Abstract
A common observation in the vertebrate testis is that new germ cell clones enter spermatogenesis proper before previously formed clones have completed their development. The extent to which the developmental advance of any given germ cell clone in any phase of spermatogenesis is dependent on that of neighboring clones and/or on the coordinating influence of associated Sertoli cells in the immediate vicinity or of others further away remains unclear. This review presents an overall synthesis of findings in an ancient vertebrate, the spiny dogfish shark and shows that, even at this phyletic level, the developmental advance of a given germ cell clone is the outcome of various processes emanating from its spatiotemporal relationship with (1) its own complement of Sertoli cells in the anatomically distinct spermatocyst and (2) Sertoli cells associated with other germ cell clones that lie upstream or downstream in the spermatogenic progression and that secrete, among others, androgen and estrogen destined for target sites upstream. Analysis of the protracted spermatogenic cycle shows the coordination in space and time of spermatogenic and steroidogenic events. Furthermore, the natural withdrawal of pituitary gonadotropin support in the dogfish causes a distinct and highly ordered gradient of apoptosis among the spermatogonial generations; this in turn is a major contributing factor to the cyclic nature of sperm production observed in this lower vertebrate. Because of the simplicity of their testicular organization, their cystic spermatogenesis and their phylogenetic position, cartilaginous fishes constitute a valid vertebrate reference system for comparative analysis with higher vertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apart from germ cells, the germinal compartment of the vertebrate testis also houses a group of supporting somatic elements, namely the Sertoli cells. Under the nurturing influence of mainly these closely positioned somatic cells, germ cells embark on their developmental journey, which encompasses several phases, i.e., several bouts of species-specific spermatogonial divisions, namely the premeiotic phase (PrM) followed by a phase of meiotic (M) divisions of the spermatocytes to form round spermatids. The latter then enter a period of morphological transformation, called spermiogenesis, culminating in the formation of mature spermatids or spermatozoa, namely the postmeiotic phase (PoM).
The extent to which the developmental advance of any given germ cell clone in any phase is dependent on that of neighboring clones and/or on the coordinating influence of associated Sertoli cells in the immediate vicinity or of others further away remains unclear. Scattered evidence indicates, however, that spermatogonial mitosis in the three mammalian orders, Rodentia, Lagomorpha and Carnivora, are synchronized with meiotic cell-cycle checkpoints at certain stages of the cycle (Blanco-Rodriguez et al. 2003). In the rat, most germ cell deaths (spermatogonia and spermatocytes) coincided with mitotic peaks of the second and fourth spermatogonial generations (seminiferous tubule stages XII, XIV-I; Blanco-Rodriguez et al. 2003). These findings suggest that events occurring in any given germ cell stage are influenced by activities of neighboring immature or mature germ cells and/or Sertoli cells. In another study, the timing of germ cell apoptosis in the pre-pubertal testis has been found to be linked to the temporal cycle of the Sertoli cycle (Timmons et al. 2002).
Studying these and other questions in vertebrates with a less complex testicular organization has supplemented our general understanding of the cell population kinetics of immature vertebrate germ cell clones as these relate to the coordination of the PrM, M and PoM phases during spermatogenesis. The cytoarchitecture of the gonads in these lower vertebrates certainly provides information about the ancient origins of one of the most crucial cellular relationships known in the body, i.e., the dependence of male germ cells on the physical and nutritive support from the somatic cell in closest proximity to them, namely the Sertoli cell (Roosen-Runge 1969; Callard 1991; Grier 1992). Such studies are of general interest because the basic principles of key processes driving all organismal life and survival, including energy (van Breukelen et al. 2010) and gamete (Matova and Cooley 2001) production, are essentially conserved and follow common themes.
Testicular organization in the spiny dogfish
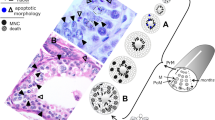
The testis of the spiny dogfish shark, Squalus acanthias (Fig. 1) and other sharks with similar testes is ideal for studying such stage-related phenomena in spermatogenesis because (1) spermatogenesis proceeds in a cystic mode, i.e., testicular cells are housed in anatomically distinct spherical structures (spermatocysts), each containing an isogeneic germ cell clone and a second clonal population of stage-synchronized Sertoli cells, (2) developing cysts are arranged in a linear maturational order across the diameter of the testis, resulting in a readily visible zonation with cysts in the premeiotic (PrM; spermatogonia and preleptotene spermatocytes), meiotic (M; primary and secondary spermatocytes) and postmeiotic (PoM; spermatids) regions and (3) Sertoli cells divide in a 1:1 ratio with spermatogonia during the first nine spermatogonial divisions and these cyst stages are therefore analogous to the cellular composition of the prenatal and neonatal rodent testis. Cysts can be dispersed from the shark testis, separated by stage under a dissecting microscope and used for in vitro study and manipulation (Dubois and Callard 1991, 1993; McClusky 2006, 2008).
