Abstract
Spermatogenesis in amphibians occurs inside spermatogenic cysts formed by Sertoli cells (SCs). SCs do not constitute a permanent population and their number increases during spermatogenic cycle and ontogeny. The number of SCs per cyst is strongly correlated with the number of germ cells in the same cyst and with the cyst volume. The number of germ and Sertoli cells and cyst volumes are species-specific and increase along with age. The mean number of primary spermatocytes per one Sertoli cell (efficiency) was the same, irrespective of age and species. Late spermatids are not attached to the Sertoli cells and do not form bundles as in other amphibians. The number of primary spermatocytes inside a cyst was the yield of several mitotic cycles of a single primary spermatogonium (stem cell) and its descendants, i.e., secondary spermatogonia. In adult Bombina bombina, the majority of cysts contained germ cells after 6–8 cycles, in juveniles after 4–6 cycles, and in adult Bombina variegata after 5–7 cycles. The mean numbers of SCs per cyst were 27.4 ± 9.9 in adult B. bombina, 6.42 ± 2.32 in adult B. variegata, and 7.1 ± 2.33 in juvenile B. bombina. The number of primary spermatocytes per one SC (SC efficiency) in B. bombina in adults was 6.5 ± 2.2, in juveniles 9.0 ± 4.2, and in adult B. variegata 7.40 ± 2.96. Spermatogenesis in Bombinatoridae is exceptional owing to the lack of intimate contact between SCs and germ cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The amphibian testes are composed of germinal and interstitial compartments separated by acellular basement membranes. The germinal compartment comprises germ cells at various stages of spermatogenesis accompanied by somatic Sertoli cells (Grier 1993) that are progenitors of the somatic external epithelium of the gonadal anlage (Gramapurohit et al. 2000; Falconi et al. 2004; Ogielska and Bartmańska 2009; Piprek et al. 2010). At the beginning of amphibian testis development, the presumptive Sertoli cells invade the space between germ cells (gonocytes) and engulf them with cytoplasmic processes enforced by desmosomes (Rastogi et al. 1988; Lopez 1989; Falconi et al. 2004; Haczkiewicz et al. 2017).
The Sertoli cells perform supportive and nutritive functions for consecutive generations of spermatogenic cells, phagocytize degenerating germ cells and residual bodies of late spermatids, as well as constitute the testis–blood barrier (Bergman et al. 1983; Tanimura and Iwasawa 1988; Grier 1993). Although they function in the same way in all chordates, their morphology, differentiation, development and mitotic activity differ between Amniota (reptiles, birds, and mammals) and Anamnia (agnathans, fishes, and amphibians). Contrary to Amniota, spermatogenesis in Anamnia is synchronic and occurs inside spermatogenic cysts (spermatocysts) formed by Sertoli cells (cystic spermatogenesis) (Pudney 1995; Exbrayat 2009; Ogielska and Bartmańska 2009; Schulz et al. 2010; Rupik et al. 2011; Uribe 2009; McClusky 2012; Haczkiewicz et al. 2017). Spermatogenic cysts in anuran amphibians are distributed along the whole length of the seminiferous tubules (Atherton 1974; Oliveira and Vicentini 1998; Oliveira et al. 2002; Rodríguez et al. 2007; Sanz et al. 2007; Ferreira et al. 2008; Ogielska and Bartmańska 2009; Haczkiewicz et al. 2017). As a rule, all germ cells inside a cyst are at the same spermatogonial stages, and the synchrony is ensured by cytoplasmic bridges (Lofts 1974; Kalt 1976; Rastogi and Iela 1980; Miething 1990; Haczkiewicz et al. 2017).
The number of Sertoli cells involved in formation of a cyst varies between species from one to several (Pudney 1993). In mammals, the number of Sertoli cells is established during fetal life. After birth, SCs do not undergo further mitotic divisions (Clermont and Perey 1957; Steinberger and Steinberger 1971). In non-amniotic vertebrates, the number of Sertoli cells does not constitute a permanent population of cells and their number changes during an annual cycle. In elasmobranchs (Stanley 1966; Parsons and Grier 1992), as well as in bony fishes, such as the Nile tilapia, African catfish (Schultz et al. 2005), and the zebra fish (Leal et al. 2009), the Sertoli cells stop dividing mitotically when germ cells enter meiosis. The cysts degenerate after spermiation and a new generation of cysts starts growing at the beginning of a successive spermatogenic wave following the breeding season and gonadal regression and quiescence (Grier 1993).
