Abstract
Intestinal villi of Caiman yacare form longitudinal folds instead of the finger-like projections of most birds and mammals. Moreover, they lack Crypts of Lieberkühn and the lamina epithelialis organization is dynamic, changing from pseudostratified to simple columnar epithelium after feeding. Because of these differences, we sought to verify whether intestinal villi of the crocodilian Caiman yacare are functionally compartmentalized along their length similarly to the finger-like villi that harbors Crypt of Lieberkühn. For this, Caiman yacare were force-fed soybean oil, the intestinal mucosa was harvested and analyzed under light microscopy after lipid staining or immunohistochemistry for the proliferative marker PCNA. Functional compartmentalization was assessed by evaluating differences in lipid absorption along intestinal villi base-to-tip axis, by localizing the proliferative enterocytes and by verifying whether such cells were capable of absorbing lipids. Histological morphometric analyses of the extent of enterocyte hypertrophy caused by lipid inclusions and the contribution of such inclusions to histological remodeling from pseudostratified to simple columnar epithelium were also evaluated. Although lacking Crypts of Lieberkühn, enterocytes present at villi base were PCNA positive and devoid of the great amount of lipid inclusions observed in the other intestinal villi domains, in a similar pattern to finger-like villi. Enterocytes doubled their volume because of lipid inclusions, and in spite of such enterocyte hypertrophy, lamina epithelialis continued to be pseudostratified within lateral sides, whereas villi tip were organized in a simple columnar epithelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal villi are asymmetrically patterned along the Crypt of Lieberkühn-to-tip axis. In mammals and in birds, for instance, the basal domain form crypts and contain proliferative and pluripotent intestinal stem cells. Enterocytes along the length of the villi are absorptive cells and at the very tip, enterocytes are necrotic cells that are being sloughed from epithelium. At various heights within intestinal villi, lamina epithelialis is organized in a simple columnar epithelium and continuous cell differentiation and migration from basal domain towards intestinal villi tip ensures renewal of the absorptive tissue (Barker 2014; Williams et al. 2015).
Although in non-avian sauropsids cell shedding occurs at villi tip similarly in mammals and birds (Starck and Beese 2001; Lignot et al. 2005; Starck et al. 2007; Helmstetter et al. 2009a, b; Borges et al. 2016) and lipid absorption occurs along the length of the villi, the domain of proliferative cells present variations among species. For instance, as demonstrated in the snake Python molurus (Helmstetter et al. 2009b), proliferative cells are randomly distributed along the length of the intestinal villi, whereas in the turtle Crysemys picta (Wurth and Musacchia 1964), proliferative cells are concentrated in the base but also scattered along the villi troughs and at the apical domains. In the lizard Sceloporus occidentalis (Johnson et al. 1967), proliferative cells are contained in the basal domain. No other non-avian sauropsids have been studied regarding the localization of proliferative cells within intestine (Jacobson 2007).
In the mammal and avian small intestine, lamina propria supporting intestinal villi is thick in the proximity to the submucosa forming the Crypts of Lieberkühn, where the proliferative and stem cells are present (Sailaja et al. 2016). The Crypts of Lieberkühn have a narrow opening to the luminal surface that hypothetically serve as a physical barrier impairing the contact of such cells to the dietary lipids and bile salts, known to be cytotoxic. Indeed, mammalian enterocytes from Crypts of Lieberkühn are capable of absorbing lipids when cultured in vitro and are more prone to membrane microruptures and to increased transcellular permeability than enterocytes from along the length of the intestinal villi (Bateman et al. 2007; Danielsen et al. 2013).
Non-avian sauropsids intestine lack intestinal crypts, harboring only a shallow groove as demonstrated in histological cross sections of intestines of the snakes Xenodon merremii (Ferri et al. 1976), Thamnophis sirtalis parietalis (Starck and Beese 2002) and Python molurus bivittatus (Secor and Diamond 2000; Starck and Beese 2001; Lignot et al. 2005; Secor 2005); of the turtles Crysemys picta (Wurth and Musacchia 1964) and Chelonia mydas (Magalhães et al. 2010); of the gecko Hemidactylus mabouia (Rodrigues-Sartori et al. 2014); and of the crocodilian Caiman yacare (Jin et al. 1991; Aleixo et al. 2011), whereas in the crocodilian Caiman latirostris the crypts are only poorly developed (Starck et al. 2007). This suggests that the proliferative enterocytes within these species are in direct contact to dietary lipids and bile salts, similarly to those species that poses proliferative cells dispersed at villi epithelium lateral sides. Therefore, it is possible that proliferative cells are also capable of absorbing lipids, and would be more resistant to deleterious effects of dietary lipids and bile salts or, alternatively, such cells do not absorb lipids due to other mechanisms at play, such as presence of a molecular barrier to lipids in membranes (Danielsen et al. 2013).
It is not known whether proliferative cells are scattered in crocodilians intestinal villi lamina epithelialis or restricted to the villus base and it is not known whether such cells are capable of absorbing lipids. Localization of proliferative enterocytes in lamina epithelialis of the crocodilian Caiman yacare intestinal villi and, importantly, whether or not the proliferative cells within Caiman yacare intestine are capable of absorbing lipids are addressed in the present work by means of immunohistochemistry with the general proliferative marker PCNA.
It was demonstrated that in some species of non-avian sauropsids, intestinal mucosa hypertrophy caused by feeding is associated to intestinal villi elongation and to lamina epithelialis histological reorganization: from pseudostratified epithelium in the fasting state to simple columnar epithelium after feeding (Starck and Beese 2001, 2002; Lignot et al. 2005; Secor 2005; Starck et al. 2007; Helmstetter et al. 2009b). The extent on how much intestinal morphological phenotypic changes occur is related to long-range feeding status, feeding frequency and also to the species considered (Secor and Diamond 2000; Secor 2005). For instance, in frequent feeders such as Thamnophis sirtalis parietalis (Starck and Beese 2002) and Caiman latirostris (Starck et al. 2007) such changes are more modest than the observed in the infrequent feeders with long episodes of fasting such as Python molurus (Starck and Beese 2001). Evidences suggest that the transition from pseudostratified to simple columnar epithelium in crocodilians is less evident because of the modest intestinal morphological changes probably caused by the frequent feeding (Secor 2005).