Representation of transversely cut spiny dogfish shark testis showing the topographical arrangement of spermatocysts and a readily visible zonation of the testis comprising spermatocysts in the premeiotic (PrM: cysts in the folliculogenic germinal zone [GZ] and cysts with peripheral layers of spermatogonia orderly arranged in one to three [early-stage, E-PrM], four to eight [mid-stage, M-PrM] and eight or more [late-stage, L-PrM] layers), meiotic (M: cysts with primary and secondary spermatocytes) and postmeiotic (PoM: cysts with spermatids at the round [early-stage, E-PoM], elongating [mid-stage, M-PoM] and mature [late-stage, L-PoM] stages of spermiogenesis) regions. Any given cyst houses a germ cell clone of only one stage of development, together with its own complement of stage-synchronized Sertoli cells whose nuclei undergo a lumen-to-base migration in the most mature spermatogonial cyst stage (L-PrM)
Hormonal regulation of spermatogenesis in the dogfish shark
As in other vertebrates, the dogfish shark testis is also under central nervous system control and thus part of the hypothalamic–pituitary–gonad axis. Unlike other vertebrates, however, no direct vascular connection exists between the hypothalamus and the ventral lobe, the hypophysial part of the dogfish pituitary that controls the gonads and gonadotrophin-releasing hormone is understood to reach the ventral lobe via the systemic circulation (for an extensive review, see Dodd 1983). Initial experiments showed that ventral lobectomy or injection of an extract of ventral lobes into hypophysectomized male dogfish significantly altered plasma androgen levels (Sumpter et al. 1978), although the decrease was small (Dobson and Dodd 1977a). Ultrastructural studies in S. acanthias showed that the Sertoli cells and not the conventional target cells of gonadotropins, i.e., the Leydig cells, possessed typical steroidogenic organelles that increased in abundance concomitant with germ cell development, such that they resembled sacs filled with agranular membranes in the mature spermatid cyst stage (Pudney and Callard 1984a, b). These observations were consistent with reports that showed that the activity of 17α-hydroxylase, a key enzyme in androgen biosynthesis, exhibited a 12-fold stage-related (PoM>M>PrM) increase (Callard et al. 1985). These results prompted investigations into steroid output of the various cyst stages. Studies of in vitro steroid secretion of spiny dogfish spermatocysts were inconclusive with regard to stage-related differences, as testosterone secretion in PrM and PoM cysts were sometimes found to be equivalent (Cuevas and Callard 1992b). Similar results were reported for European spotted dogfish spermatocysts (Sourdaine et al. 1990; Sourdaine and Garnier 1993). Further studies on Squalus testis showed, however, that the stage-related distribution patterns for androgen receptors were the exact opposite of that of 17α-hydroxylase activity, i.e., PrM>M>>PoM (Cuevas and Callard 1992a). Since the direction of blood flow via the dogfish genital artery is from the mature to immature pole of the testis (Cuevas et al. 1992), an overall picture emerged that seemed to imply that androgen secreted at the mature pole of the testis might be destined for a role upstream in the spermatogenic sequence where androgen receptors are concentrated. A difficulty experienced with these cyst culture experiments was the variable reproducibility of results from one year to the next, all of which was later shown to be related to the near absence of androgen-secreting mature PoM cysts in spiny dogfish caught over the summer months (see below), the time of year when cyst culture experiments could be performed.
Spermatogonial cell population kinetics within the PrM region
A salient feature of vertebrate spermatogenesis is the synchrony of germ cell development. This is in a large part attributable to the presence of intercellular bridges between the progeny of any given committed spermatogonial stem cell in higher (Dym and Fawcett 1971; Weber and Russell 1987; Ehmcke et al. 2005) and lower (Pudney 1995; Loppion et al. 2008) vertebrates. This clonal type of development persists until extremely late in spermiogenesis. Since a single germ cell clone can consist of many hundreds in a germ cells, synchronous germ cell development in any given clone probably involves intraclonal signaling mechanisms, from the germ cells themselves and/or from the associated Sertoli cells.
