Abstract
There are relatively few published illustrations of ovipositors for harvestmen belonging to the suborder Laniatores. As a result, the ovipositor of these harvestmen has largely been ignored as a source of informative characters. We used scanning electron microscopy to examine the ovipositor of eight species representing several major lineages (Gonyleptoidea, Phalangodoidea, Samooidea, and Zalmoxoidea). We observed interspecific variation with respect to the number of external lobes on the distal tip, the surface texture of the distal tip, and the morphology of the peripheral setae. The ovipositors of Bishopella (Phalangodoidea) and Stygnomma (Samooidea) were similar in appearance and differed with respect to the number and position of peripheral setae. We observed significant interspecific variation among the gonyleptoidean species, especially with respect to the peripheral setae. The ovipositors of Zalmoxoidea harvestmen had smooth, spatulate peripheral setae but differed with respect to the surface texture of the distal tip. The unusual morphology of these setae has not been observed previously and may represent a new synapomorphy for the family. Characters based upon ovipositor morphology have the potential to illuminate phylogenetic relationships within and between families and genera in these harvestmen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Order Opiliones, harvestmen belonging to the clade Phalangida (suborders Eupnoi + Dyspnoi + Laniatores: Giribet and Kury 2007) transfer immotile sperm from male to female via a penis, with internal fertilization occurring within the terminal end of the ovipositor (Machado and Macías-Ordóñez 2007). The morphology of the ovipositor varies dramatically among species belonging to suborders Cyphophthalmi, Eupnoi, Dyspnoi, and Laniatores, and this difference in reproductive anatomy is reflected behaviorally in the selection of oviposition sites by females (Martens et al. 1981). In the Cyphophthalmi and Eupnoi, the ovipositors are segmented, muscular, sclerotized, and relatively long, often exceeding total body length (Macías-Ordóñez et al. 2010). These harvestmen lay eggs in crevices or beneath the surface of the soil (Machado and Macías-Ordóñez 2007). In contrast, the ovipositors of harvestmen in the suborders Dyspnoi and Laniatores are fleshy (lightly sclerotized), considerably shorter, and have more limited musculature (Martens et al. 1981; Martens 1986; Macías-Ordóñez et al. 2010). These harvestmen lay eggs only upon the exposed surfaces of leaves, logs, rocks, or the soil (Machado and Macías-Ordóñez 2007; Macías-Ordóñez et al. 2010). In species of neotropical laniatorean harvestmen representing several families, the eggs are often guarded by one adult, usually the female (reviewed by Machado and Macías-Ordóñez 2007; Proud et al. 2011). In comparison with the ovipositors of other harvestmen, those of species in the suborders Dyspnoi and Laniatores are also thought to have a much lower density of sensory organs (Machado and Macías-Ordóñez 2007). In cross section, the lumen of the vagina within the ovipositor of a laniatorean harvestman is X-shaped and has seminal receptacles, usually in multiples of four (Martens et al. 1981; Machado and Macías-Ordóñez 2007).
Modern species descriptions for taxa in the suborders Cyphophthalmi and Eupnoi include illustrations of both male and female reproductive anatomies. Characters based upon penis morphology are also used to differentiate genera, subfamilies, and families in the suborder Laniatores (DaSilva and Gnaspini 2009; Hara and Pinto-da-Rocha 2010; Sharma and Giribet 2011; Pinto-da-Rocha et al. 2012; Kury 2014; Bragagnolo et al. 2015). However, systematic works of laniatorean harvestmen only rarely (Sharma and Giribet 2011) include general illustrations and descriptions of the ovipositor. Of the 28 families currently placed in the Laniatores (Kury 2003; Sharma and Giribet 2011; Pinto-da-Rocha et al. 2014), ovipositor morphology has only been described for three families of Insidiatores (Briggs 1971a, b; Martens et al. 1981; Maury 1988; Hunt and Hickman 1993; Giribet and Kury 2007) and seven families of Grassatores (Martens et al. 1981; Goodnight and Goodnight 1983; Sharma and Giribet 2011; Bennett and Townsend 2013; Walker and Townsend 2014).