Intestinal mucosa lamina epithelialis reorganization caused by feeding is accompanied by enterocyte hypertrophy that in turn is supposedly caused by the hygroscopic properties of absorbed amino acids and sugars (Secor et al. 2002). Although the high amounts of cytoplasmic lipid inclusions after a meal also causes enterocyte hypertrophy (Secor et al. 2002), it is believed that such inclusions do not cause intestinal lamina epithelialis reorganization. The last aim of the present work is to test whether cytoplasmic lipid inclusions that cause enterocyte hypertrophy are sufficient for histological reorganization of C. yacare intestinal lamina epithelialis.
For this, and to avoid interferences of other dietary components, a vegetable oil alone was injected into Caiman yacare stomach. Lipid absorption by enterocytes positioned at different locations of intestinal villi was evaluated in histological sections, to verify whether proliferative enterocytes absorb lipids, as well as the distribution and quantification of proliferative cells within intestinal lamina epithelialis. Moreover, it evaluated the degree of enterocyte hypertrophy caused by lipid inclusions and the contribution of this event to histological organization of intestinal lamina epithelialis.
Materials and methods
Animal housing
The four juvenile (31.7 ± 3.8 cm jaw-cloaca length) specimens used herein are a subset of the animals used in a previous experimentation published in Borges et al. (2016). Animals were kindly donated by the Cooperativa dos Criadores de Jacaré do Pantanal (COOCRIJAPAN), Cáceres, Mato Grosso, Brazil. Animals were kept in cement tanks with water at 24 °C and a dry area (air temperature 30 °C) in February. They were fed crude bovine offal mixed with vitamins and mineral mix (Aleixo et al. 2002) until being fasted for 5 days before beginning experimentations. Animals were force-fed 5 ml of soybean oil or mineral water with the aid of a silicone rubber tube passing through the mouth and killed with a dart shot in the skull frontal bone 24 h after the intragastric injection.
Animal handling and experimentation were approved by the committee on animal welfare and ethical experimentation from Institute of Biomedical Sciences, University of Sao Paulo (protocol number 131, page 110, book 02; issued September 20th, 2011) and by Brazilian environmental agency (ICMBIO/MMA/SISBIO: authorization number 30509-2; issued August 30th, 2011).
Histology
Five millimeter thick intestinal cross sections were harvested from small intestine (duodenum, cranial jejunum and caudal jejunum), fixed in 10% formalin and afterwards in 2% osmium tetroxide. Samples were embedded in glycol methacrylate Historesin (Leica Microsystems), sectioned at 2 µm thickness and stained with 0.1% Safranin as described in details in Borges et al. (2016).
Immunohistochemistry
Intestine transversal segments (duodenum, cranial jejunum and colon) of 1 cm were embedded in paraffin and cut to 5 µm thickness. Whole cross sections were harvested in silanized glass slides, de-paraffinized, re-hydrated in decreasing ethanol grade series and washed in phosphate buffer saline (PBS). Antigen retrieval was performed with 0.1 M citrate buffer at 90 °C for 10 min (Pressinotti et al. 2013). Endogenous peroxidase was blocked by treating the sections with 3% of hydrogen peroxide diluted in methanol (30 min). The proliferating cell nuclear antigen (PCNA) rabbit polyclonal IgG primary antibody (Santa Cruz Biotechnology, 1:200), used for identification of proliferating cells present in the intestinal villi, was diluted in PBS containing 20% Bovine Serum Albumin (BSA). Slides were incubated with primary antibody for 12 h at 4 °C. Novolink Novocastra Polymer Detection System (Leica Microsystems), which uses DAB as chromogen, was used for detecting primary antibody binding. The remaining steps were performed according to the manufacturer’s manual. The slides were dehydrated in graded series of ethanol and mounted permanently in Entellan. Negative control of immunohistochemistry was performed by omitting primary antibody in the reaction and positive reactions were monitored by staining of intraepithelial lymphocytes (Pressinotti et al. 2013) and background level was monitored by the presence of negative stained nuclei within lamina epithelialis.
Lamina epithelialis lipid inclusions quantification
Lamina epithelialis of different intestinal villi functional domains (intestinal villi base, lateral side and villi tip) were evaluated for lipid inclusions with the aid of ImageJ 1.43 m software (NIH, Bethesda, USA) as described in Borges et al. (2016). Twenty intestinal villi functional domains (base, lateral and tips) from small intestine (duodenum, cranial jejunum or caudal jejunum) of the two animals that received soybean oil that had the most intense osmiophilic lipid staining were evaluated. Data are presented as mean and standard deviation area of each lamina epithelialis intestinal villi domains that were occupied by lipid inclusions.
Quantification of cell proliferation
Five intestinal villi from each intestinal segment (duodenum, cranial jejunum and colon) of the two animals that received soybean oil and the two control animals were evaluated after PCNA immunohistochemistry. Because PCNA immunostaining of enterocytes gradually faded towards intestinal villi tips, without distinct borders between positive and negative PCNA enterocytes, all enterocytes that were brownish irrespective of intensity were considered as positive. Only oval nuclei were counted to avoid counting PCNA-positive intraepithelial lymphocytes. Data are presented as median and interquartile ranges [Q2(Q1–Q3)] of PCNA positive enterocytes in relation to total of enterocytes present in each intestinal segment.
Intestinal mucosa morphometry
Enterocyte hypertrophy was estimated by three parameters: (1) cytoplasmic total length; (2) cytoplasmic length in relation to nucleus length; and (3) cytoplasmic diameter (Jackson and Perry 2000; Lignot et al. 2005; Andrew et al. 2015). Only duodenal enterocytes that were present at lateral sides of lamina epithelialis were evaluated. Slides were photographed under 100× objective (ZEISS EC Plan-NEOFLUAR 100x/1.3 oil ph 3). Measurements were performed in micrographs of semi-thin resin histological sections with the aid of ImageJ (v. 1.43 m), after drawing a straight line from cell base to apex of the cells, parallel to the greater axis of the nucleus that was also measured. Cell cytoplasmic diameter was estimated by measuring a straight line at the supranuclear cytoplasm and tangential to the nucleus. Cell volume was estimated by applying cell length and diameter measurements to the equation of a cylinder, according to Jackson and Perry (2000), Lignot et al. (2005) and Andrew et al. (2015).