A striking finding in hypophysectomized male dogfish is the coordinated massive breakdown of spermatocysts at the spermatogonia–spermatocyte transition and a gradient of degeneration in the PrM region (Dobson and Dodd 1977b). Cysts containing the final spermatogonial division degenerate and the dying spermatogonia are phagocytosed by the associated Sertoli cells following hypophysectomy. These changes are only observed when the water temperature of the holding tanks resembles that of the summer sea temperature of 13°C and warmer (Dobson and Dodd 1977c). These findings in Scyliorhinus have drawn attention to an earlier report of Simpson and Wardle (1967) who describe their observation of a naturally occurring zone of cyst breakdown of similar quality and quantity in summer-caught S. acanthias of the Scottish-Norwegian stock, an observation the latter authors ascribed to a pause in pituitary gonadotropin secretion during the winter months. Subsequent biochemical, TUNEL (terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling) immunohistochemical, morphologic and acridine-orange vital-staining analyses of cysts in the zone of degeneration and the PrM region (Callard et al. 1995; McClusky 2005, 2006) of summer-caught S. acanthias have confirmed the mode of death in these dying spermatogonial cysts as apoptosis. Moreover, TUNEL analysis of paraffin sections of the zonated testis has revealed a gradient of apoptosis (germinal zone [GZ] < early-stage PrM [E-PrM] << mid-stage [M-PrM]) in the PrM region (Fig. 2).
TUNEL immunohistochemistry of testicular cross-sections of Squalus acanthias caught in late spring showing the qualitative differences in labeling in the immature (a) and mature (b) PrM regions. Although the GZ and E-PrM regions (a) generally show no TUNEL-labeling, a few large rounded spermatogonia may occasionally be TUNEL-positive at the height of apoptosis activation. By contrast, an apoptosis gradient is clearly visible in the M-PrM region (b), with the incidence of TUNEL-labeling being proportional to increasing cyst size (top right to bottom left). Bars 25 μm (L.M. McClusky, unpublished)
To explore further the apoptosis gradient in the PrM region and the quality of spermatogonial apoptotic death in S. acanthias as revealed by TUNEL immunohistochemistry, all TUNEL-related death phenomena observed in the PrM region in spring–summer were quantified substage-by-substage (L.M. McClusky, unpublished). Given that the shark spermatocyst is an anatomically distinct spherical structure and that germ cells within a cyst are cytoplasmically linked, we can reasonably expect that the spreading of cellular signals within the spermatocyst will be synchronized and all-or-none. Various staining patterns have, however, been observed (see Fig. 3). We can conclude that the observed TUNEL-related variations in spermatogonial death most likely reflect the protracted sequence of events in dying, coalesced cells, i.e., coalescence (not TUNEL-labeled) → fragmentation of nuclear DNA (labeled by TUNEL) → leakage of DNA fragments into the cytoplasm (TUNEL-labeling of large rounded mass), phenomena that are known also to occur in teleosts (McClusky et al. 2008). To determine whether the various substages within the PrM region differ in their response to the death stimulus, the various TUNEL-related death phenomena have been analyzed stage-by-stage within the PrM region (Fig. 4). The total number of TUNEL-labeled nuclei and the various forms of TUNEL-related death phenomena increase in a stage-dependent manner (E-PrM<M-PrM<<condemned M-PrM [zone of degeneration, ZD]). The total number of multinucleate giant cells (TUNEL-positive and TUNEL-negative) in both E-PrM and M-PrM cysts is higher than the number of individual single TUNEL-positive nuclei in these cyst stages (Fig. 4). One interpretation of the latter results could be that the death signal is transmitted in equal measure through intercellular bridges in several subsections of a spermatogonial clone, regardless of whether it might be an immature or mature spermatogonial clone. This implies that other factors are involved in the coordinated propagation of the apoptotic stimulus among the 13 spermatogonial generations of the spiny dogfish such as to produce an apoptosis gradient in the PrM region. Interestingly though, the entire demise of immature spermatogonial cysts can occur but is extremely rare, as is the observation of apoptotic spermatogonia amongst GZ cysts (Fig. 3). Moreover, mature spermatogonial clones (M-PrM) may sometimes show simultaneous activation of mitosis and cell death (Fig. 3), a phenomenon also observed in the blue shark testis (McClusky 2011). Mitosis is, however, never observed in severely apoptotic cysts. All in all, the available evidence suggests the presence of clone-intrinsic mechanisms involved in assessing the magnitude of the apoptosis-inducing signal, with clone fate (life or death) being decided accordingly. Indeed, asynchronous cell death in a population of cells is a well-known observation and some cells might even undergo mitosis in the presence of a death stimulus when the survival mode predominates (Messam and Pittman 1998; Wilson et al. 2000). Mouse spermatogonial clones are able to alter the numbers of single apoptotic versus chains of apoptotic spermatogonia depending on the magnitude of the apoptosis-inducing signal, which in this case was radiation (Hamer et al. 2003).