Not surprisingly, relatively little is known about interspecific variation in the external morphology of the ovipositor for laniatorean harvestmen (Martens et al. 1981). The distal end of the ovipositor is externally divided into two or four lobes (Martens et al. 1981) and adorned with relatively large setae distributed around the periphery of the distal tip (Martens et al. 1981). The bilobed condition is generally considered plesiomorphic for laniatorean harvestmen (Martens et al. 1981; Giribet and Kury 2007). The peripheral setae on the distal tip insert into sockets that occur on the ventral surface or on a slightly more dorsal position (Sharma and Giribet 2011). The peripheral setae of the ovipositor are hypothesized to interact with the lateral setae on the ventral plate of the pars distalis of the penis during copulation (Macías-Ordóñez et al. 2010). Illustrations of ovipositor morphology in the form of line drawings are available for relatively few laniatorean harvestmen (e.g., Goodnight and Goodnight 1976; Šilhavỳ 1979; Martens et al. 1981; Goodnight and Goodnight 1983; Hunt and Hickman 1993). Unfortunately, most of the illustrations and the supporting descriptions provide only limited details (number of lobes and number of peripheral setae only), thus preventing interspecific comparisons of surface details on the distal tip and the morphology of peripheral setae. Recently, studies using scanning electron microscopy (SEM) of ovipositor morphology (Sharma and Giribet 2011; Bennett and Townsend 2013; Walker and Townsend 2014) have yielded significant insights into variation in the morphology and position of peripheral setae and the surface texture of the external lobes on the distal tip. Bennett and Townsend (2013) found considerable intraspecific variation with respect to the number of peripheral setae on the distal tips of the ovipositors of Cynortula granulata (Cosmetidae), Phareicranaus calcariferus (Gonyleptidae, Cranainae), and Rhopalocranaus albilineatus (Gonyleptidae, Manaosbiinae). The results of Bennett and Townsend (2013) indicated that strictly meristic counts of peripheral setae were not useful for distinguishing taxa. Kury et al. (2007) reported similar observations regarding the number of tarsomeres on leg I for cosmetid harvestmen and recommended against the use of tarsomere counts for diagnosing genera. Bennett and Townsend (2013) observed relatively little intraspecific variation in surface texture on the distal tip or the morphology of the peripheral setae; however, they observed significant, consistent interspecific variation in the morphology of the distal tips of the peripheral setae and with respect to the presence of setae (denticles) and folds on the surface of the distal tip of the ovipositor. Walker and Townsend (2014) examined the ovipositors of 12 species of cosmetid harvestmen and reported considerable interspecific variation in the relative size, shape, and surface texture of the peripheral setae. They also observed interspecific variation in surface texture (smooth or denticulate) and the number of lobes present (two or four).
In this study, we investigated variation in ovipositor morphology among eight laniatorean species representing several major lineages within the infraorder Grassatores, including Gonyleptoidea, Phalangodoidea, Samooidea, and Zalmoxoidea. Specifically, we used SEM to examine and compare the microanatomical features of the peripheral setae on the distal tip of the ovipositor as well as surface texture on the external surface of the lobes. In an attempt to provide further context for variation in ovipositor morphology in suborder Laniatores, we compared the morphology of the species that we examined with published descriptions for ovipositors for species representing several additional families from infraorders Insidiatores and Grassatores (Tables 1, 2).
Materials and methods
We examined 2–5 ovipositors of Avima intermedia (Goodnight and Goodnight 1947) (Agoristenidae), Bishopella laciniosa (Crosby and Bishop 1924) (Phalangodidae), Ethobunus albitrochanteris (Roewer 1933) (Zalmoxidae), Glysterus sp. (Gonyleptidae, Ampycinae), Pachylicus spinatus Goodnight and Goodnight 1983 (Zalmoxidae), Panopiliops reimoseri (Roewer 1949) (Zalmoxidae), Stygnomma fuhrmanni Roewer 1912 (Stygnommatidae), and Stygnoplus clavotibialis (Goodnight and Goodnight 1947) (Stygnidae). The specimens of A. intermedia and S. clavotibialis were collected in 2005–2006 from the Northern Range of Trinidad, W.I. Adult females of B. laciniosa were collected in June 2014 from Anvil Cave and Jim’s Cave in Morgan County, Alabama, USA. Specimens of all three zalmoxid species and S. fuhrmanni were collected in July 2010 from La Selva Biological Station, Costa Rica.
