue arrows = ovipositor loop. N1 pronotum, N2 mesonotum, pl2 mesopleuron.
Abdominal sternum 3–7
Sterna 3–6 are approx. twice as wide as long, concave dorsally (Fig. 5a), the posterior part slightly overlaps the succeeding sternum. In Orussobaius minutus, there is a prominent, posteriorly directed spine posteromedially on sternum 3. Paired median apodemes are situated on a common stem (Figs. 5b, 6a) and project at an angle from medially on the anterior margin of each sternum; a groove between the apodemes accommodates the ovipositor sack and the ovipositor inside it. Lateral apodemes are situated on the anterolateral corners of the sterna. The median apodemes become progressively shorter posteriorly; they are shorter in P. henschii than in O. fulvostigma. Sternum 7 is less concave and has lower width/length ratio, the median apodemes are elongate and almost horizontal, not angled relative to the rest of the sternum. Posteriorly, the apodemes are continuous with raised horizontal flanges extending the length of sternum 7 and accommodating the ovipositor trough between them (Figs. 5c, 6c, d, 7a). Externally, the median part of sternum 7 is traversed by three longitudinal weakly sclerotized lines corresponding to the median and lateral parts of the trough; the lateral lines diverge slightly halfway across sternum 7, but converge again towards the posterior margin. In O. fulvostigma, the trough has shallow transverse grooves proximally, a transition zone approx. halfway with a triangular patch of distally oriented ctenidia continuing as a narrow median band of ctenidia almost to the posterior end of sternum 7 (Fig. 5d, e); the sculpture in the trough of P. henschii appears to be less differentiated, with ctenidia not much developed (Fig. 6d). In Orussus abietinus, there is a transition zone medially in the trough, with a dense mat of distally directed microtrichia (Fig. 7c); these are more or less absent proximally and distally of the zone (Fig. 7b, d). The posterior ends of the flanges are continuous with the narrow sternal projection situated medially on the posterior margin of sternum 7 (Figs. 5f, 6c). The configuration of the abdominal sterna with median apodemes and a sternum 7 with an ovipositor trough, flanges and a sternal projection is overall very similar in all Orussidae dissected (see Table 1).
Ophrynopus fulvostigma, abdominal sterna. a Overview, anterior to the right; b median apodeme on sternum 5 in posterior view; c sternum 7, posterodorsal view; d ovipositor trough, approx. mid-length; e ovipositor trough, distal part; f projection on sternum 7, dorsal view. Yellow arrows = ovipositor trough on sternum 7. lap[n] = lateral apodeme on sternum [n]; map [n] median apodeme on sternum [n]; S[n] = sternum [n]
Pseudoryssus henschii, abdominal sterna. a Median apodeme on sternum 5 with remnant of ovipositor sack, posterior view; b detail of ovipositor sack; sack lumen (left) is anatomically external to the body and contains ovipositor in resting position, body cavity (right) is anatomically internal; c abdominal sterna 6 and 7, dorsal view; d distal part of ovipositor sack with ovipositor trough in middle, dorsoposterior view. Yellow arrow = ovipositor trough. S[n] = sternum [n]
Ovipositor sack
The sack extends anteriorly through the body all the way into the thorax from its attachments on sternum 7 and around the proximal parts of the ovipositor apparatus (Figs. 1c, 2a–c). It consists of membranous cuticle throughout. Dorsally, it is difficult to observe where the sack attaches, but it seems to be along the dorsal margins of tergum 9 as well as the lateral margins of the dorsally displaced the first and second valvifers. Posteroventrally, the sack is attached along the lateral margins of the horizontal flanges lateral to the ovipositor trough on sternum 7 (Fig. 6c, d). The posterior part of the sack is expanded to form a funnel-like opening (Fig. 6d). Further anteriorly, the sack ventrally passes between the median apodemes on sterna 3–6 but does not fuse with them (Fig. 6a). In P. henschii, the ventral part of the sack has the anatomically external surface towards the sack cavity lined with transverse swellings covered with distally oriented ctenidia (Fig. 6b); on the opposite side towards the body lumen, the sack is lined with transverse swellings without ctenidia. Distally in the sack, there are no ctenidia on the external surface either (Fig. 6d).
Ovaries
The paired ovaries lie laterally of the ovipositor sack, extending through most of the length of the abdomen. Most of the lumen of the ovaries is occupied by the eggs; in O. fulvostigma, the eggs reach a length of approx. 4 mm (Fig. 1b), extending into the oviduct. The eggs are shaped somewhat similar to a human sperm cell, with an expanded, slightly curved head anteriorly and a long tail posteriorly. In O. fulvostigma, each ovary contains 6–7 eggs.