Enterocyte relative length was estimated to avoid biased measurements due to variations in histological sectioning planes. Measurements of lipid-free enterocytes present in intestinal villi without any sign of lipid inclusions of animals that received soybean oil and measurements of enterocytes from mineral water injected animals were both used as controls. This was done to have a second point of comparison for control (lipid-free enterocytes) and avoid bias that could emerge from differences of intestinal muscular contractions among sampled animals. At least 100 cells were measured for each condition (two animals that received soybean oil and two control animals) distributed in 33 intestinal villi. For ensuring independency of the measurements taken, groups of enterocytes were measured from distinct intestinal villi. Data are presented as mean and standard deviation of cell cytoplasm-to-nucleus length ratio, cytoplasmic length and cytoplasmic diameter for experimental or the both control conditions (Jackson and Perry 2000).
Statistics
Two-level nested analysis of variance (McDonald 2014) was performed for testing: (1) differences of lipid loading among intestinal villi domains; and (2) differences in enterocyte morphometry measurements among lipid-loaded enterocytes and enterocytes free of lipids, measured from control animals and from animals force-fed soybean oil.
For testing differences in lipid inclusions in different functional domains of lamina epithelialis, the groupings considered were intestinal villi base, lateral domain or villi tip and the subgroupings were measurements taken from each of the two animals that received soybean oil injection. For enterocyte morphometry, different treatments were grouped with subgroups being considered the intestinal villi that had multiple enterocytes measured. Normality and homoscedastic tests were evaluated with aid of Statistica software to authorize ANOVA and Tukey post-test.
Differential effect of treatment (soybean oil versus mineral water) in PCNA positive enterocytes in a given intestinal segment was evaluated by the non-parametric Mann–Whitney U test and the difference in PCNA positive enterocytes among intestinal segments irrespective of treatment was evaluated by Kruskal–Wallis followed by Dunn’s multiple comparisons test.
In all analysis, α level was set at 0.05. Statistical analysis was performed using GraphPad Prism for Windows (v.5.01, 2007) and Microsoft Office Excel for Windows (v2007).
Results
Functional compartmentalization of intestinal villi lamina epithelialis
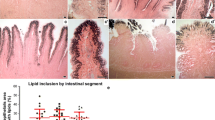
Lipid inclusions were observed in the cytoplasm of enterocytes present at intestinal villi tips (Fig. 1a). Lamina epithelialis localized at intestinal villi tips had 35.7 ± 7.8% of their areas occupied by lipids. Lipid-absorptive enterocytes were also present along the length of the villi (Fig. 1b), where 27.7 ± 4.9% of lamina epithelialis area were occupied by lipid inclusions. On the contrary, almost every intestinal villus analyzed (68.9% of intestinal villi observed) lacked lipid inclusions in their base (Fig. 1c, d), albeit lacking Crypts of Lieberkühn. Whenever lipid inclusions were observed in the enterocytes at the intestinal villi base, lipid inclusions were much less, never occupying more than 10.9% (mean 2.2 ± 2.9%) of lamina epithelialis area, and the particle sizes observed were much smaller. Differences between enterocytes localized in intestinal villi base and enterocytes positioned laterally in the intestinal villi or in the apices were statistically significant, as well as between lateral located and apical enterocytes F(2,3) = 95.60, p < 0.005.
Functional compartmentalization of the Caiman yacare intestinal villi. Enterocytes present at the villus tip (a) and along the length of the villus trough (b) are absorptive for lipids, whilst the enterocytes present at intestinal villi base do not show lipid staining (c). Mean and standard deviation for lamina epithelialis area occupied by lipid inclusions in different intestinal villi domains. Different letters indicate statistically significant differences (d). a–c Historesin sections stained with safranin and osmium tetroxide. Bar: 50 µm (a, b); 100 µm (c)
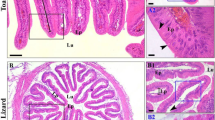
Although lacking typical intestinal crypts, the cell proliferative marker PCNA were observed in intestinal villi base, a region where enterocytes were devoid of lipid inclusions (Fig. 2a, b). PCNA immunostaining, however, was not very restricted to the shallow groove, but extended laterally to the troughs of intestinal villi at some extent from the base, a place where also absorptive enterocytes were observed (Fig. 2a, b). After 24 h of soybean oil injection, the median frequencies of enterocytes PCNA positive in the intestine of treated animals were 20.5% (10.4–26.6), 25.1% (20.8–33.7) and 47.5% (29.2–64.5), respectively, for duodenum (Fig. 3a, j), cranial jejunum (Fig. 3b, j) and colon (Fig. 3c, j). For duodenum, cranial jejunum and colon of control animals, PCNA positive enterocytes were, respectively, 14.0% (10.2–18.6), 25.2% (19.0–42.4) and 30.9% (24.8–40.7). Differences observed between a given intestinal segment of the animals that received oil and their counterpart animals that received mineral water were not statistically significant by Mann–Whitney U test: U = 36.00, p = 0.32 (duodenum); U = 47.00, p = 0.85 (cranial jejunum); U = 14.00, p = 0.21 (colon). Absence of significant difference does not mean equality of data.
Proliferative enterocytes are present in the intestinal villi base domain and do not absorb lipids (a). In this domain, enterocytes are positive for PCNA immunohistochemistry (b). There were some enterocytes in between these two domains that were either absorptive enterocytes and also positive for PCNA. In this region there are cells with nuclei stained brown and showing supranuclear vacuoles, places of lipid inclusions that were washed out because of immunohistochemistry processing. Note that cells between red dotted lines do not show such vacuoles. In (a), dotted line denotes the limits of the tunica submucosa and the tunica muscularis to indicate that the enterocytes were indeed close to the intestinal villi base and in (b), indicate the domain where possibly the true stem cells that do not absorb lipids are hypothetically located. a Historesin sections stained with safranin and osmium tetroxide; b PCNA immunohistochemistry. Bar: 50 µm
Soybean oil absorption did not increase enterocytes proliferation. Immunohistochemistry for PCNA in the intestinal mucosa of Caiman yacare killed 24 h after soybean oil oral injection (a–c) or of animals that received mineral water (d–f). Duodenum, cranial jejunum and colon are depicted, respectively, from the first to the third columns. The PCNA positive enterocytes were restricted to the villus base and the proliferative domain did not expand towards the villus tip after the soybean oil oral injection. Control of immunohistochemistry specificity reaction was performer by omitting primary antibody: duodenum (g), cranial jejunum (h) and colon (i). Differences of PCNA positive enterocytes observed between treatments within a given intestinal segment were not found to be statistically significant (j). Regarding the Antero-Posterior axis of intestine, however, colon had significantly more proportion of PCNA positive enterocytes than duodenum but not in comparison to jejunum (k). Median and interquartile ranges of PCNA positive cells in relation to total enterocytes counted per intestinal villi within a given segment are shown. Different letters indicate statistical differences. Bar: 250 µm
Colonic intestinal villi appeared to have immunostaining pattern expanded more laterally in the villi troughs of both soybean oil treated animals (Fig. 3c) and controls (Fig. 3f), in comparison to duodenum or to cranial jejunum segments. To corroborate this view, PCNA positive enterocytes of control and oil treated groups from each segment irrespective of treatment were summed up and re-analyzed amplifying the power resolution of the test. Median PCNA positive enterocytes respective for duodenum, cranial jejunum and colon were 16.7% (10.6–25.1); 25.1% (20.4–35.2); 38.0% (28.3–60.8). The difference between colon and duodenum was statistically different by Kruskal Wallis/Dunn´s post-test [H(2) = 20.18, p < 0.0001], but not between colon and cranial jejunum (Fig. 3k). Control of immunohistochemistry reaction specificity was obtained by omitting the primary antibody (Fig. 3g–i).