Prior to dissection, we observed that three specimens (one B. laciniosa, one Glysterus sp, and one E. albitrochonteris) had ovipositors that were everted and readily visible with the stereomicroscope. For these specimens, we removed the legs, but did not disturb the ventral surface. For the other specimens, we carefully excised the ovipositor and removed the connective tissue surrounding the ovipositor with the aid of forceps under a Leica EZ4 stereomicroscope. Intact specimens and dissected ovipositors were placed in 70 % ethanol and sonicated for 2–5 min to remove any remaining debris. They were dehydrated in a graded series of ethanol and dried using hexamethyldisilizane (Nation 1983). We mounted the excised ovipositors so that they were perpendicular to the surface of the aluminum stub. Intact specimens were mounted to aluminum stubs with the ventral surface and the ovipositor visible from above. We sputter-coated the specimens with 15–20 nm of gold and examined them with the Hitachi S-3400 N SEM at Virginia Wesleyan College. For ovipositor morphology, we generally followed the terminology used by Martens et al. (1981), Sharma and Giribet (2011), and Walker and Townsend (2014).
Results
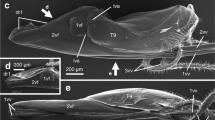
Of the three intact specimens that we observed with a visible ovipositor, two were almost fully everted (Fig. 1a, b). The ovipositors of these harvestmen were relatively short in comparison with total body length (Fig. 1). Ovipositors were bilobed (Fig. 1a, c) or had four external lobes on the distal tip (Fig. 1b). The distal tips were generally asymmetric with respect to the distribution of the peripheral setae (Fig. 1), with the anterior surface (Fig. 1a), anterior lobes (Fig. 1b), or the posterior surface (Fig. 1c) having more setae. Most peripheral setae inserted into sockets with a well-developed basal membrane. Most sockets occurred along the periphery of the ventral surface along the border of the inflatable tip (Fig. 1a, b). However, we observed three patterns with respect to socket location of the peripheral setae (Fig. 2). Ovipositors had peripheral setae inserted into sockets that were ventral (along the periphery of the inflatable tip: Fig. 2a), dorsal (sockets occurred below the inflatable tip: Fig. 2b), or a mixture of dorsal and ventral sockets (Fig. 2c, d). For ovipositors with both ventral and dorsal sockets, the medial setae on the anterior and posterior borders were usually dorsal (two anterior or two posterior) and the remaining sockets were ventral. The bases of the peripheral setae were generally not in contact, although the degree of spacing varied between species (Fig. 1). The surface texture of the distal tip was generally smooth or had shallow folds (Fig. 1a, b), although in the zalmoxid taxa, there were extensive folds and denticles (Fig. 1c). The number and shape of the peripheral setae also exhibited considerable interspecific variation (Figs. 1, 2, 3, 4, 5). In several specimens, one or more of the peripheral setae were broken, revealing a hollow lumen within the shaft (Figs. 4a, 5d, 6d, g). This hollow lumen morphology in association with a well-developed basal membrane associated with the socket is consistent with that of sensory setae (Guffey et al. 2000; Willemart et al. 2007, 2009).