Tergum 9 and first valvifer
Tergum 9 resembles a boat that has been cut in half in outline (Fig. 8a) and is situated below tergum 8, which partly overlaps it laterally and posteriorly. The medioventral margins of tergum 9 abut in a straight longitudinal line, concealing the third valvulae. In O. fulvostigma, a pair of longitudinal carinae is situated submedially lateral to the median line (Fig. 2d); these carinae are not developed in P. henschii, where the area posteromedially on tergum 9 is slightly concave. Prominent carinae are also present in Guiglia schauinslandi, Ophrella amazonica and Orussus abietinus (Fig. 2e). The tergo-valvifer articulation with the first valvifer is situated on the anterodorsal corner of tergum 9 (Fig. 8a). Internally, the prominent anterior flanges extend from the tergo-valvifer articulations posteriorly for most of the length of tergum 9; the flanges are wide anteriorly but taper posteriorly (Fig. 8c). The cordate apodemes are continuous with the anterior flanges dorsally and extend posteroventrally at an angle towards the ventral margin of tergum 9. The first valvifers are triangular in lateral view, wide and rectangular in anterior view (Fig. 8a, b). The tergo-valvifer articulation is situated at the posterior corner, the intervalvifer articulation with the second valvifer at the anteroventral corner of the first valvifer (Fig. 8a). Dorsally, the first valvifers are continuous with the base of the first valvulae. The configuration of tergum 9 and the first valvifer is overall very similar in all taxa dissected.
Pseudoryssus henschii ovipositor apparatus, distal/posterior to the left. a–c Ovipositor apparatus base, lateral, posterior and dorsal views; d first valvula, median view; e first valvula surface, lateral view; f second valvula, ventral view; g, h tip of first and second valvulae, dorsal and lateral view. Orange arrows = sensilla basally on second valvulae; green arrows = incisura praearticularis; red arrow = aulax on first valvula; blue arrows = rhachis on second valvula; yellow arrows = tooth on first valvula. 1vf first valvifer, 1vv first valvula, 2vf second valvifer, 2vv second valvula, afl anterior flange, bar basal articulation, iva intervalvifer articulation, spa sensillar patch, tva tergo-valvifer articulation
Second valvifer and third valvula
The second valvifers are L-shaped in lateral view; the anterior, vertical part lies anterior to tergum 9 and the first valvifers, the posterior horizontal part lies ventral to them. Throughout their extent, the second valvifers are situated mostly medial to tergum 9 and the first valvifers (Fig. 8a, b). The intervalvifer articulations with the first valvifers are situated some distance down the posterior margin of the anterior part of the second valvifer; a sensillar patch of sensory setae is situated anterior to the intervalvifer articulation. The wide dorsal flanges extend ventrally and then posteriorly along the posterior/dorsal margin of the second valvifer, tapering gradually. Dorsally, the second valvifers are continuous with the second valvulae. Except for their most proximal parts, the second valvifers and the second valvulae are separated by the incisurae praearticulares, which lie just proximal to the basal articulations between the second valvifers and the second valvulae (Fig. 8a, b); just proximal to the incisurae, elongate rows of sensilla extend along the base of the second valvulae. The second valvifers are continuous with the third valvulae ventrally, separated by a narrow vertical line dorsally. The third valvulae are elongate, tapering to a point distally. They are mostly concealed above the medioventral margins of tergum 9. The lateral parts of the third valvulae are covered with distally pointing setae; medially, they are comparatively smooth. The configuration of the second valvifer and the third valvula is overall very similar in all taxa dissected.