Photomicrographs of methyl-green-counterstained tissue sections showing the various types of TUNEL-related death phenomena and qualitative deviations from the stage-dependency of TUNEL-labeling in spermatogenically active S. acanthias. a TUNEL immunohistochemistry of dying spermatogonia in a zone of degeneration (ZD; condemned M-PrM) cyst showing single TUNEL-positive nuclei, large rounded TUNEL-positive masses (#) and multinucleate giant cells whose nuclei are either TUNEL-negative (arrowhead) or TUNEL-positive (asterisk). b In a rare example of asynchronous development in a spermatogonial clone, both mitosis and apoptosis are simultaneously activated in this tangentially sectioned M-PrM cyst. Note that the number of intense methyl-green-stained metaphase figures, with one immediately adjacent to a TUNEL-positive nucleus (arrow), is approximately double that of the number of the TUNEL-positive nuclei. c In another rare case of asynchrony in the stage-dependency of apoptosis in Squalus, the entire spermatogonial clone is completely TUNEL-labeled in an E-PrM cyst, with the perilumenar Sertoli nuclei being totally unaffected by the demise of the germ cell clone. Bars 16 μm (a, b), 20 μm (c; L.M. McClusky, unpublished)
Stage-related changes in the number of the various forms of TUNEL-labeling in the PrM region. Values represent the mean number ± SEM of counts of TUNEL-related death phenomena. Germinal zone cysts were rarely TUNEL-labeled and not scored, whereas L-PrM cysts were absent from the spermatogenic progression during this time of the year (see Fig. 5; L.M. McClusky, unpublished)
Another explanation for the occasionally observed asynchrony in life–death decisions in these cytoplasmically linked germ cell clones could be the purported surveillance activity of immature Sertoli cells and their role as putative cell counters within these cysts. Sertoli cell proliferation occurs when the E-PrM cyst spermatogonia are quiescent and no further Sertoli cell proliferation occurs after the E-PrM stage (see below). Therefore, upregulated spermatogonial cell-cycle activities in awakening E-PrM cysts might be monitored by Sertoli cells, which by the summer have entered a post-mitotic state (see below). Since Squalus Sertoli cells never become apoptotic in vivo and since the germ-cell-carrying capacity of the Sertoli cell is finite (for a review, see Sharpe et al. 2003), it is argued that Sertoli cell proliferative activities in each E-PrM cyst during late autumn through the winter months are exact and require no post-winter adjustment. Any required adjustment of the cell numbers of a cyst to optimize the Sertoli:germ cell ratio would affect the spermatogonia, which are in the cell cycle during spring–summer (see below); this agrees in principle with the known density-dependent regulation of spermatogonia in mammals (de Rooij and Janssen 1987; de Rooij and Lok 1987; Tadokoro et al. 2002). Since the Sertoli cells of E-PrM cysts are in essence naive or inexperienced cells, they might not be as efficient in their support of germ cells as experienced Sertoli cells, a conclusion that has similarly been made to explain the common degenerative phenomena in the pubertal rodent testis (for a review, see Sharpe 1994). For these reasons, the resumption of testicular activities in S. acanthias in the spring–summer transition has also been likened to a “seasonal” puberty.
Role of environmental cues in the seasonal variations of germ cell clone numbers
Given that all PrM cysts dispersed from summer-caught spiny dogfish continue with DNA synthetic activities on schedule and respond to various regulators in vitro (Dubois and Callard 1991, 1993; Piferrer et al. 1993; Piferrer and Callard 1995), the demise of especially large numbers of mature spermatogonial cysts via apoptosis at the resumption of testicular activities in spring–summer is indeed intriguing.