General ovipositor morphology of laniatorean harvestmen, ventral views. a Bishopella laciniosa (Phalangodidae). b Glysterus sp. (Gonyleptidae). c Ethobunus albitrochanteris (Zalmoxidae). al anterior lobe, ar anterior row, ds seta with dorsal socket, gp genital plate, pl posterior lobe, vr ventral row, versus seta with ventral socket, arrows indicate spatulate setae on distal tip of ovipositor. Scale bars 200 μm
Position of peripheral setae on distal tip of the ovipositor. a Lateral view of the ovipositor of Bishopella laciniosa showing ventral sockets (anterior and posterior) of peripheral setae. b Posterior view of the ovipositor of Panopiliops reimoseri showing dorsal sockets of peripheral setae. c Anterolateral view of the ovipositor of Stygnomma fuhrmanni showing medial dorsal and lateral ventral sockets of peripheral setae. d. Posterolateral view of the ovipositor of S. fuhrmanni showing medial dorsal and lateral ventral sockets of peripheral setae. ar anterior row of peripheral setae inserting into ventral sockets, d seta inserting into a dorsal socket, pr posterior row of peripheral setae inserting into ventral sockets, v seta inserting into a ventral socket. Scale bars 50 μm
Ovipositor morphology of Bishopella laciniosa (Phalangodidae). a Ventral view of distal tip revealing bilobed condition with smooth surface and groups of four anterior and three posterior peripheral setae on each lobe. b Bases of ventral peripheral setae inserting into sockets that are not physically in direct contact, and cylindrical shafts have fine striations on surfaces. c Relatively straight shafts of ventral peripheral setae. d Distal undivided tips of ventral peripheral setae. Scale bar 40 μm
a–c Ovipositor morphology of Avima intermedia (Agoristenidae), d–f ovipositor morphology of Glysterus sp. (Gonyleptidae), g–i ovipositor morphology of Stygnoplus clavotibialis (Stygnidae), ventral views. a Bilobed distal tip with smooth surface. b Distal tips of peripheral setae. c Striations on straight, cylindrical shaft of a peripheral seta. d Distal tip with four lobes and smooth surface. e Undivided distal tip of a peripheral seta. f Striations on straight, cylindrical shaft of a peripheral seta. g Distal tip with four lobes and smooth surface. h Curled setae with divided distal tips. i curled peripheral seta with divided tip. Al anterior lobe, pl posterior lobe; arrows indicate anterior peripheral setae. Scale bars 100 μm for a, d, g; 10 μm for b, c, e, f, h, i
Ovipositor morphology of Stygnomma fuhrmanni (Stygnommatidae), ventral views. a Distal tip with two lobes with smooth surface. b Base of peripheral seta inserting into socket, cylindrical shaft has fine striations. c Straight, cylindrical shafts of peripheral setae. d Undivided distal tips of peripheral setae. Scale bars 110 μm for a; 10 μm for b and d; 25 μm for c
a–c Ovipositor morphology of Ethobunus albitrochanteris (Zalmoxidae), d–f ovipositor morphology of Panopiliops reimoseri (Zalmoxidae), g–i ovipositor morphology of Pachylicus spinatus (Zalmoxidae), ventral views. a Bilobed distal tip with coarse surface and denticles. b Distal tips and flattened (lanceolate), smooth shafts of peripheral setae. c Denticulate surface of distal tip. d Bilobed distal tip with coarse surface. e Flattened, smooth shafts of peripheral setae with undivided distal tips. f Denticulate surface of distal tip of ovipositor with denticles. g Bilobed distal tip with coarse surface. h Distal tips and flattened, smooth shafts of peripheral setae. i Course surface of distal tip of ovipositor without denticles. Scale bars 100 μm for a, d, g; 10 μm for b, c, e, f, h, i
The bilobed ovipositor of Bishopella laciniosa (Fig. 3) featured an anterior group of four relatively large peripheral setae and three slightly smaller posterior setae on each lobe (Fig. 3a). The surface texture of the distal tip was generally smooth, lacking denticles or extensive folding (Fig. 3a). The sockets of the setae were not in direct contact (Fig. 3b), and the shafts had very fine striations (Fig. 3b, c). The tips of the peripheral setae were relatively straight and undivided (Fig. 3d).