Histological remodeling of intestinal villi lamina epithelialis during lipid absorption
Enterocyte nuclei from duodenum and cranial jejunum of oil-treated group were lined close to basement membrane and formed an evident supranuclear cytoplasm mass (Fig. 4a, b), whereas the nuclei of control group were dispersed within lamina epithelialis and there were fewer supranuclear cytoplasm (Fig. 4d, e), suggesting hypertrophy of the enterocytes and/or reorganization of lamina epithelialis histology of the animals that received dietary lipids. In the colon such differences were not observed (Fig. 4c, f).
Greater magnification of intestinal villi base with immunohistochemistry for PCNA epitope to reveal localization of proliferative enterocytes. First row in the panel are representative pictures of intestinal villi of animals that received soybean oil. Note that enterocyte nuclei of small intestine segments (duodenum: a; cranial jejunum: b) are restrained to the villi base and are organized closer to basal portion of the tissue, when compared to the more diffuse aspect of the intestinal villi obtained from control animals and appear to have thinner supranuclear cytoplasm (duodenum: d; cranial jejunum: e). This histological organization is less obvious in the colon (lipid loaded: c; control: f). Bar: 25 µm
Mean cell volume of enterocytes greatly loaded with lipids was (4.2 ± 2.0) × 103 µm3, a 100% volume increase in relation to enterocytes of control animals (2.1 ± 0.6) × 103 µm3, and 133.33% increase in relation to enterocytes lacking lipid inclusions of soybean oil force-fed animals (1.8 ± 0.8) x 103 µm3. Mean differences between lipid-loaded enterocytes and enterocytes without lipid inclusions of soybean oil injected animals or control animals injected with mineral water were both statistically significant F(2,30) = 7.09, p = 0.003 (Table 1; Fig. 5a).
Histoarchitectural organization of intestinal villi lamina epithelialis is not changed by lipid inclusions. Intestinal villi domains closer to intestinal villi tips are predominantly composed of a simple columnar epithelium either when devoid of lipid inclusions as in the control animal intestinal mucosa (a) or when mucosa is loaded with lipids (b). The lamina epithelialis closer to the intestinal villi base formed a pseudostratified epithelium, independent of the lipid accumulation as seen in the control intestinal mucosa (c) and in the intestinal mucosa of the animals that received soybean oil oral injection (d). Example of an intestinal villi base with the greatest accumulation of lipid inclusions measured (E). This domain is pseudostratified. Historesin sections stained with safranin and osmium tetroxide (a–e). Bar: 10 μm
Total length and cytoplasmic-to-nucleus relative length of enterocytes with lipid inclusions (Table 1) were both greater than the enterocytes of lipid-free enterocytes measured from control animals and from animals that received soybean oil. This suggests that the greater length observed in the enterocytes with lipid inclusions was not due to differences in sectioning planes. Enterocyte width did not change significantly between lipid-loaded enterocytes and from control animals (Table 1).
Spatial distribution of enterocytes nuclei within lamina epithelialis varied in relation to their positions along the length of the intestinal villi, but not in relation to the amount of lipid inclusions (Fig. 5). While in the lateral domains closer to villi tip there was a prevalence of simple columnar epithelium, either in the controls and in the intestines with presence of great amount of lipid inclusions (Fig. 5a, b), further in direction to villus base, the prevalent organization of lamina epithelialis was pseudostratified columnar epithelium, even when no lipid inclusions were observed (Fig. 5c, d). Intestinal villi base epithelia were always pseudostratified (Fig. 5e).
Discussion
Functional compartmentalization of intestinal villi lamina epithelialis
Caiman yacare intestinal mucosa internal relief pattern is formed by lengthwise folds, interconnected by lower orthogonal folds, giving the internal relief a zigzag pattern (Parsons and Cameron 1977; Aleixo et al. 2011). Moreover, in histological cross sections, typical crypts of Lieberkühn of mammals and birds do not form and only a shallow groove can be observed. Both morphological aspects are the most common condition for non-avian sauropsids, whereas the finger-like projections and presence of intestinal crypts resembling mammals, and some birds are restricted to only some species of squamates in the non-avian sauropsids group (Parsons and Cameron 1977; Lignot et al. 2005; Helmstetter et al. 2009a, b). Whenever crypts of Lieberkühn are present in non-avian sauropsids such as in Caiman latirostris (Starck et al. 2007), it is considered underdeveloped in comparison to mammals. These two chief intestinal morphological differences led us to investigate whether functional organization of the longitudinal folds without Crypts of Lieberkühn of the Caiman yacare is similar to the well-described finger-like projections that present intestinal crypts.
In Caiman yacare, intestinal proliferative cells, as identified herein by PCNA immunohistochemistry, were located in intestinal villi base and some cells laterally to the basal domains of villi troughs, in a similar pattern to the lizard Sceloporus occidentalis (Johnson et al. 1967). Proliferative cells are randomly distributed along the length of the intestinal villi lamina epithelialis of Python molurus (Helmstetter et al. 2009b) or concentrated in the base and presenting large number of cells along the length of the lamina epithelialis and also reaching the villi tip of the turtle Crysemys picta (Wurth and Musacchia 1964). No other non-avian sauropsids were studied; therefore, it is not known which of the three conditions is more frequent within the group. Python molurus presents finger-like projections of the mucosa and in the turtle Crysemys picta, the villi form longitudinal and parallel folds, the same pattern observed in Caiman yacare and lizards intestinal mucosae. Therefore, there is not a correlation between type of intestinal villi projection (villi versus fold) and pattern of localization of proliferative intestinal cells.