In amphibians, the Sertoli cells were described in Gymnophiona (Exbrayat 2009), Urodela: the newt Trituroides hongkongensis (Tso 1974) and the fire salamander Salamandra salamandra (Bergman et al. 1983), and Anura: the toads Xenopus laevis (Reed and Stanley 1972) and Bufo arenarum (Burgos and Vitale-Calpe 1967a), and frogs: Rana temporaria (van Oordt and Brands 1970), Lithobates (Rana) pipiens (Lofts 1964) R. arenarum (Cavicchia and Moviglia 1983), Lithobates (Rana) catesbeianus (Sprando and Russell 1988), Odontophrynus cultripes (Báo et al. 1991), and Pelophylax esculentus (Rana esculenta) (Rough 1939), P. ridibundus and P. lessonae (Haczkiewicz et al. 2017). In all the species investigated so far, the cysts display common features. The cyst size increases along with the formation of consecutive generations of secondary spermatogonia that finally transform into primary spermatocytes and enter meiosis. Consequently, the Sertoli cells become extremely tenuous. During spermiogenesis, spermatids elongate and condense into bundles that become associated with Sertoli cells. The heads of spermatids are embedded in hypertrophied apical parts of the Sertoli cells that are rich in invaginations of the cell membrane. Soon after, the cyst opens and tails of spermatozoa protrude to the seminiferous tubule lumen until they finally detach while the Sertoli cells stay incorporated into the walls of seminiferous tubules (Burgos and Vitale-Calpe 1967b; Pudney 1993).
Spermatogenesis in Bombinatoridae is the only known exception, which does not conform the above description. As documented in light microscopy (Obert 1976), developing spermatids do not form bundles, but mature en masse what the author explained as the result of the absence of Sertoli cells in seminiferous tubules. This interpretation was invalidated by a more detailed study of the gonad differentiation and development in transmission and scanning microscopy that gave unequivocal evidence of the Sertoli cells presence in this species (Piprek et al. 2010). In the present study, we aimed to investigate the structure of spermatogenic cysts and the way germ cells contact the Sertoli cells in Bombina bombina and Bombina variegata, the two European sister species of the family Bombinatoridae (Pabijan et al. 2013). Besides the qualitative study, we also quantified the number of Sertoli cells involved in cysts formation, the volume of the cysts, as well as the number of primary spermatocytes present during early steps of active spermatogenesis in juvenile and mature males. To our knowledge, this is the first quantitative study on the number of Sertoli cells constituting spermatogenic cysts in amphibians.
Materials and methods
Bombina bombina L., 1761 were collected in Pruszowice (Lower Silesia N: 51°10′20″ E: 17°8′27″), and Bombina variegata L., 1758 were collected in Skawce (Beskid Makowski N: 49°47′17″ E: 19°34′53″) and Jurków (Beskid Wyspowy N: 49°40′53″ E: 20°14′25″) in Poland. Adult males were collected during active seasons (May–September) and juveniles before their first hibernation (September) in 1998, 1999, and 2005. In total, we analyzed eight individuals (three adult and two juvenile of B. bombina, and three adult B. variegata). All specimens were acquired according to Polish legal regulations for the protection of wild species (Dz. U. nr 33, poz. 289, 2005) and with the permission from the Polish Ministry of Environment Protection and Forestry, and approval from the Local Commission for Ethics in Experiments on Animals.
Light microscopy
Before dissection of gonads, the animals were anesthetized by immersion in 0.25% aqueous solution of 3-aminobenzoic acid ethyl ester (MS 222, Sigma Chemical Co., St Louis, USA). The testes were fixed in Bouin’s solution, dehydrated, embedded in paraplast (Sigma) and sectioned on Leica RM 2255 microtome at 5 or 6 μm. Sections were stained with HE or Mallory’s trichrome. Images were taken with a Nikon Eclipse E600 light microscope and processed with Corel Photo-Paint 11.