To acquire a better understanding of the significance of these findings, all cyst stages in monthly samples of testicular cross-sections have been quantified over a full spermatogenic cycle. The arrival each year of spiny dogfish in the inshore waters of New England with the onset of spring, following their northward migration from locations near Cape Hatteras (North Carolina) and offshore waters, occurs when sea water temperatures rise to 13°C and higher in New England (Stehlik 2007). This environmental cue is associated with cyst breakdown at the spermatogonia–spermatocyte transition such as to form a ZD behind any spermatocyte cyst that might be present in the spermatogenic sequence (Fig. 5). Further advancement into meiosis remains blocked for the duration of the summer and resumes only in the early autumn, followed one month later by the appearance of round spermatid cysts. Resumption of meiosis and spermiogenesis ensures the displacement of the ZD band toward the mature pole of the testis until it disappears from the spermatogenic sequence in winter. Interestingly, the changing seasons also result in spermatocyst cyst death via apoptosis at the spermatogonia–spermatocyte transition in cystic spermatogenesis in the Japanese newt, with a natural fall in ambient temperature at the onset of winter and accompanying elevated levels of prolactin being the inducers of apoptosis (Yazawa et al. 1999). By all accounts, the interval between the release of the apoptosis-inducing signal and cyst breakdown appears to be shorter in the newt than in the dogfish shark. The environmental cue that profoundly influences spermatogenic arrest in dogfish is water temperature and not photoperiod (Dobson and Dodd 1977c). This implies that the prevailing sea water temperature regime most probably underlies the cyclical pituitary gonadotropin activity and/or the responsiveness of germinal clones to gonadotropins, a notion that is consistent with the known effects of water temperature on fish gonadotropin synthesis and release (Fraser et al. 2002) and refractoriness of newt spermatogonial cysts to gonadotropic stimulation when ambient temperature falls (Yazawa et al. 2001).
Seasonal changes in the proportions of the major cyst stages comprising the spiny dogfish testis. The spatial arrangement of the various cyst stages and spermatogenic sequence (arrow) are also shown (GZ germinal zone, PrM premeiotic phase, M meiotic phase, ZD zone of degeneration, E-PoM early postmeiotic phase, M-PoM mid-postmeiotic phase, L-PoM late postmeiotic phase). Values represent the mean percentages of cysts at a given stage relative to the total number per testicular cross-section at each time point (see Fig. 6 for actual values; blue bar, red bar same months as indicated in Fig. 6; Yr year). Adapted from McClusky (2005) with permission
Since androgen reduces the rate of apoptosis in E-PrM cysts dispersed from spring-caught sharks in vitro (McClusky 2008), local androgen deprivation might also be a contributing factor to spermatogonial apoptosis in Squalus in vivo (see below). The time of year when the TUNEL-index of all PrM cysts is at its nadir (McClusky 2005) and when the mitosis–meiosis transition is re-established, corresponds exactly with the same months (October–December) when the proportion of mature spermatid cysts is at its highest (Fig. 6). As habitation in cold waters is thought to underlie the slow metabolism in spiny dogfish (Mattingly et al. 2004), the effects of androgen deprivation during the winter months might only manifest as an increase in the rates of apoptosis when rising sea water temperatures stimulate testicular recrudescence. All of this is consistent with the seasonal analysis of cyst numbers (Fig. 6), which shows a gradual protracted wave-like movement of peaks in cyst numbers over time. For instance, the formation of peak numbers of late-stage PoM (L-PoM) cysts (containing mature spermatids) once every year in November–December actually reflects the peak numbers of L-PrM cysts formed one year earlier during November–December (Fig. 6). Taken together, massive apoptotic death among mature spermatogonial generations is thus responsible for the cyclical nature of sperm production in S. acanthias. Squalus Sertoli cells themselves are not affected (Fig. 3) and instead mediate the death signals to the M-PrM cyst spermatogonia, indicating that Squalus Sertoli cells also have a role, though in an indirect manner, in the cyclical nature of spermatogenesis in the spiny dogfish shark. These findings agree in principle with the observed link between the timing of germ cell apoptosis and the temporal cycle of the Sertoli cell activities in a higher vertebrate (Timmons et al. 2002).