First and second valvula
The first and second valvulae are basally continuous with the first and second valvifers, respectively. The valvulae are firmly connected for their entire length by the olistheter system, consisting of a groove in the dorsal part of each first valvula, the aulax (Figs. 8b, 9b), into which corresponding paired longitudinal lists ventrally on the second valvula, the rhachises (Fig. 8f), are inserted. Together the first and second valvulae form the ovipositor proper that accommodate the egg canal between them. The ovipositor proper extends in an elongate hairpin loop (Fig. 2b, c) from the base of the ovipositor apparatus anteriorly and dorsally into the thorax before turning and running posteriorly and ventrally. Throughout its length, the ovipositor proper is accommodated inside the ovipositor sack. The stretch of the ovipositor proper extending ventrally along the abdominal sterna lies in the groove between the median apodemes of the sterna. The first valvulae are smooth laterally, only revealing very fine irregular sculpture consisting of small raised areas at very high magnification in P. henschii (Fig. 8e); in O. abietinus there are sensilla at intervals on the first valvula, and there is slightly rougher sculpture on the second valvula (Fig. 9a). Medially, the dorsal part of the first valvula microtrichia are assembled into slightly curving and slanted distally pointing ctenidia (Figs. 8d, 9b); the ventral part is comparatively smooth with only scattered microtrichia. The exact pattern of distally directed microtrichia differs slightly between O. fulvostigma (Fig. 9c), O. abietinus (Fig. 9b) and P. henschii (Fig. 8d), but they are most dense in the ventral part of the median wall that forms the main part of the egg canal. Distally, the first valvulae taper to narrow points with 3–4 symmetric teeth that are sharp on both edges (Fig. 8g, h). The second valvulae are fused throughout their length; distally, they terminate in four transverse anteriorly directed teeth that appear at progressively shorter intervals towards the tip (Fig. 8g, h). There is considerable variation in the relative length of the ovipositor proper in the taxa dissected (see Table 2); most of them have the simple hairpin loop configuration observed in O. fulvostigma and P. henschii (Fig. 2b), but Orussus spp. and Pedicrista hyalina have a safety pin configuration with two (P. hyalina; Fig. 3e, f) or more (O. occidentalis; Fig. 2a) circular whorls in the prothorax.
Discussion
The observations of the ovipositor system in Vilhelmsen et al. (2001) were mostly focused on Orussus spp.; the expanded taxon sample examined for the present paper indicates that the modes of internalization and operation are essentially the same in all Orussidae examined so far. The basal parts of the ovipositor apparatus, in particular the first and second valvifers and the first and second valvulae are rotated anteriorly to various degrees and/or extended, inverting the base of the ovipositor proper which is housed in the invaginated ovipositor sack that extends all the way into the thorax; the sack is flared posteriorly, probably to allow for the rotation of the valvifers, both of which are quite broad. The similarity of the configuration of the abdominal sterna 3–7 with the presence of median apodemes, and in the case of sternum 7, an ovipositor trough that together can grip the ovipositor proper during extension and retraction indicates that the mechanism for oviposition is the same in all taxa examined.
The mechanism for the invagination of the ovipositor in Orussidae has not been explored in detail. Rohwer and Cushman (1917) illustrated the pupa of a female Orussus occidentalis (pl. xi, Figs. 4, 7) with part of the ovipositor bended dorsally and extending externally along the back all the way to the head; furthermore they stated (p. 91) in their description of the pupa that ‘internally [the ovipositor] extends forward into the mesothorax where it makes a simple loop’. However, they also stated (p. 94) that ‘in the prepupa [i.e., the last immovable larval stage prior to pupation] the ovipositor is coiled as it is in the adult’. Clearly, the ontological sequence of ovipositor development in Orussidae needs further clarification.
The oviposition mechanism is probably a ground plan feature of the Orussidae; this is indicated by the similarity of the parts of the ovipositor apparatus that can be observed externally across the entire family, e.g., the presence of three weakly sclerotized subparallel lines and a posterior projection on sternum 7 (Vilhelmsen 2003; character 148:1), and the abutting medioventral margins of tergum 9 which usually conceal the third valvulae (character 154:1). Both these traits are putative autapomorphies of the Orussidae. The true extent of the ovipositor proper is occasionally revealed in pinned specimens (e.g., Blank and Vilhelmsen 2016, fig. 8a–b), but these instances are clearly artefacts of preservation.
Nevertheless, some variation in the ovipositor system in the Orussidae can be observed, more specifically in the configuration of the profurca and the coiling of the ovipositor when not extended (Table 2). Two states are displayed in the configuration of the prophragma: (1) presence of a narrow median cleft extending dorsally all the way to the anterior margin of the mesonotum; (2) mostly entire medially, but posteriorly having a narrow groove flanked by short septa accommodating the anterior part of the ovipositor loop when at rest. The latter state is observed exclusively in members of the ‘ophrynopine clade’ (e.g., Vilhelmsen et al. 2013), making it a putative synapomorphy for the ophrynopine genera (Fig. 10). The median groove on the ventral, interior side of the mesoscutum occurs less consistently within the ophrynopine clade. Perhaps the presence of the groove is correlated with the presence of a median, external ridge on the mesoscutum, a character that varies considerably within the ophrynopine clade (Vilhelmsen 2003, character 66).