Transmission (TEM) and scanning (SEM) electron microscopy
For TEM, the gonads were dissected and fixed in Karnovsky’s fixative, rinsed in cacodylate buffer and postfixed in 1% osmium tetroxide solution (Karnovsky 1965). After dehydration, samples were embedded in Epon 812, cut into semi-thin sections (0.5 μm), and stained with methylene blue and Azure. Selected fragments were cut for ultra-thin sections stained with uranyl acetate and lead citrate. The sections were analyzed with a JEOL JEM-1005× transmission electron microscope. For SEM, the gonads were also fixed in the Karnovsky’s fixative, dehydrated, and dried in a LADD critical point drier, fractured and sputter-coated with gold. Samples were viewed with a JEOL JSM 5410 scanning electron microscope.
Cell counting and statistics
For cell counting, we used 5 µm-thick sections stained with HE and examined using a Zeiss Axioskop 20 microscope. The volume of a cyst was estimated by multiplying areas of each section (the AxioVision Zeiss software) by the thickness and the number of sections. Cell counting was performed interactively on serial sections of complete cysts. We counted the number of nuclei as equivalents to the number of cells. Cysts were chosen randomly and the only technical constrain was completeness (integrity) of the selected cysts and clear-cut borders between the adjacent ones. Because the number of primary spermatocytes was estimated on serial sections and some of the nuclei could be counted twice, we applied the Abercrombie’s formula (Abercrombie 1946):
where N number of primary spermatocytes, n number of nuclei visible on each of the serial sections, d mean diameter of nuclei measured in 30 randomly chosen primary spermatocytes at the bouquet stage, separately for each species, adults and juveniles, S section thickness (μm).
The results were rounded up to the nearest 1.
The number of Sertoli cells was counted in the same cysts, in which the number of spermatocytes was already estimated. We counted the actual number of nuclei (one by one) on consecutive sections, so the use of the Abercrombie’s formula was not necessary.
We used the AxioVision Zeiss software for all counts. Analyses were performed with the use of statistical software package Statistica (version 12.0 StatSoft PL). Because the analyzed samples were small, we used non-parametric tests for analyzing differences of medians in two (Mann–Whitney U) independent groups. Additionally, Spearman correlation coefficient between variables was calculated. In this study the accepted level of significance was p ≤ 0.05.
Results
Morphology
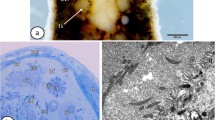
The testes in both species were elongated and circular in cross sections, and composed of large seminiferous tubules (Figs. 1a, 2a). Sertoli cells formed capsules that enclosed germ cells at various stages of spermatogenesis, beginning from clusters of secondary spermatogonia and meiocytes, until spermatids (Figs. 1b, c, 2b). The capsule of Sertoli cells together with germ cells inside constituted a spermatogenic cyst (spermatocyst). The external surface of the Sertoli cells was covered by the basal lamina. The Sertoli cells at all stages of spermatogenesis were flattened, had irregular shape, and formed a single-layer cyst wall (Fig. 3a, b). Analysis under SEM revealed that edges of the adjacent Sertoli cells tightly adhered to each other (Fig. 2c) and the inner surfaces of the Sertoli cells faced germ cells (Fig. 2b), but no specialized connections between the two types of cells were visible. Beginning from secondary spermatogonia until round spermatid stages, the clusters of germ cells were connected by cytoplasmic bridges (Fig. 3b). During spermiogenesis, late spermatids and early spermatozoa were loosely arranged and separated by space that appeared between adjoining germ cells, and between the Sertoli and germ cells. Spermatids did not form bundles as in other anurans, but constituted a mass of randomly distributed cells (Figs. 1c, 2a). The rupture of the cyst wall resulted in release of spermatozoa into the testis tubule lumen (Fig. 1a, b). The shape of spermatozoon was exceptional and resembled a spindle; the head and tail lied parallel to each other and were connected by a wide undulating membrane (Fig. 2d).