Temporal spermatogenic wave in S. acanthias as shown by the month-to-month changes in the number of spermatocysts in the major cyst stages, with the months exhibiting the testicular condition just prior to spermiation (blue). This testicular condition (h) also features peak numbers of M-PrM (c) and L-PrM cysts (d), and increasing numbers of cysts attempting the mitosis–meiosis transition (e). As indicated by the dashed lines, peak production of L-PoM cysts (h), which occurs once in a calendar year (Yr), is the culmination of the peak production of L-PrM cysts exactly one year earlier after the resumption of meiosis 4–6 months prior to that. The spent testicular condition (red) is associated with the peak production of cysts containing spermatocytes (e), followed one month later by a peak production of round spermatids (f). An inverse relationship between the peak occurrences of spermatocytes and mature spermatids is also known in two other shark species: Mustelus manazo and M. griseus (Teshima 1978). Adapted from McClusky (2005) with permission (lowercase letters indicate statistically significant differences)
Quantitative spermatogenic cycle data and other indicators of testicular activity for other elasmobranch species are fragmentary and incomplete. A comparison of the available data shows, however, that the cycle of the spiny dogfish shark is protracted compared with, for example, stingrays and bonnethead sharks found in temperate and tropical waters. Large portions of the round stingray testis contain spermatocysts at a similar stage of development (Mull et al. 2008). Furthermore, the developmental advance from spermatocyte to spermatozoa is completed within 3–4 months in both the Atlantic stingray (Maruska et al. 1996) and bonnethead shark (Parsons and Grier 1992). Given the stingray’s particular reproductive strategy, which includes a homogeneous and rapid type of spermatogenic development resulting, a few months later, in a testis almost entirely filled with mature spermatid cysts, the PrM region in this species is not surprisingly devoid of any TUNEL-positive staining (Mull et al. 2008). However, at the time of peak sperm production in the round stingray, the Sertoli cells of mature spermatid cysts near the efferent ducts are TUNEL-positive (Mull et al. 2008), a finding reminiscent of the spotted ray (Prisco et al. 2003) but not of any dogfish species testis. Taken together, these results illustrate the differences in the testicular activities and expression of testicular apoptosis among elasmobranchs, all of which reflect different reproductive strategies in different habitats.
Advance of spermatogonial clones after removal of premeiotic blockage
In most vertebrates, testicular regression is associated with massive germ cell death and the downregulation of all other indices of testicular activity, including DNA synthesis and steroidogenic activity. Massive spermatogonial apoptotic death at the mitosis–meiosis transition associated with testicular recrudescence and stage-related differences in seasonal variations in cyst numbers are, however, intriguing findings.
The diametric testis of S. acanthias has a distinct premeiotic region comprising 13 spermatogonial generations. Unlike in adult mammals, Sertoli cells are not permanent elements in the adult shark testis. During each spermatogenic wave across the diameter of dogfish testis, new Sertoli cells proliferate in a 1:1 ratio with spermatogonia over the first nine of the 13 spermatogonial divisions, i.e., in E-PrM cysts (McClusky 2005).
Given that enhanced DNA synthesis is a well-known effect of testicular steroid action, the next goal was to place the observed massive apoptotic death in vivo, cyst steroid output data and DNA synthesis and apoptosis activity determinations in cultured E-PrM cysts within an overall context of an in vivo analysis of PrM-cyst cell-cycle status (by using proliferative cell nuclear antigen [PCNA] immunohistochemistry) over an annual cycle (Fig. 7). Quantitative PCNA immunohistochemical analysis showed that close to 100% of M-PrM cysts were PCNA-positive at a time of year (summer months) when their numbers were the lowest during the annual cycle (McClusky 2005). An overall interpretation of Figs. 6, 7 revealed that the observed peak numbers of the next spermatogonial stage (L-PrM cysts, Fig. 6d) in the autumn months were the outcome of increased cell-cycle activity in an earlier spermatogonial stage (M-PrM cysts) during the summer months (McClusky 2005). Likewise, the peak in the numbers of M-PrM cysts in the autumn (Fig. 6c) was the result of increased cell-cycle activity in the previous spermatogonial stage (E-PrM cysts) during the summer, as shown by the intense PCNA-immunostained spermatogonia (Fig. 7c).
Seasonal and stage-related changes in the percentage of PrM spermatocysts a with PCNA immunoreactivity of spermatogonia (filled circles, open arrows) and Sertoli cells (open circles, black arrows) in E-PrM cysts b, c. Sertoli cells are in a postmitotic state after the 9th spermatogonial division (after the E-PrM stage) and were therefore not recorded in the M- and L-PrM cysts. Hatched bars above (a) indicate the months when stem-cell proliferation in the germinal ridge is known to occur (not quantified). The blue-shaded area indicates the same months as those highlighted in Fig. 6. a Adapted from McClusky (2005) with permission. b, c L.M. McClusky, unpublished (lowercase letters indicate statistically significant differences)
Furthermore, PCNA-immunostaining patterns of E-PrM cysts showed cell-type-specific changes according to the time of year, i.e., immunostaining patterns of spermatogonia and their Sertoli cells were not coincident but alternated (Fig. 7a) over the annual cycle. Sertoli cells of E-PrM cysts were in the cell cycle at a time of year (October-January) when the associated spermatogonia were quiescent (Fig. 7b). Indeed, this testicular condition in the PrM region during the winter months was coincident with the presence of peak numbers of mature spermatid cysts (blue shaded area, Figs. 6, 7) in the testis and presumably also with the peak operation of the mature→immature androgen-gradient via the vascular pathway of PoM→M→PrM (Cuevas et al. 1992).