The ovipositors of the gonyleptoidean harvestmen exhibited considerable interspecific variation (Fig. 4). The bilobed ovipositor of Avima intermedia (Fig. 4a–c) had six anterior and four posterior peripheral setae. The medial setae on each lobe (anterior and posterior) had dorsal sockets, whereas the remaining setae inserted into ventral sockets (Fig. 4a). The surface texture of the distal tip of the ovipositor was generally smooth (Fig. 4a). The distal tips of the peripheral setae were subdivided into 5–9 smaller fimbrial processes, and many were broken in the specimens examined (Fig. 4b). Examinations of the broken processes on the distal tip of the setae revealed no internal lumen, indicating that these processes are only cuticular extensions and are not innervated. The shafts of the setae had well-developed striations (Fig. 4c). In Glysterus sp., the distal tip of the ovipositor had four external lobes with a smooth external surface (Fig. 4d). There were 8–10 peripheral setae present on the distal tip, with only the medial setae on each lobe having dorsal sockets (Figs. 1b, 4d). In most specimens, each anterior lobe had three peripheral setae and each posterior lobe had two setae (one specimen had two setae on each lobe). The peripheral setae had undivided tips (Fig. 4e) and well-defined striation on the shafts (Fig. 4f). The distal tip of the ovipositor of Stygnoplus clavotibialis (Fig. 4g–i) had 9–10 small, curled peripheral setae and a relatively smooth surface. There were three peripheral setae on each anterior lobe and two peripheral setae on each posterior lobe. Of the ten setae, only the medial setae on each lobe inserted into dorsal sockets (Fig. 4g). In addition to being curled, each of the peripheral setae had 2–3 tips (Fig. 4h, i) and small surface striations.
The bilobed ovipositor of Stygnomma fuhrmanni (Fig. 5) had a relatively smooth external surface. There were three peripheral setae on each anterior lobe and two peripheral setae on each posterior lobe (Fig. 5a). The medial setae on each lobe (anterior and posterior surfaces) inserted into ventral sockets and had fine striations on the shafts (Fig. 5b) with undivided distal tips (Fig. 5c, d).
The ovipositors of the zalmoxid harvestmen exhibited considerable interspecific variation (Fig. 6). In general, each ovipositor had a bilobed distal tip (Fig. 6a, d, g) surrounded by 8–10 peripheral setae, with all setae inserting into sockets that were dorsal in position. In comparison with the other ovipositor setae examined in this study, the peripheral setae differed in general morphology in that they were spatulate, with a smooth surface (no visible striations), and had attenuated undivided tips (Fig. 6b, e, h). They differed with respect to the coarseness and presence of denticles on the distal surface of the ovipositor (Fig. 6c, f, i). In Ethobunus albitrochanteris (Fig. 6c), the distal surface had many folds with prominent denticles. In Pachylicus spinatus (Fig. 6f), the distal surface had fewer surface folds and fewer and less prominent denticles, whereas in Panopiliops reimoseri (Fig. 6i), the distal surface was folded, but lacked denticles.
Discussion
Our results, in combination with a literature review (Tables 1, 2), indicate that there is considerable interspecific variation in ovipositor morphology among the relatively small sample of laniatorean harvestmen that have been examined. Comparisons between taxa are further limited by the fact that the majority of published illustrations are line drawings and usually only depict the lateral perspective, rather than scanning electron microscopy-based photomicrographs of the ventral surface and setae on the distal tip. While the number of external lobes and peripheral setae can be determined from most line drawings, other features such as surface texture and the morphology of peripheral setae are only readily observable with SEM. Thus, even for species with published ovipositor illustrations or descriptions, there remain a number of potential characters that need to be reexamined before the evolution and diversification of the laniatorean ovipositor can be fully appreciated.