Morphology of testes in adult Bombina bombina (a, b) and B. variegata (c). A Cross section of the whole gonad with rete testis (arrows) and seminiferous tubules (outlined yellow) with spermatocysts and spermatozoa. b Cross section of a seminiferous tubule of B. bombina with spermatocysts. c Cross section of a seminiferous tubule of B. variegata. SgI primary spermatogonia (spermatogonial stem cells), SgII secondary spermatogonia, ScI primary spermatocytes, ScII secondary spermatocytes, Sz spermatozoa, Sd cysts with spermatids at different stages of spermiogenesis, arrowheads Sertoli cells nuclei. Scale bar 100 µm. Mallory’s trichrome (a), HE (b, c) staining
Structure of testes in adult Bombina variegata. a Seminiferous tubule (outlined yellow) packed with spermatogenic cysts (outlined white) at different stages of spermatogenesis. b, c Inner surface of a spermatogenic cyst; arrows indicate tightly adjoining edges of the Sertoli cells. d A single spermatozoon with a head, tail, and undulating membrane (arrowhead). S Sertoli cells, Sd a cyst with spermatids, Sz spermatozoa, Scale bar in a 100 µm, in b D 50 µm. SEM
Ultrastructure of spermatogenic cyst containing secondary spermatogonia (a) and early spermatids (b) in Bombina variegata. S nuclei of the Sertoli cells, Sd spermatids, SgII secondary spermatogonia, Sz spermatozoa in the seminiferous tubule lumen, arrows indicate a cytoplasmic bridge between two spermatids. Scale bar 5 µm. TEM
Spermatogenic cyst volume and number of germ and Sertoli cells
Cysts sizes increased during ontogeny (Table 1). In B. bombina, the mean volume of cysts was smaller in juveniles (204,287.00 µm3 ± 153,822.3) than in adults (906,169.41 µm3 ± 499,909.33) (Mann–Whitney U test, U = 8.0, p < 0.001). We also found that cysts in adults differed in sizes between the species and were larger in B. bombina than in B. variegata (160,607.35 µm3 ± 86,503.72) (Mann–Whitney U test, U = 0.0, p < 0.001) (Fig. 4).
The number of primary spermatocytes within cysts also varied between ages and species (Table 1). We counted the number of germ cells at the same spermatogenic stage, i.e., primary spermatocytes at the bouquet stages (zygotene-early pachytene), which were easily distinguishable from other meiotic stages in the adjacent cysts (Fig. 1a, c). They also assured that the last cycle of secondary spermatogonial mitoses had been completed, and thereby the number of meiocytes would not increase until the first and second meiotic divisions. In B. bombina, the mean number of spermatocytes in adults was higher (186.6 ± 118.74) than in juveniles (67.70 ± 49.92). In adult B. variegata, the number of germ cells was lower than in B. bombina (50.30 ± 30.34) (Fig. 5) and the difference between adults of the two species was highly significant (Mann–Whitney U test, U = 24.0, p < 0.001). The number of primary spermatocytes inside a cyst was the yield of several mitotic cycles of a single primary spermatogonium (SSC) and its descendants, i.e., secondary spermatogonia. In adult B. bombina, the majority of cysts contained germ cells accumulated after 6–8 cycles and in juveniles the numbers were lower (4–6 cycles). In adult B. variegata, the respective numbers were 5–7. The remaining cysts contained less germ cells (Table 2).
As the next step, we counted the number of Sertoli cells and estimated its correlation with the number of primary spermatocytes and volume of the same cyst (Table 1). The mean numbers of Sertoli cells per cyst were 27.4 ± 9.9 in adult B. bombina, 6.42 ± 2.32 in adult B. variegata, and 7.1 ± 2.33 in juvenile B. bombina. The number of Sertoli cells that constituted the cyst walls was bigger in adult B. bombina than in adult B. variegata (Mann–Whitney U test, U = 0.0, p < 0.001 (Fig. 6) and strongly correlated with the number of germ cells, as well as with the cyst volume (Table 3). In juveniles, there was no correlation between the number of Sertoli cells and the number of germ cells (R = 0.57; p = 0.08) and cyst volume (R = 0.57; p = 0.08) (Table 3). Then we analyzed the mean number of primary spermatocytes per one Sertoli cell (Sertoli cell efficiency). In B. bombina, the value for adults was 6.5 ± 2.2, for juveniles 9.0 ± 4.2, and for adult B. variegata 7.40 ± 2.96. We found no statistically significant differences neither between adults of the two species (Mann–Whitney U test, U = 171.0, p = 0.33) (Fig. 7) nor between adults and juveniles of the same species (B. bombina) (Mann–Whitney U test, U = 73.0, p < 0.14) (Fig. 8).