Interestingly, the intratesticular distribution pattern of the Squalus androgen receptors (Cuevas and Callard 1992a) and estrogen receptors (Callard and Mak 1985; Callard et al. 1985) is PrM>M>>PoM. On the basis of the changing cellular relationships in the various zones, these receptors are probably located in the Sertoli cells themselves, because of the observed decrease in androgen receptor and estrogen receptor numbers through successive stages of cyst maturation (Callard et al. 1985). Therefore, androgen itself or its aromatized form (estrogen) has been hypothesized to have the presumed proliferative effect on Sertoli cells in E-PrM cysts during October-January (blue shading, Fig. 7a). Added estradiol does increase [3H]thymidine incorporation in Sertoli cells dispersed from E-PrM cysts (obtained in May-September) by more than two-fold (Dubois and Callard 1989). These in vitro findings are consistent with the analysis of in vivo quantitative seasonal data that show that the peak production of spermatocyte cysts (Fig. 6e), the source of estrogen biosynthetic enzymes (Callard et al. 1985), corresponds spatiotemporally exactly with the time of year (winter months) when the Sertoli cells in the immature PrM region are intensely PCNA-immunoreactive (Fig. 7b). In their study of the steroidogenic cycle in male European spotted dogfish, Garnier et al. (1999) have also observed a winter peak in intratesticular testosterone levels, which they have linked to “spermatogonial mitosis”, an understandable conclusion in the absence of any quantitative seasonal analysis of the cell-cycle status of the PrM region of the European spotted dogfish. Nevertheless, the data from these two Northern hemisphere dogfish species unequivocally indicate that androgen production reaches peak levels in winter.
The possibility that androgen itself might be responsible for Sertoli cell proliferation in winter E-PrM cysts cannot be completely excluded. Androgens were traditionally thought not to be implicated in Sertoli cell proliferation in mammals, although a role for androgen in the maturation process of Sertoli cells was mooted by some rodent studies (Sharpe et al. 2003; Hill et al. 2004). Subsequently, studies on mice lacking a functional androgen receptor (Johnston et al. 2004), microarray analysis of androgen-regulated transcripts in neonatal mice (Zhou et al. 2005) and investigations in which androgen production/action in neonatal rats were manipulated (Atanassova et al. 2005) suggested that androgens most likely play a physiological role in promoting Sertoli cell proliferation in fetal and newborn rodents. Furthermore, the complete ablation of androgen action in androgen receptor knockout mice reduces Sertoli cell numbers in adults (Tan et al. 2005). All in all, the findings of these intricate experiments in conventional laboratory mice agree in principle with the notion that, in Squalus at least, androgen emanating from mature spermatid cysts in winter probably underlies Sertoli cell proliferation in E-PrM cysts during those months when spermatogonia are quiescent. Further studies, such as the immunolocalization of androgen and estrogen receptors, are required to confirm this.
The overall synthesis of all the available data indicates therefore that the absence of mature spermatid (L-PoM) cysts from the testis during the winter and summer (Fig. 6h), coupled with the pause in pituitary gonadotropin output over the winter months, results in little to no diffusible androgen reaching the M-PrM cysts during the winter period of spermatogenic arrest and when these cysts awaken and enter the cell cycle (PCNA-positive) in late spring. This would explain the vulnerability of the spermatogonia in M-PrM cysts to apoptosis and, ultimately, the demise of M-PrM cysts and ZD formation (Fig. 8). E-PrM cysts in winter and summer are subjected to the same steroidal milieu as M-PrM cysts but even less of the minute amounts of available androgen, if any, will reach the E-PrM cysts, which are further upstream than the M-PrM cysts. Even though E-PrM cysts show slightly increased vulnerability to apoptosis in vivo in the awakening testis, they are relatively apoptosis-resistant compared with M-PrM cysts in vivo (McClusky 2005). The data therefore suggest that the proliferation of immature spermatogonia in Squalus E-PrM cysts during the spermatogenically active period (summer) is associated with an environment of low intratesticular androgen concentration, a conclusion that is consistent with similar findings reported in teleost fish (Chaves-Pozo et al. 2007; Corriero et al. 2007) and frogs (Sasso-Cerri et al. 2005). As shark cyst harvesting procedures predominantly select for E-PrM cysts (McClusky 2006, 2008), it is therefore no surprise that co-culture of shark PrM cysts with androgen-secreting PoM cysts in the summer results in a marked reduction in DNA synthesis in E-PrM cysts (Piferrer and Callard 1992). The addition of testosterone in the presence of 1% fetal bovine serum to cultured E-PrM cysts harvested from the awakening Squalus testis initially stimulates DNA synthesis but the effects become muted and even reversed in subsequent culture experiments later during the summer months (McClusky 2008).