Even with this limitation, however, there appear to be several potential characters based upon ovipositor morphology that could provide useful phylogenetic information when evaluating relationships among different clades of laniatorean harvestmen (Tables 1, 2). With the exception of the laterally compressed distal tip of the ovipositor reported for the Oncopodidae (Schwendiger and Martens 2002), the distal tip of most taxa is cylindrical. In addition, our observations lend further support to the hypothesis that the bilobed condition of the distal tip is plesiomorphic for laniatorean harvestmen, and thus, having four external lobes is evolutionarily derived (Giribet and Kury 2007). There are also at least two families (Cosmetidae and Phalangodidae) and one superfamily (Travunioidea) that include species with ovipositors that have two lobes and others that have four lobes (Table 1). Surface texture of the distal tip is readily observable with SEM, but difficult to infer from line drawings; thus, we are limited in our comparisons to SEM-based studies only. In the Petrobunidae (Sharma and Giribet 2011) and Zalmoxidae, there is interspecific variation in the surface texture, ranging from coarse (but not visible denticles) to highly denticulate. In at least two other lineages (Cosmetidae and Gonyleptidae), there are species that have denticles or small conical projections on the distal tip and others that have a smooth surface texture (Table 1). Most taxa examined with SEM have a smooth texture to the ventral surface of the distal tip of the ovipositor (Table 1).
Perhaps the most variable structures associated with the distal tip of the ovipositor are the peripheral setae (Table 2). At least four peripheral setae are present on the distal tip in all laniatorean harvestmen examined, with the exception of the Travuniidae (Martens et al. 1981). In most taxa, each seta has a straight shaft that terminates in an undivided tip (Table 2). However, in two families of gonyleptoideans (Cosmetidae and Stygnidae), the shafts may be curled. The peripheral setae insert into sockets that are generally apart and on the ventral surface, although most species have setae that insert into sockets that occupy a slightly more dorsal position and all of the setae of the zalmoxid species inserted into sockets that were dorsal to the inflatable tip (Table 2). In the Triaenonychidae, there are species that have peripheral setae that are bifid near the base (Hunt and Hickman 1993). Among gonyleptoideans, there are three families (Agoristenidae, Cosmetidae, and Stygnidae) that have species with peripheral setae with multiple divisions at the distal tip. For taxa examined with the SEM (Table 2), the shafts of the peripheral setae are usually finely striated or, in the case of a few cosmetid species, grooved (Walker and Townsend 2014). However, in the Zalmoxidae, the shafts of the peripheral setae are highly flattened (lanceolate) and generally very smooth. The morphology of the peripheral setae that we observed in three genera of zalmoxid harvestmen has not been described for any other laniatorean harvestmen. A review of Goodnight and Goodnight (1983) revealed additional species of Ethobunus and Pachylicus that have similar shapes of peripheral setae, yet we could not discern texture of the setae from the line drawings. We believe that the morphology of the peripheral seta (smooth and lanceolate) on the distal tip of the ovipositor may represent a previously overlooked synapomorphy for the Zalmoxidae.
The functional significance of the peripheral seta on the distal tip of the ovipositor has not been previously investigated. Martens et al. (1981) provided a detailed survey of the internal anatomy of the ovipositor for a diverse variety of harvestmen, including multiple species of laniatorean harvestmen, but did not include ultrastructural observations of the innervation of the peripheral setae. Our observations indicate that these setae are structurally similar to other sensory setae in that they have lumens within the shafts as well as well-developed basal membranes associated with the sockets (Willemart et al. 2007, 2009). However, there have been no ultrastructural studies that have examined the innervation of these setae; thus, the question remains do peripheral setae on the ovipositor function as mechanoreceptors, chemoreceptors, or dual receptors? If the main function of these setae is to interact with the setae on the ventral plate of the penis during copulation (Macías-Ordóñez et al. 2010), then an innervation similar to those observed for other mechanoreceptors in harvestmen should be expected (Guffey et al. 2000). However, if the peripheral setae on the distal tip of the ovipositor are also important in the selection of an appropriate substrate for oviposition (Bennett and Townsend 2013; Walker and Townsend 2014), then these setae may also have an innervation consistent with that of other types of chemoreceptors or dual receptors (Guffey et al. 2000). Observations of copulation and oviposition under field or laboratory conditions as well as additional ultrastructural studies of these peripheral setae are needed to establish the function of these setae and will also enable better comparisons between the peripheral setae and the diverse assortment of setae that have been described for other body regions, e.g., pedipalps (Spicer 1987), legs (Willemart and Gnaspini 2003), and dorsal scutum (Willemart et al. 2007, 2009; Rodriguez et al. 2014).