Discussion
The present study revealed that the structure of testes in both B. bombina and B. variegata is typical of anuran amphibians, with walls of spermatocysts formed by a monolayer of flattened Sertoli cells. There are two approaches to define a spermatogenic cyst. Manochantr et al. (2003) proposed that a cyst is formed as early as when a single primary spermatogonium, i.e., a gonocyte in juveniles and a spermatogonial stem cell (SSC) in adults (for details see Haczkiewicz et al. 2017) is engulfed by one or several Sertoli cells. According to our former study (Haczkiewicz et al. 2017), as well as to Rastogi et al. (1983, 1988), and Pierantoni et al. (2002), the cyst forms when a single SSC start dividing mitotically to form a cluster of secondary spermatogonia. Initially two daughter cells, then more generations stay connected by cytoplasmic bridges formed as a result of incomplete cytokineses. The number of primary spermatocytes within a cyst is the result of several mitotic cycles of a single SSC that give rise to a cluster of secondary spermatogonia, which finally transform (without additional mitotic divisions) into primary spermatocytes. Thus, the number of secondary spermatogonia should theoretically equal the number of primary spermatocytes. The expected number of primary spermatocytes is 2n, where n is the number of cell cycles undergone by secondary spermatogonia. In adult B. bombina the majority of cysts contained the yield of 6–7 cell cycles of a single SSC to form a final cluster of secondary spermatogonia, in B. variegata 5–7 cycles, and these numbers were comparable to other amphibian species studied in this respect (Table 4). In B. variegata and juvenile B. bombina, three cycles is a minimum, as in the frogs Pelophylax lessonae and P. ridibundus. The lowest number of primary spermatocytes inside a cyst also defines the minimal number of cell cycles necessary to develop into preleptotene meiocytes, i.e., the meiosis entry. However, usually the number of primary spermatocytes is lower, most likely due to germ cell death.
The number of cell cycles of a single primary spermatogonium (SSC) differs also in other representatives of cystic spermatogenesis. In a cartilaginous fish Squalus acanthias (McClusky 2012), the number represents 13 generations, and in two bony fish Danio rerio at least 4–5 (Schulz et al. 2010) up to 8–9 cell cycles (Leal et al. 2009), and in Oreochromis niloticus 8 cycles (Schultz et al. 2005) However, comparison of the numbers of spermatogonial cycles between species may be vitiated by an error caused by various methodologies used by the authors. One of the difficulties is discrimination between SSC that divide mitotically and thereby renew the pool, and those that enter the pathway of differentiation into secondary spermatogonia and are committed to enter meiosis. In frogs, the first category is represented by “pale”, and the latter by “dark” SSC (Haczkiewicz et al. 2017). The other difficulty is a definition of secondary spermatogonia. The term “secondary spermatogonia” is the most general and comprises various morphological cell types, e.g., spermatogonia A, In, B in mammals (de Rooij 2001) and A differentiated and B in fishes (Schultz et al. 2005; Leal et al. 2009). The common features of all types of secondary spermatogonia (even if not defined in such a way) are incomplete cytokineses and formation of cellular bridges beginning with the first division of a single SSC until meiosis entry. We used the latter naming in our former (Haczkiewicz et al. 2017) and present studies.
Sertoli cell number is the main factor determining the magnitude of sperm production, as it was experimentally shown for a fish species, the Nile tilapia Oreochromis niloticus (Matta et al. 2002). The ratio between the number of germ cells and the number of Sertoli cells of the same cyst determines the Sertoli cell efficiency. In Danio rerio, one Sertoli cell serves for about 95 spermatids (Leal et al. 2009) and in Oreochromis niloticus for about 100 (Schultz et al. 2005). In an anuran amphibian Bombina (this study), from 3 to 15 primary spermatocytes theoretically give rise to a maximum of 12–60 spermatids that fall on one Sertoli cell and thus the efficiency is lower than in fishes.
As was shown for fishes, the Sertoli cells divide mitotically along with proliferation of secondary spermatogonia and their number stabilizes after meiotic entry to about 6 (Schultz et al. 2005). As we demonstrated in this study, the number of Sertoli cells constituting spermatogenic cyst walls in B. bombina and B. variegata is higher (the minimum of 5, the maximum of 44, depending on age and species, see Table 1). In fishes (Schultz et al. 2005) and amphibians (Haczkiewicz et al. 2017), cyst volume increases until pachytene stage, when the testis–blood barrier is formed. We also demonstrated that the number of Sertoli cells is positively correlated with the spermatocyte number per cyst and thus also along with the volume of the cyst. These results suggest that Sertoli cells divide mitotically during cyst size increase. The proliferation of Sertoli cells was experimentally proved for the fishes Danio rerio (Leal et al. 2009), Oreochromis niloticus and Clarias gariepinus (Schultz et al. 2005). In that way all Sertoli cells of a given cyst are progenitors of a single somatic cell, which originally wrapped a single SSC.