PCNA immunostaining for quantification of proliferative cells is frequently criticized because it is also expressed in cells going through DNA repair. However, it gives an instant picture of the proliferating and post-proliferating cells. Proliferation assessment by means of Thymidine/BRDU incorporation has also its controversy, especially because the pattern of proliferative cells can be altered depending on the period of incubation with the thymidine analogue, Regardless the fact that counting of proliferative intestinal cells labeled with PCNA is usually overestimated in comparison to short pulse BRDU/thymidine incorporation, there is no reason to believe that the localization pattern of such cells are dissimilar when both approaches are compared, i.e., restricted to the intestinal villi base versus randomly distributed in lamina epithelialis (Kubben et al. 1994; Yu et al. 2010). That being said, we believe that the comparison tailored above about distribution pattern of BRDU/thymidine labeled proliferative cells described in the intestine of Python molurus, Crysemys picta, and Sceloporus occidentalis with the PCNA immunostaining in Caiman yacare (our data) is possible to be made. It is also important to stress out that the samples herein studied were from young animals and drastic tissue remodeling can be associated to ontogenetic diet shifts in other species. Santos et al. (1996) did not find a correlation of food items preyed and size classes of Caiman yacare, concluding that there is not major ontogenetic diet shifts in this species. Therefore, although the number of PCNA positive enterocytes and the extent to which those cells localize laterally to the intestinal villi troughs may be different in adult animals, there is not a priori reason to believe that the pattern of proliferative cells will be different in such animals.
Although lacking Crypts of Lieberkühn, a morphological characteristic of intestinal mucosa that hypothetically may hinder lipids to reach intestinal base in mammalian/avian intestine, intestinal proliferative cells in Caiman yacare lacked the great concentrations of lipid inclusions typical of enterocytes located along the length of intestinal villi lamina epithelialis, in a similar pattern to Crypt-harboring species. Current view about dietary lipid absorption by enterocytes states that it is a passive process, not requiring cell membrane transporters (Abumrad and Davidson 2012). Because lacking the physical barrier promoted by Crypt of Lieberkühn and because free fatty acids and bile salts are cytotoxic (Bateman et al. 2007; Danielsen et al. 2013), such species may present an alternative molecular mechanism to impair lipid absorption by proliferative cells and their deterioration. Interestingly, in the colon, where comparatively much less lipids are absorbed and domains of absorption is restricted to intestinal villi tips (Borges et al. 2016), the domain of PCNA-positive enterocytes were more expanded laterally in the villi troughs, maybe because of the very little dietary lipids and bile salts that reach this intestinal segment.
A possible mechanism of protection for intestinal stem cells restricted at the intestinal villi base would be the differential expression of members of fatty acid binding proteins (FABP) between enterocytes located along the length of the intestinal villi and at intestinal base. FABPs are cytoplasmic lipid transporters that bind free fatty acids and direct fatty acids from juxtamembrane domains to endoplasmic reticulum (Gajda and Storch 2015). In reticulum, the large cytoplasmic lipid droplets are assembled, avoiding free fatty acids cytotoxic effects (Alpers et al. 2000; Guilmeau et al. 2007; Levy et al. 2009; Hayashi et al. 2013).
One may speculate that the efficiency of enterocytes along intestinal villi for lipid absorption would function as a barrier preventing lipids reaching the proliferative cells located at the very base; however, within 24 h lipids are observed in colon of the treated animals (Borges et al. 2016), suggesting that the amount of lipids used were not a limiting factor and probably reached the basal portion of intestinal villi. Indeed, some lipid drops were observed in the intestinal lumen near intestinal villi base (data not shown).
Histological remodeling of lamina epithelialis during lipid absorption
We observed that histological organization of Caiman yacare intestinal mucosa lamina epithelialis was related to the position along intestinal villi length. Lateral sides and basal enterocytes were organized in a pseudostratified epithelium, whereas the villi tip was organized in a simple columnar epithelium. This is clearly different from the organization described for mammals and birds, which presents simple columnar epithelium irrespective of the position along finger-like intestinal villi. As discussed below, in crocodilians and other non-avian sauropsids that harbor intestines with fold-like intestinal villi, the histological organization of lamina epithelialis may differ from the non-avian sauropsids that harbor from the finger-like intestinal villi.
It was demonstrated that the histological organization of intestinal mucosa lamina epithelialis of some snakes is correlated to intestinal mucosa hypertrophy that in turn is influenced by feeding status. Infrequent feeders with long periods of aphagia, the Python molurus being the most prominent representative, present very drastic intestinal morphological changes upon feeding (Lignot et al. 2005; Secor 2005; Helmstetter et al. 2009b). Such morphological alterations provoked by feeding are intestinal mucosa thickening, enterocyte hypertrophy and elongation of intestinal villi along basal-to-tip axis that reorganizes enterocytes within lamina epithelialis changing from pseudostratified to simple columnar epithelium. Restoration to pseudostratified epithelium occurs in post-feeding condition when mucosal thickness restores to pre-feeding values. On the other hand, the non-avian sauropsids that are frequent feeders such as the turtles Chelydra serpentina, Trachemys scripta and Sternotherus odoratus, intestinal mucosa hypertrophy is not observed (Secor and Diamond 1999) and presumably, according to this model, histological organization of lamina epithelialis would not change upon feeding. Accordingly, Wurth and Musacchia (1964) showed that organization of Chelydra serpentina intestinal mucosa lamina epithelialis of fed animals is pseudostratified in lateral and basal domains, although simple columnar epithelium in the tip.
Caiman yacare is a frequent feeder, although experiencing large periods of aphagia during aestivation in the dry seasons (Campos et al. 2006). Crocodilians are considered as modest regulators of intestinal phenotype (Secor 2005), and therefore, the mucosa thickness of animals that are frequently fed indeed show such moderate mass increase (Starck et al. 2007) and consequently, histological reorganization would not be so drastic. Frequently fed Caiman latirostris intestinal hypertrophy restores to the fasting condition within 5–6 days after feeding (Starck et al. 2007). Animals used herein had been fed every other day and fasted for 5 days before experimentation. These data let us to conclude that both experimental and control animals herein described had intestinal mucosa phenotype similar to the fasting state. Therefore, the simple columnar epithelium observed in intestinal villi tips may occur independent of the feeding status, similarly to the turtle Chelydra serpentine (Wurth and Musacchia 1964). The histological organization pattern herein described can also be observed in Aleixo et al. (2011), where subadult Caiman yacare were fasted for a week after a frequent feeding regimen and in postprandial Alligator mississippiensis and Crocodylus porosus (Tracy et al. 2015).