We believe that the significant variation in ovipositor morphology between families within the Gonyleptoidea (Tables 1, 2) as well as within the Cosmetidae (Walker and Townsend 2014) warrants further study as well as the consideration of the inclusion of ovipositor morphology in future taxonomic descriptions, especially SEM images of non-type materials when possible. Assessment of the usefulness of ovipositor morphology for evaluating phylogenetic relationships between families and within genera will require examinations of considerably more taxa.
References
Bennett MK, Townsend VR Jr (2013) Reproductive morphology of three species of Neotropical harvestmen (Opiliones, Laniatores, Gonyleptoidea). J Morphol 274:1415–1424
Bragagnolo C, Hara MR, Pinto-da-Rocha R (2015) A new family of Gonyleptoidea from South America (Opiliones, Laniatores). Zool J Linn Soc 173:296–319
Briggs TS (1971a) The harvestman family Triaenonychidae in North America (Opiliones). Occ Pap Calif Acad Sci 90:1–43
Briggs TS (1971b) Relict harvestmen from the Pacific Northwest (Opiliones). Pan-Pac Entomol 47:165–178
DaSilva MB, Gnaspini P (2009) A systematic revision of the Goniosomatinae (Arachnida: Opiliones: Gonyleptidae), with cladistics analysis and biogeographic notes. Invert Syst 23:530–624
Giribet G, Kury AB (2007) Phylogeny and Biogeography. In: Pinto-da-Rocha R, Machado G, Giribet G (eds) Harvestmen: the biology of Opiliones. Harvard University Press, Cambridge, pp 62–87
Goodnight ML, Goodnight CJ (1976) Observations on the systematics, development, and habits of Erginulus clavotibialis (Opiliones: Cosmetidae). Trans Am Micro Soc 95:654–664
Goodnight CJ, Goodnight ML (1983) Opiliones of the family Phalangodidae found in Costa Rica. J Arachnol 11:201–242
Guffey CA, Townsend VR Jr, Felgenhauer BE (2000) External morphology and ultrastructure of the prehensile region of the legs of Leiobunum nigripes (Arachnida, Opiliones). J Arachnol 28:231–236
Hara MR, Pinto-da-Rocha R (2010) Systematic review and cladistics analysis of the genus Eusarcus Perty 1833 (Arachnida, Opiliones, Gonyleptidae). Zootaxa 2698:1–136
Hunt GS, Hickman JL (1993) A revision of the genus Lomanella Pocock and its implications for family level classification in the Travunioidea (Arachnida: Opiliones: Triaenonychidae). Rec Aust Mus 45:81–119
Kury AB (2003) Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones). Rev Ibérica Aracnol 1:1–337
Kury AB (2014) Why does the Tricommatinae position bounce so much within Laniatores? A cladistics analysis, with description of a new family of Gonyleptoidea (Opiliones, Laniatores). Zool J Linn Soc 172:1–48
Kury AB, Villareal Manzanilla O, Sampaio C (2007) Redescription of the type species of Cynorta (Arachnida, Opiliones, Cosmetidae). J Arachnol 35:325–333
Machado G, Macías-Ordóñez R (2007) Reproduction. In: Pinto-da-Rocha R, Machado G, Giribet G (eds) Harvestmen: the biology of Opiliones. Harvard University Press, Cambridge, pp 414–454
Macías-Ordóñez R, Machado G, Pérez-González A, Shultz JW (2010) Genitalic evolution in Opiliones. In: Leonard J, Cordoba-Aguilar A (eds) The evolution of primary sexual characters in animals. Oxford University Press, New York, pp 285–306
Martens J (1986) Die Großgliederung der Opiliones und die evolution der Ordnung (Arachnida). In: Barrientos JA (ed) Proceedings of the 10th international congress of arachnology. Instituto Pirenaico de Ecología & Grupo de Aracnología, Barcelona, Spain, pp 289–310
Martens J, Hoheisel U, Götze M (1981) Comparative anatomy of the ovipositors of the Opiliones as a contribution to the phylogeny of the order (Arachnida). Zool Jb Anat 105:13–76