Conclusion
Although spermatogenesis and cyst formation in B. bombina and B. variegata (this study) and B. orientalis (Yi and Lee 2015) are similar to the same processes in other amphibians, their peculiarity lies in the lack of intimate contact between Sertoli and germ cells. As a rule, the Sertoli cells in vertebrates form numerous invaginations that provide niches for individual late spermatid heads at the time when the tail is forming (Grier 1993). In amphibians, the arrangement of niches of the Sertoli cells results in formation of regular bundles of late spermatids. As we reported in this study, although the Sertoli cells exist in Bombinatoridae, their function in arranging spermatids and spermatozoa into bundles is lacking, and this is a premise to claim that the germ cells differentiation, at least spermiohistogenesis, is not critically depended on intimate contact with the Sertoli cells. The lack of contact between germ and Sertoli cells may be connected with an exceptional shape of spermatozoa described in B. bombina and B. variegata (Furieri 1975; Folliot 1979; this study) and B. orientalis (Lee and Kwon 2005). The head and tail of the Bombina spermatozoa are not linearly arranged, as is a rule in anuran amphibians (Scheltinga and Jamieson 2003), but lie parallel to each other embedded in a thick layer of cytoplasm. The tail is juxtaposed to the convex side of the nucleus along the length of the head that altogether gives a fusiform shape of a spermatozoon.
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Atherton RW (1974) A gradient analysis of spermatogenesis in the toad Bufo woodhousei Girard (1854). Herpetologica 30:240–244
Báo SN, Dalton GC, de Oliveira SF (1991) Spermiogenesis in Odontophrynus cultripes (Amphibia, Anura, Leptodactylidae): ultrastructural and cytochemical studies of proteins using E-PTA. J Morphol 207:303–314
Bergman M, Greven H, Schindelmeiser J (1983) Observations on the blood-testis barrier in a frog and salamander. Cell Tissue Res 232:189–200
Burgos MH, Vitale-Calpe R (1967a) The fine structure of the Sertoli cell-spermatozoan relationship in the toad. J Ultrastruct Res 19:221–237
Burgos MH, Vitale-Calpe R (1967b) The mechanism of the spermiation in the toad. Am J Anat 120:227–252
Cavicchia JC, Moviglia GA (1983) The blood–testis barrier in the toad (Bufo arenarum Hensel): a freeze-fracture and lanthanum tracer study. Anat Rec 205:387–396
Clermont Y, Perey B (1957) Quantitative study of the cell population of the seminiferous tubules in mature rat. Am J Anat 100:241–267
De Rooij DG (2001) Proliferation and differentiation of spermatogonial stem cells. Reproduction 121:347–354
Exbrayat JM (2009) Spermatogenesis and male reproduction system in Gymnophiona. In: Ogielska M (ed) Reproduction of amphibians. Science Publishers, Enfield, pp 125–152
Falconi R, Dalpiaz D, Zaccanti F (2004) Ultrastructural aspects of gonadal morphogenesis in Bufo bufo (Amphibia, Anura). 1. Sex differentiation. J Exp Zool A Comp Exp Biol 301A:378–388
Ferreira A, Mehanna M, Prado CP (2008) Morphologic and morphometric analysis of testis of Pseudis limellum (Cope, 1862) (Anura, Hylidae) during the reproductive cycle in the Pantanal, Brazil. Biocell 32:185–194
Folliot R (1979) Ultrastructural study of spermiogenesis of the anuran amphibian Bombina variegata. In: Fawcett DW, Bedford JM (eds) The Spermatozoon. Urban and Schwarzenberg, Baltimore-Munich, pp 333–339
Furieri P (1975) The peculiar morphology of the spermatozoon of Bombina variegata (L.). Monit Zool Ital 9:185–201
Gramapurohit NP, Shanbhag BA, Saidapur SK (2000) Pattern of gonadal sex differentiation, development, and onset of steroidogenesis in the frog, Rana curtipes. Gen Comp Endocrinol 119:256–264
Grier HJ (1993) Comparative organization of the Sertoli cells including the Sertoli cell barrier. In: Russell LD, Griswold MD (eds) The Sertoli Cell. Cache River Press, Clearwater, pp 703–739