Our observation do not exclude the model of histological organization transitional changes due to feeding, especially for animals fed after a 3 month fasting, when wide morphological intestine upregulations take place (Starck et al. 2007). It is proposed an additional complexity, i.e., whereas enterocytes located along the length of the intestinal villi and possibly at intestinal base at some extent change histological organization upon feeding, enterocytes at intestinal villi tips may be less prone to reorganization and constantly maintain the simple columnar epithelium organization. Because enterocytes present closer to intestinal villi tip were arranged in a simple columnar epithelium and are the enterocytes to be sloughed from lamina epithelialis in the physiological process of epithelial turnover, it is tempting to speculate the relationship between the two phenomena to explain physiology in relation to morphology.
Enterocyte shedding in mammal intestine occurs not restricted to the intestinal villi tip, but also in the lateral domain somewhat closer to the tip, at the upper fourth length of villi (Kiesslich et al. 2007; Watson et al. 2009). This represents grossly the same position of the simple columnar epithelium organization observed in Caiman yacare lamina epithelialis tip. The mechanism that actively drives enterocyte shedding is partially known for mammals that, interestingly, present a simple columnar epithelium organization. Therefore, this epithelial organization may be important for the process, as cells that are neighbors to the cell to be shed protrudes lamellipodia laterally and form points of cell–cell contacts in a zipper-like fashion underneath the cell to be excluded to actively exclude the cell and to seal the gap left by the excluded enterocyte, maintaining the integrity of lamina epithelialis barrier (Kiesslich et al. 2007; Watson et al. 2009). The presence of lipids in the intestinal villi tip enterocytes, which are also the cells most likely to engage in cell shedding, represents a potential paradox, especially because it is believed that enterocytes represent a temporary storage for recently assimilated lipids (McCue et al. 2015). However, even in an event of great amount of lipid ingestion, very few amounts of lipids are lost in the feces. Therefore, we can hypothesize that the cell shedding will occur only after enterocytic lipid clearance by means of lipoprotein secretion. Additionally, if the lipid loaded cells happened to be excluded before secreting the lipoproteins, it is possible that this content (sloughing cells, cell debris and their intracellular lipid contents) will be digested within the neighboring intestinal villi enterocytes by the membrane-associated lipases present in the brush borders and absorbed again downstream of the intestinal segment, until very few lipid will reach the colon.
As an effort to corroborate the view that lipid alone is not sufficient for causing intestinal villi hypertrophy in non-avian sauropsids (Secor et al. 2002), and thus do not cause histological reorganization of lamina epithelialis, dimensions of enterocytes with great amounts of lipid inclusions were compared to enterocytes free of such inclusions. Despite the great lipid inclusions in enterocyte cytoplasm, which made the volume to double, and despite the great extension of lipid-absorbing enterocytes along crypt-to-tip axis, lamina epithelialis organization did not change from pseudostratified to simple columnar epithelium. In agreement to this, nuclei were localized close to the base membrane of lamina epithelialis and the enterocyte length and volume increased, but not the width. Enterocyte width is the axis that corresponds to the intestinal villi length, and the axis which would cause the villi elongation and changing lamina epithelialis reorganization. Indeed, Lignot et al. (2005) demonstrate that after a feeding, enterocytes of Python molurus have the length shortened and the width increased, increasing the volume by 80%. This suggests that although causing enterocyte hypertrophy, lipids alone are not sufficient for intestinal villi increase and reorganization of lamina epithelialis histoarchitecture in this model of non-avian sauropsida frequent feeder that presents mild morphological alterations after a feeding, similarly to the stated by others about the infrequent feeders that presents a wide range of morphological changes (Secor et al. 2002; Lignot et al. 2005; Starck and Wimmer 2005; Ott and Secor 2007). The colon displayed only very few lipid droplets in the lamina propria and, as observed by others (McCue et al. 2017), there was no evidence of histological remodeling in this segment.
One may be concerned that the lacking of lamina epithelialis histological reorganization in Caiman yacare intestinal villi after soybean oil absorption could be attributed to hyperplasia. Cells proliferating in intestinal villi base domain would migrate through lamina epithelialis towards villi tip and increase cell density within lamina epithelialis, impairing observation of intestinal villi reorganization; however, this suggestion can be ruled out because mean proliferation of enterocytes of control animals and soybean oil injected animals were similar after 24 h of injection.
All the above data and discussion about differences in histology and physiology of the intestinal villi, i.e., finger-like versus folds led us to raise a hypothesis to be further tested: that there is a correlation of internal relief pattern of mucosa and the degree of morphological changes after a feeding, especially the model of telescope elongation of intestinal villi upon feeding and transition from pseudostratified lamina epithelialis to simple epithelium. The wide intestinal morphological changes are observed in snakes of the families Pythonidae, Boidae and Viperidae (Secor 2005; Ott and Secor 2007) that present finger-like intestinal villi (Parsons and Cameron 1977; Lignot et al. 2005; Helmstetter et al. 2009a, b), whereas the modest alterations on histological reorganization would occur in frequent feeding species that possess intestinal mucosa internal relief in the form of longitudinal projections or zigzag folds. Indeed, in a great variety of turtles so far analyzed, some families of snakes and the crocodilians, intestinal mucosa internal relief is of the longitudinal fold-like pattern projections (Parsons and Cameron 1977) and present modest morphological alterations (Secor and Diamon 1999; Secor 2005), and/or no lamina epithelialis remodeling upon feeding (Wurth and Musacchia 1964). Of course, it will be necessary to test this hypothesis upon feeding, because although lipids cause great enterocyte hypertrophy, other parameters influence intestinal mucosa hypertrophy such as amino acids (Secor et al. 2002) and augmented fluid pressure within lamina propria lymphatic and blood vessels (Starck and Wimmer 2005).
Conclusions
Caiman yacare intestine lacks the finger-like intestinal villi projections of the mucosa. Instead, intestinal villi are formed by longitudinal folds and lack Crypts of Lieberkühn. Despite these differences, intestinal villi are organized in a similar pattern to other vertebrates that presents both morphological aspects: enterocytes are being shed from the villi tip and lipid absorption by enterocytes present at the tip and along the length of the lamina epithelialis, but not by enterocytes present in the intestinal villi base. Within the intestinal villi base devoid of lipid inclusions, lamina epithelialis concentrate the proliferative enterocytes.