Phylogeny of the crown group genera of Orussidae, adapted from Fig. 12 in Vilhelmsen and Zimmermann (2014). The occurrence of hair pin-type and safety pin-type of ovipositor is indicated to the right of the terminals examined. The two conditions of the prophragma are shown to the left; the examined terminals are color coded according to the state they display. The inferred change from deep (green) to shallow (red) prophragmal cleft at the base of the ‘ophrynopine clade’ is indicated
Most taxa examined have the profurcal bridge straight with a short median groove for the ovipositor and the ovipositor stored in a simple, hairpin-like loop. However, in Orussus and Pedicrista, the profurcal bridge is bent posteriorly in the middle, forming a depression anteriorly in which the ovipositor may coil safety pin-wise. These characters seem to be correlated and may in turn reflect the relative length of the ovipositor, the bent profurca allowing storage of a longer ovipositor in coils. Indeed, the longest relative ovipositor length was observed in Orussus (Table 2); unfortunately, measurements for Pedicrista could not be obtained.
Phylogenetic analyses of Orussidae (e.g. Vilhelmsen 2003 and Vilhelmsen et al. 2013, 2017) indicate that Orussobaius is the most basal taxon in the taxon sample for the present paper (Fig. 10). The presence of the straight profurcal bridge and the hairpin configuration of the ovipositor in this and most of the other taxa examined suggest that these are the plesiomorphic conditions for Orussidae. Orussus and Pedicrista are usually not retrieved as closely related. The sister group of Orussus is usually Pseudoryssus, which has an unmodified profurca and the ovipositor in a hairpin loop; the sister to Pedicrista is usually Chalinus + Mocsarya, genera that have not been included in the present survey. Based on this distribution, it seems most likely that the bent profurcal bridge and safety pin coiled ovipositor are independently derived in the taxa that have these traits, but more taxa need to be investigated to confirm this. Given the rarity of most species of Orussidae, it will be difficult to obtain material for dissection of representatives of all genera. However, microCT scannings of dry mounted specimens might make it possible to obtain at least some information without having to perform dissection; preliminary results are promising (Vilhelmsen unpubl.). This technique can also be applied to amber fossils of female orussids, should they become available; unfortunately, the best preserved orussid fossils are males (Vilhelmsen and Zimmermann 2014).
The detailed observations on Ophrynopus and Pseudoryssus make it possible to supplement the information and correct a few inaccuracies. Vilhelmsen et al. (2001: p. 71) stated that the ovipositor sack “is attached dorsally and laterally to the area around the base of the ovipositor apparatus and ventrally to the posterior margin of sternum 7”. As shown here, the posterior part of the ovipositor sack is funnel-shaped; ventrally, it is continuous with the flanges on sternum 7 lateral to the ovipositor trough, not the posterior margin. In the trough of O. fulvostigma and the median side of the ventral part of the sack in P. henschii, i.e., parts that are in direct contact with the ovipositor proper during extension, the surface is covered with distally directed microtrichia. This is probably to obtain better grip and prevent slippage when pushing the ovipositor out of the body; during retraction, less force is required as the ovipositor is not drilling into the wood anymore, so presumably it matters less that the movement is against the direction of the microtrichia. The subparallel weakly sclerotized lines that extend longitudinally across sternum 7 form flexion lines that allows the ovipositor trough to accommodate the ovipositor proper.
The sculpture of the first and second valvulae is revealed to some extent in P. henschii. The minute and shallow sculpture of the outer surface of the first valvula together with the circular cross-section of the ovipositor proper (Quicke et al. 1994: Fig. 25) ensures that it can move with minimum friction through the narrow tunnel drilled through the wood. The distally directed ctenidia on the ventral part of the inner surface of the first valvulae helps pushing the egg distally through the ovipositor and into the wood; this is a mechanism found in other Hymenoptera (e.g., Braconidae; Rahman et al. 1998a, b) and considered to be widespread in insects (see Austin and Browning 1981). Pushing the large egg through the egg canal constitutes a major challenge; it is only possible because of the apparent elasticity of the egg allows it to be temporarily deformed during passage (see Cooper 1953).
Vilhelmsen et al. (2001: p. 81) wrote of the tips of the first valvulae: “we did not observe anything but weakly developed annuli in the species we examined”. This seems to be an oversight, as in P. henschii small but distinct teeth could be discerned at the very tip of the first valvulae; the teeth are symmetric and sharp on both sides, allowing them to cut both during extension and retraction. In contrast, the transverse teeth at the tip of the second valvulae are asymmetric and taper toward the tip, so they probably chiefly serve to anchor the tip of the ovipositor in the wood during drilling, preventing it from slipping backwards as the first valvulae push into the wood.