Haczkiewicz K, Rozenblut-Kościsty B, Ogielska M (2017) Prespermatogenesis and early spermatogenesis in frogs. Zoology. pii: S0944-2006(16)30125–8. doi:10.1016/j.zool.2017.01.003
Kalt MR (1976) Morphology and kinetics of spermatogenesis in Xenopus laevis. J Exp Zool 195:393–407
Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137A
Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW (2009) Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod 81:177–187
Lee J-H, Kwon J-K (2005) Sperm ultrastructure of Bombina orientalis. Korean J Electron Microsc 35:1–11
Lofts B (1964) Seasonal changes in the functional activity of the interstitial and spermatogenetic tissues of the green frog, Rana esculenta. Gen Comp Endocrinol 4:550–562
Lofts B (1974) Reproduction. In: Lofts B (ed) Physiology of the amphibia. Academic Press, New York, pp 107–218
Lopez K (1989) Sex differentiation and early gonadal development in Bombina orientalis (Anura: Discoglossidae). J Morphol 199:299–311
Manochantr S, Sretarugsa P, Wanichanon C, Chavadej J, Sobhon P (2003) Classification of spermatogenic cells in Rana tigerina based on ultrastructure. Sci Asia 29:241–254
Matta SLP, Vilela DAR, Godinho HP, França LR (2002) The goitrogen 6-n-propyl-2-thiouracil (ptu) given during testis development increases Sertoli and germ cell numbers per cyst in fish: the tilapia (Oreochromis niloticus) model. Endocrinology 143:970–978
McClusky LM (2012) Coordination of spermatogenic processes in the testis: lessons from cystic spermatogenesis. Cell Tissue Res 349:703–715
Miething A (1990) Intercellular bridges between germ cells in the immature golden hamster testis: evidence for clonal and non-clonal mode of proliferation. Cell Tissue Res 262:559–567
Obert HJ (1976) Die Spermatogenese bei der Gelbbauchunke (Bombina variegata variegata L.) im Verlauf der järlichen Aktivitatatsperiode und die Korrelation zur Paarungsrufaktivität (Discoglossidae, Anura). Z Mikrosk Anat Forsch 90:908–924
Ogielska M, Bartmańska J (2009) Spermatogenesis and male reproductive system in Anura. In: Ogielska M (ed) Reproduction of amphibians. Science Publishers, Enfield, pp 34–99
Oliveira C, Vicentini CA (1998) Anatomical description of the Scinax fuscovarius fat bodies and testes (Anura, Hylidae). Biociências 6:79–88
Oliveira C, Zanetoni C, Zieri R (2002) Morphological observations on the testes of Physalaemus cuvieri (Amphibia, Anura). Rev Chil Anat 20:263–268
Pabijan M, Wandycz A, Hofman S, Węcek K, Piwczyński M, Szymura JM (2013) Complete mitochondrial genomes resolve phylogenetic relationships within Bombina (Anura: Bombinatoridae). Molec Phylog Evol 69:63–74
Parsons GR, Grier HJ (1992) Seasonal changes in the shark testicular structure and spermiogenesis. J Exp Zool 261:173–184
Pierantoni R, Cobellis G, Meccariello R, Palmiero C, Fienga G, Minucci S, Fasano S (2002) The amphibian testis as model to study germ cell progression during spermatogenesis. Com Biochem Physiol B, Biochem Mol Biol 132:131–139
Piprek RP, Pecio A, Szymura JM (2010) Differentiation and development of gonads in the yellow-bellied toad, Bombina variegata L. 1758 (Amphibia: Anura: Bombinatoridae). Zool Sci 27:47–55
Pudney J (1993) Comparative cytology of the non-mammalian vertebrate Sertoli cell. In: Russell LD, Griswold MD (eds) The Sertoli Cell. Cache River Press, Clearwater, pp 611–657
Pudney J (1995) Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 32:459–497
Rastogi RK, Iela L (1980) Steroidogenesis and spermatogenesis in anuran Amphibia: a brief survey. In: Delrio G, Brachet J (eds) Steroids and their mechanism of action in non-mammalian vertebrates. Raven Press, New York, pp 131–146
Rastogi RK, Iela L, Di Meglio M, Di Matteo L, Minucci S, Izzo-Vitiello I (1983) Initiation and kinetic profiles of spermatogenesis in the frog, Rana esculenta (Amphibia). J Zool 201:515–525