Lamina epithelialis is organized in a pseudostratified epithelium in the intestinal villi base and lateral sides, and simple columnar epithelium in the villi tip, in an arrangement probably typical of non-avian sauropsida species that possess intestinal folds instead of finger-like villi. The great amount of lipid inclusions, although causing enterocyte hypertrophy, is not sufficient for reorganizing lamina epithelialis histoarchitecture, corroborating the view that lipid alone is not sufficient for intestinal villi hypertrophy.
References
Abumrad NA, Davidson NO (2012) Role of the gut in lipid homeostasis. Physiol Rev 92:1061–1085. https://doi.org/10.1152/physrev.00019.2011
Aleixo VM, Cotta T, Logato PVR, Oliveira AIG, Fialho ET (2002) Efeitos da adição de diferentes teores de farelo de soja na dieta sobre o desenvolvimento de filhotes de jacaré-do-pantanal [Caiman yacare(Daudin, 1802)]. Cienc Agrotec 26:411–417
Aleixo VM, Pressinotti LN, Campos DVS, Menezes-Aleixo RC, Ferraz RHS (2011) Histologia, histoquímica e histometria do intestino de jacaré-do-pantanal criado em cativeiro. Pesq Vet Bras 31:1120–1128. https://doi.org/10.1590/S0100-736X2011001200014
Alpers DH, Bass NM, Engle MJ, DeSchryver-Kecskemei (2000) Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim Biophys Acta 1483:352–362. https://doi.org/10.1016/S1388-1981(99)00200-0
Andrew AL, Card DC, Ruggiero RP, Schield DR, Adams RH, Pollock DD, Secor SM, Castoe TA (2015) Rapid changes in gene expression direct rapid shifts in intestinal form and function in the Burmese python after feeding. Physiol Genom 47:147–157. https://doi.org/10.1152/physiolgenomics.00131.2014
Barker N (2014) Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15:19–33. https://doi.org/10.1038/nrm3721
Bateman PA, Jackon KG, Maitin V, Yaqoob P, Willians CM (2007) Differences in cell morphology, lipid and apo B secretory capacity in caco-2 cells following long term treatment with saturated and monounsaturated fatty acids. Biochim Biophys Acta 1771:475–485. https://doi.org/10.1016/j.bbalip.2007.02.001
Borges RM, Pressinotti LN, Aleixo VM, Borges JCS, Bérgamo AS, Iunes RS, Silva JRMC. (2016) Dietary lipid absorption and lipoprotein secretion by the intestine of the crocodilianCaiman yacare(Daudin, 1802). Zoomorphology. https://doi.org/10.1007/s00435-015-0300-9
Campos Z, Coutinho M, Magnusson WE (2006) Caiman crocodilus yacare (Pantanal caiman). Aestivation Herpetol Rev 37:343–344
Danielsen EM, Hansen GH, Rasmussen K, Niels-Christiansen LL (2013) Permeabilization of enterocytes induced by absorption of dietary fat. Mol Membr Biol 30:261–272. https://doi.org/10.3109/09687688.2013.780642
Ferri S, Junqueira LC, Medeiros LF, Medeiros LO (1976) Gross, microscopic and ultrastructural study of the intestinal tube of Xenodon merremiiWagler, 1824 (Ophidia). J Anat 121:291–301
Gajda AM, Storch J (2015) Enterocyte fatty acid binding proteins (FABPs): different functions of liver- and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids 93:9–16. https://doi.org/10.1016/j.plefa.2014.10.001
Guilmeau S, Niot I, Laigneau JP, Devaud H, Petit V, Brousse N, Bouvier R, Ferkdaji L, Besmond C, Aggerbeck LP, Bado A, Samson-Bouma ME (2007) Decreased expression of intestinal I- and F-FABP levels in rare human genetic lipid malabsorption syndromes. Histocehm Cell Biol 128:115–123. https://doi.org/10.1007/s00418-007-0302-x
Hayashi H, Maruyama S, Fukuoka M, Kozakai T, Nakajima K, Onaga T, Kato S (2013) Fatty acid-binding protein expression in the gastrointestinal tract of calves and cows. Anim Sci J 84:35–44. https://doi.org/10.1111/j.1740-0929.2012.01038.x
Helmstetter C, Reix N, T´Flachebba M, Pope RK, Secor SM, Le Maho Y, Lignot JH (2009a) Functional changes with feeding in the gastro-intestinal epithelia of the Burmese pyton (Python molurus). Zool Sci 26:632–638. https://doi.org/10.2108/zsj.26.632
Helmstetter C, Pope RK, T´Flachebba M, Secor SM, Lignot JH (2009b) The effects of feeding on cell morphology and proliferation of the gastrointestinal tract of juvenile Burmese pythons (Python molurus). Can J Zool 87:1255–1267. https://doi.org/10.1139/Z09-110
Jackson K, Perry G (2000) Changes in intestinal morphology following feeding in the brown treesnake, Boiga irregularis. J Herpetol 34:459–462. https://doi.org/10.2307/1565371
Jacobson ER (2007) Overview of reptile biology, anatomy and histology. In: Jaratoncobson ER (ed) Infectious diseases and pathology of Reptiles, color atlas and text. CRC Press, Boca Raton, pp 1–30. https://doi.org/10.1201/9781420004038.ch1
Jin SM, Maruch SMG, Rodrigues MAM, Pacheco P (1991) Histologia geral dos intestinos do Caiman crocodilus yacare(DAUDIN, 1802) (Crocodilia:Reptilia). Rev Bras Zool 7:111–120. https://doi.org/10.1590/S0101-81751990000200011
Johnson TS, Dornfeld EJ, Conte FP (1967) Cellular renewal of intestinal epithelium in the western fence lizard, Sceloporus occidentalis. Can J Zool 45:63–71. https://doi.org/10.1139/z67-008
Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, Allen T, Neurath MF, Shroeyer NF, Montrose MH, Watson AJM (2007) Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133:1769–1778. https://doi.org/10.1053/j.gastro.2007.09.011
Kubben FJ, Peeters-Haesevoets A, Engels LG, Baeten CG, Schutte B, Arends JW, Stockbrüggers RW, Blijham GH (1994) Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut 35:530–535
Levy E, Ménard D, Delvin E, Montoudis A, Beaulieu JF, Mailhot G, Dubé N, Sinnett D, Seidman E, Bendayan M (2009) Localization, function and regulation of the two intestinal fatty acid-binding protein types. Histochem Cell Biol 132:351–367. https://doi.org/10.1007/s00418-009-0608-y
Lignot JH, Helmstetter C, Secor SM (2005) Postprandial morphological response of the intestinal epithelium of the Burmese python(Python molurus). Comp Biochem Physiol A Mol Integr Physiol 141:280–291. https://doi.org/10.1016/j.cbpb.2005.05.005