The ovipositor proper of Orussidae can be regarded as a prime example of an insect ‘multi-element probe’ (Cerkvenik et al. 2019) that is employed in targeting hosts in a dense substrate, i.e., wood. The solutions to the challenges involved in this context that have been evolved by the family are unique and somewhat idiosyncratic in that they are apparently not transferable to other habitats. This may at least in part explain why the Orussidae does not have very high diversity (Vilhelmsen et al. 2001), in contrast to its putative sister group, the Apocrita, which may also initially have evolved targeting woodboring insect larvae, but later radiated dramatically on a multitude of insect hosts (Vilhelmsen and Turrisi 2011).
References
Austin AD, Browning TO (1981) A mechanism for movement of eggs along insect ovipositors. Int J Insect Morphol 10:93–108
Blank SM, Vilhelmsen L (2016) Two new parasitoid wasp species of the Australasian genus Orussobaius (Hymenoptera: Orussidae). Arthropod Syst Phylogeny 74:83–103
Cerkvenik U, Dodou D, van Leuwen JL, Gussekloo SWS (2019) Functional principles of steerable multi-element probes in insects. Biol Rev Cambridge Philos Soc 94:555–574. https://doi.org/10.1111/brv.12467
Cooper KW (1953) Egg gigantism, oviposition, and genital anatomy: their bearing on the biology and phylogenetic position of Orussus (Hymenoptera: Siricoidea). Proc Rochester Acad Sci 10:38–68
Quicke DLJ, Fitton MG, Tunstead JR, Ingram SN, Gaitens PV (1994) Ovipositor structure and relationships within the Hymenoptera, with special reference to the Ichneumonoidea. J Nat Hist 28:635–682
Rahman MH, Fitton MG, Quicke DLJ (1998a) Ovipositor internal microsculpture in the Braconidae (Insecta, Hymenoptera). Zool Scr 27:319–331
Rahman MH, Fitton MG, Quicke DLJ (1998b) Ovipositor internal microsculpture and other features in doryctine wasps (Insecta, Hymenoptera, Braconidae). Zool Scr 27:333–343
Rohwer SA, Cushman RA (1917) Idiogastra, a new suborder of Hymenoptera with notes on the immature stages of Oryssus. Proc Entomol Soc Wash 19:89–98
Vilhelmsen L (2000) Cervical and prothoracic skeleto-musculature in the basal Hymenoptera (Insecta): comparative anatomy and phylogenetic implications. Zoologischer Anzeiger 239:103–136
Vilhelmsen L (2003) Phylogeny and classification of Orussidae (Insecta: Hymenoptera), a basal parasitic wasp taxon. Zool J Lin Soc 139:337–418
Vilhelmsen L (2004) The old wasp and the tree: fossils, phylogeny and biogeography in the Orussidae (Insecta, Hymenoptera). Biol J Lin Soc 82:139–160
Vilhelmsen L, Turrisi GF (2011) Per arborem ad astra: morphological adaptations to exploiting the woody habitat in the early evolution of the Hymenoptera. Arthropod Struct Dev 40:2–20
Vilhelmsen L, Zimmermann D (2014) Baltorussus total makeover: rejuvenation and sex change in an ancient parasitoid wasp lineage. PLoS ONE 9:e98412
Vilhelmsen L, Isidoro N, Romani R, Basibuyuk HH, Quicke DLJ (2001) Host location and oviposition in a basal group of parasitic wasps: the subgenual organ, ovipositor apparatus, and associated structures in the Orussidae (Hymenoptera, Insecta). Zoomorphology 121:63–84
Vilhelmsen L, Blank SM, Costa VA, Alvarenga TM, Smith DR (2013) Phylogeny of the ophrynopine clade revisited: review of the parasitoid sawfly genera Ophrella Middlekauff, Ophrynopus Konow and Stirocorsia Konow (Hymenoptera: Orussidae). Invertebr Syst 27:450–483
Vilhelmsen L, Blank SM, Sechi D, Ndiaye MM, Niang AA, Guisse A, van Noort S (2017) The Orussidae (Insecta: Hymenoptera) of Africa. Proc Entomol Soc Washington 119(Special Issue):879–930
Acknowledgements
Daniele Baiocchi, Fabrizio Turrisi, Braet Yves, and Dave Smith all provided valuable material for the present study. Simon van Noort gave permission to include images from https://www.wasp.org. Two anonymous referees provided useful comments to the submitted version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
No animals were harmed during the production of this paper. The study was based entirely on dead specimens deposited in zoological collections.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vilhelmsen, L. From hair pin to safety pin: evolution of the ovipositor apparatus in Orussidae (Insecta: Hymenoptera). Zoomorphology 139, 37–49 (2020). https://doi.org/10.1007/s00435-019-00468-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-019-00468-y