Rastogi RK, Bagnara JT, Iela L, Krasovich MA (1988) Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor. IV. Spermatogenesis: a light and ultrasonic study. J Morphol 197:277–302
Reed SC, Stanley HP (1972) Fine structure of spermatogenesis in the South African clawed toad Xenopus laevis Daudin. J Ultrastruct Res 41:277–295
Rodríguez Y, Segura ML, Jiménez LF, Sanz A, Lara R (2007) Ultrastructure of sexual cells of Eleutherodactylus riparius (Anura: Leptodactylidae). Microsc Acta 16:187–188
Rough R (1939) The reproductive processes in the male frog Rana pipiens. J Exptl Zool 80:81–105
Rupik W, Huszno J, Klag J (2011) Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)—structural and ultrastructural studies. Micron 42:833–839
Sanz A, Rodríguez Y, Segura ML, Jiménez LF (2007) TEM of Bufo longinasus (Anura: Bufonidae) gonad. Microsc Acta 16:185–186
Scheltinga DM, Jamieson BGM (2003) Spermatogenesis and the mature spermatozoa: Form, function and phylogenetic implications. In: Jamieson BGM (ed) Reproductive biology and phylogeny of anura. Science Publisher Inc, Enfield, Plymouth, pp 119–251
Schultz RW, Menting S, Bogerd J, França LR, Vilela DAR, Godinho HP (2005) Sertoli cell proliferation in the adult testis—evidence from two fish species belonging to different orders. Biol Reprod 73:891–898
Schulz RW, De França LR, Lareyre JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Sprando RL, Russell LD (1988) Spermiogenesis in the bullfrog (Rana catesbeiana): a study of cytoplasmic events including cell volume changes and cytoplasmic elimination. J Morphol 198:303–319
Stanley HP (1966) The structure and development of the seminiferous follicle in Scyliorhinus caniculus and Torpedo marmorata (Elasmobranchii). Z Zellforsch Mikrosk Anat 75:453–468
Steinberger A, Steinberger E (1971) Replication patterns of Sertoli cells in maturing rate testis in vivo and organ culture. Biol Reprod 4:84–87
Takamune K, Kawasaki T, Ukon S (2001) The first and the second mitotic phases of spermatogonial stage in Xenopus laevis: secondary spermatogonia which have differentiated after completion of the first mitotic phase acquire an ability of mitosis to meiosis conversion. Zoolog Sci 18(4):577–583. doi:10.2108/zsj.18.577
Tanimura A, Iwasawa H (1988) Ultrastructural observations on the origin and differentiation of somatic cells during gonadal development in the frog Rana nigromaculata. Dev Growth Differ 30:681–691
Tso E (1974) An ultrastructural study on the Sertoli cells in the newt (Trituroides hongkongensis). Acta Zool 55:217–223
Uribe MCA (2009) Spermatogenesis and male reproductive system in Urodela. In: Ogielska M (ed) Reproduction of amphibians. Science Publishers, Enfield, pp 100–124
Van Oordt PGWJ, Brands F (1970) The Sertoli cells in the testis of the common frog, Rana temporaria. Proc Soc Endocrinol 119 M Meet J Endocrinol 48:100
Yazawa T, Yamamoto T, Nakayama Y, Hamada S, Abé S (2000) Conversion from mitosis to meiosis: morphology and expression of proliferating cell nuclear antigen (PCNA) and dmc 1 during newt spermatogenesis. Dev Growth Differ 42:603–611
Yi M-J, Lee J-H (2015) Seminiferous epithelium cycle in Bombina orientalis. Dev Reprod 19:1–10
Acknowledgements
We would like to thank the staff of the Laboratory of Scanning Electron Microscopy Institute of Zoology, Jagiellonian University for technical assistance with (JEOL JSM 5410 (JEOL, Tokyo, Japan) and Beata Lorenc for valuable help with cell counting. This work was supported by the Polish Ministry of Sciences and Higher Education (Grant Numbers 2020/BW/IZ, 1018/DS/IZ, and 5429/B/P01/2010/38).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rozenblut-Kościsty, B., Piprek, R., Pecio, A. et al. The structure of spermatogenic cysts and number of Sertoli cells in the testes of Bombina bombina and Bombina variegata (Bombinatoridae, Anura, Amphibia). Zoomorphology 136, 483–495 (2017). https://doi.org/10.1007/s00435-017-0362-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-017-0362-y