Magalhães MS, Freitas ML, Silva NB, Moura CEB (2010) Morfologia do tubo digestório da tartaruga verde (Chelonia mydas). Pesq Vet Bras 30:676–684. https://doi.org/10.1590/S0100-736X2010000800012
McCue MD, Guzman M, Passement CA (2015) Digesting pythons quickly oxidize the proteins in their meals and save the lipids for later. J Exp Biol 218:2089–2096
McCue MD, Passement CA, Meyerholz DK (2017) Maintenance of distal intestinal structure in the face of prolonged fasting: a comparative examination of species from five vertebrate classes. Anat Rec 300:2208–2219
McDonald JH (2014) Handbook of biological statistics, 3rd (ed). Sparky House Publishing, Baltimore
Ott BD, Secor SM (2007) Adaptive regulation of digestive performance in the genus Python. J Exp Biol 210:340–356. https://doi.org/10.1242/jeb.02626
Parsons TS, Cameron JE (1977) Internal relief of the digestive tract. In: Gans C, Parsons TS (eds) Biology of the reptilian. Academic Press, New York, pp 159–224
Pressinotti LN, Borges RM, Alves de Lima AP, Aleixo VM, Iunes RS, Borges JC, Bogliati B, Cunha da Silva JR (2013) Low temperature reduces skin healing in the jacaré do pantanal (Caiman yacare, Daudin 1802). Biol Open 2:1171–1178. https://doi.org/10.1242/bio.20135876
Rodrigues-Sartori SS, Nogueira KOPC., Rocha AS, Neves CA (2014) Functional morphology of the gut of the tropical house gecko Hemidactylus mabouia(Squamata: Gekkonidae). Anim Biol 64:217–237. https://doi.org/10.1163/15707563-00002443
Sailaja BS, He XC, Li L (2016) Regulatory niche in intestinal stem cells. J Physiol. https://doi.org/10.1113/JP271931 (Accepted Author manuscript)
Santos SA, Nogueira MS, Pinheiro MS, Campos Z, Magnusson WE, Mourão GM (1996) Diets of Caiman crocodilus yacare from different habitats in the Brazilian Pantanal. Herpetological J 6:111–1117
Secor SM (2005) Evolutionary and cellular mechanisms regulating intestinal performance of amphibians and reptiles. Integr Comp Biol 45:282–294. https://doi.org/10.1093/icb/45.2.282
Secor SM, Diamond J (1999) Maintenance of digestive performance in the turtle Chelydra serpentina, Sternotherus odoratus, and Trachemys scripta. Copeia 1:75–84. https://doi.org/10.2307/1447387
Secor SM, Diamond JM (2000) Evolution of regulatory responses to feeding in snakes. Physiol Biochem Zool 73:123–141. https://doi.org/10.1086/316734
Secor SM, Lane JS, Whang EE, Ashley SW, Diamond J (2002) Luminal nutrient signals for intestinal adaptation in pythons. Am J Physiol Gastrointest Liver Physiol 283:G1298–G1309. https://doi.org/10.1152/ajpgi.00194.2002
Starck JM, Beese K (2001) Structural flexibility of the intestine of Burmese python in response to feeding. J Exp Biol 204:325–335
Starck JM, Beese K (2002) Structural flexibility of the small intestine and liver of garter snakes in response to feeding and fasting. J Exp Biol 205:1377–1388
Starck JM, Wimmer C (2005) Patterns of blood flow during the postprandial response in ball pythons, Python regius. J Exp Biol 208:881–889. https://doi.org/10.1242/jeb.01478
Starck JM, Cruz-Neto AP, Abe AS (2007) Physiological and morphological responses to feeding in broad-nosed caiman (Caiman latirostris). J Exp Biol 210:2035–2045. https://doi.org/10.1242/jeb.000976
Tracy CR, McWhorter TJ, Gienger CM, Starck JM, Medley P, Manolis SC, Webb GJ, Christian KA (2015) Alligators and crocodiles have high paracellular absorption of nutrients, but differ in digestive morphology and physiology. Integr Comp Biol 55:986–1004. https://doi.org/10.1093/icb/icv060
Watson AJM, Duckworth CA, Guan Y, Montrose MH (2009) Mechanisms of epithelial cell shedding in the mammalian intestine and maintenance of barrier function. Ann N Y Acad Sci 1165:135–142. https://doi.org/10.1111/j.1749-6632.2009.04027.x
Williams JM, Duckworth CA, Burkitt MD, Watson AJ, Campbell BJ, Pritchard DM (2015) Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol 52:445–455. https://doi.org/10.1177/0300985814559404
Wurth MA, Musacchia XJ (1964) Renewal of intestinal epithelium in the freshwater turtle Chrysemys picta. Anat Rec 148:427–439. https://doi.org/10.1002/ar.1091480302
Yu T, Chen QK, Gong Y, Xia ZS, Royal CR, Huang KH (2010) Higher expression patterns of the intestinal stem cell markers Musashi-1 and hairy and enhancer of split 1 and their correspondence with proliferation patterns in the mouse jejunum. Med Sci Monit 16:BR68–74
Acknowledgements
The authors would like to thank: COOCRIJAPAN (Cooperativa dos Criadores de Jacaré-do-Pantanal) for donating the animals used in the experiments and for sharing the facilities; Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)/São Paulo Research Foundation for supporting this work.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) contract Grant Number 715823/2008 and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)/São Paulo Research Foundation contract Grant Number 2010/04527-5. RMB has received a scholarship from FAPESP, contract Grant Number 09/52884-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Animal handling and experimentation were approved by the committee on animal welfare and ethical experimentation from Institute of Biomedical Sciences, University of Sao Paulo (Protocol Number 131, page 110, book 02; issued September 20th, 2011) and by Brazilian environmental agency (ICMBIO/MMA/SISBIO: authorization number 30509-2; issued August 30th, 2011).
Rights and permissions
About this article
Cite this article
Borges, R.M., Pressinotti, L.N., Marcus, F.A. et al. Histological organization of intestinal villi in the crocodilian caiman yacare (Daudin, 1802) during dietary lipid absorption. Zoomorphology 137, 419–432 (2018). https://doi.org/10.1007/s00435-018-0401-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-018-0401-3