Maury EA (1988) Triaenonychidae sudamericanos. V. Un nuevo género de opiliones cavernícolas de la Patagonia (Opiliones, Laniatores). Mém Biospéol 15:117–131
Nation JL (1983) A new method for using hexamethyldisilazane for preparation of soft insect tissue for scanning electron microscopy. Stain Technol 58:347–351
Pinto-da-Rocha R, Benedetti AR, de Vasconcelos EG, Hara MR (2012) New systematic assignments in Gonyleptoidea (Arachnida, Opiliones, Laniatores). ZooKeys 198:25–68
Pinto-da-Rocha R, Bragagnolo C, Marques FPL, Junior MA (2014) Phylogeny of harvestmen family Gonyleptidae inferred from a multilocus approach (Arachnida: Opiliones). Cladistics 30:519–530
Proud DN, Víquez C, Townsend VR Jr (2011) Paternal care in a Neotropical harvestman (Arachnida: Cosmetidae) from Costa Rica. J Arachnol 39:497–499
Rodriguez AL, Townsend VR Jr, Johnson MB, White TB (2014) Interspecific variation in the microanatomy of cosmetid harvestmen (Arachnida, Opiliones, Laniatores). J Morphol 275:1386–1405
Schwendiger PJ, Martens J (2002) Penis morphology in Oncopodidae (Opiliones, Laniatores): Evolutionary trends and relationships. J Arachnol 30:425–434
Sharma PP, Giribet G (2011) The evolutionary and biogeographic history of the armoured harvestmen—Laniatores phylogeny based on ten molecular markers, with the description of two new families of Opiliones (Arachnida). Invert Syst 25:106–142
Šilhavỳ V (1977) Further cavernicolous opilionids from Mexico. Quad Acc Naz Lincei 171:219–233
Šilhavỳ V (1979) Opilionids of the suborder Gonyleptomorphi from the American caves, collected by Dr. Pierre Strinati. Rev Suisse Zool 86:321–334
Spicer GS (1987) Scanning electron microscopy of the palp sense organs of the harvestman Leiobunum townsendi (Arachnida: Opiliones). Trans Am Micro Soc 106:232–239
Walker EA, Townsend VR Jr (2014) Ovipositor morphology of cosmetid harvestmen (Arachnida, Opiliones, Laniatores): a new source of informative characters. J Morphol 275:1376–1385
Willemart RH, Gnaspini P (2003) Comparative density of hair sensilla on the legs of cavernicolous and epigean harvestmen (Arachnida: Opiliones). Zool Anz 242:353–365
Willemart RH, Chelini MC, De Andrade R, Gnaspini P (2007) An ethological approach to a SEM survey on sensory structures and tegumental gland openings of two Neotropical harvestmen (Arachnida, Opiliones, Gonyleptidae). Ital J Zool 74:39–54
Willemart RH, Farine J-P, Gnaspini P (2009) Sensory biology of Phalangida harvestmen (Arachnida, Opiliones): a review, with new morphological data on 18 species. Acta Zool 90:209–227
Acknowledgments
This research was supported by the VWC Science Undergraduate Research Fund (MSB), the VWC Electron Microscopy Lab, and summer faculty development grants (VRT). Specimens were collected in the field with help from D. Proud (University of Louisiana at Lafayette), C. Víquez (INBio), M. Moore (Mercer University), J. Campbell (High Point University), A. Abbate, B. Campbell (University of Florida), and Stephen Broadbridge (Caribbean Discovery Tours). Special thanks to Andrea Rodriguez for assistance with specimen preparation and the operation of the SEM, and Daniel Proud and two anonymous reviewers for insightful comments on earlier versions of this manuscript. Specimens were legally exported from Trinidad (001339) and Costa Rica (01607). Voucher specimens were deposited into the natural history collection at the American Museum of Natural History, New York.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Townsend, V.R., Bertram, M.S. & Milne, M.A. Variation in ovipositor morphology among laniatorean harvestmen (Arachnida: Opiliones). Zoomorphology 134, 487–497 (2015). https://doi.org/10.1007/s00435-015-0269-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-015-0269-4