Cross-sectional representation of the spatiotemporal changes in the zonated testis of S. acanthias during the winter, spring and summer months in the northern hemisphere. Cysts containing spermatids and spermatozoa are omitted for the sake of clarity. After a summer season of full spermatogenic activity, all spermatogenic stages, albeit in different proportions, are present in the spermatogenic sequence when the autumn months arrive. Note that a major feature of the autumn months is the peak production of cysts containing spermatozoa (L-PoM), which are major sources of androgen. A gradual decline follows, especially in spermatogonial proliferative activities after spermiation (red-shaded area, Fig. 6), such that the winter months are characterized by spermatogenic arrest, presumably as a result of the cessation of pituitary gonadotropin support. Upon resumption of the developmental advance in spring, the effects of gonadotropin and androgen deficiency during the past winter months are manifested as a gradient in apoptosis (black spots apoptotic spermatogonia) in the PrM region, with M-PrM and L-PrM cysts subsequently aborting their development and forming a zone of degeneration (ZD) at the spermatogonia–spermatocyte transition. Continued developmental advance of cysts during the summer months and continued abortion of M-PrM cysts subsequently result in deficits in the number of L-PrM (spring and summer) and M stages (summer) in the spermatogenic progression. Degenerative processes in ZD cysts continue; these cysts become oval and flattened as the formation of newer cysts compresses them. They persist in the testis until they reach the mature pole at the end of January (see Fig. 5)
Although focused quantitative analyses of the population dynamics of rodent spermatogonia are not as common as the analyses of other germ cell stages, active spermatogonial divisions are generally thought to occur when testosterone levels are relatively low; high testosterone levels might negatively affect spermatogonial differentiation (for a review, see de Rooij and Russell 2000; Meistrich and Shetty 2003). Low levels of testosterone characterize rat seminiferous epithelial stages IX-XII (Parvinen and Huhtaniemi 1990), the same stages that also feature peak spermatogonial mitotic activity (Blanco-Rodriguez et al. 2003). The transplantation of spermatogonia also favors an environment of low testosterone (Ogawa et al. 1998). Studies primarily in adult rodents indicate that the regulation of spermatogonia is multifaceted, involving density-dependent (de Rooij et al. 1989; de Rooij 2001; Meng et al. 2000; Tadokoro et al. 2002) and hormone-controlled (McLachlan et al. 1994; Meachem et al. 1999; Matthiesson et al. 2006) mechanisms mediated by Sertoli cells and operating in an age-dependent and spermatogonial-stage-dependent manner. Findings from organ cultures of immature rodent testes also indicate that developing Sertoli cells regulate the cell population dynamics of gonocytes, i.e., undifferentiated and differentiated spermatogonia, depending on the age of the Sertoli cell (Boitani et al. 1993, 1995; van Dissel-Emiliani et al. 1993; Sariola and Immonen 2008). Thus, a picture emerges showing that the regulation of the vertebrate spermatogonial population is indeed complex, most likely involving a signaling network of clone-extrinsic and clone-intrinsic factors mediated through the closely positioned Sertoli cells.
Concluding remarks
These findings indicate that the developmental advance of male germ cell clones and their response to various internal and external stimuli, including those from other more mature stages, occur in the context of an intimate three-dimensional spatiotemporal relationship with associated Sertoli cells. Since the Sertoli cell of the spiny dogfish shark nurtures only one anatomically enclosed germ cell clone at any one time and is simultaneously the source of steroids within the testis of this shark species at least, we might be tempted to speculate that the distinct stage-related functions of Sertoli cells in the shark testis will provide us with information about the ancient roles of Sertoli cells in vertebrates in general. Unconventional animal models, such as the ancient cartilaginous fishes, have a utility in the study of the close relationship between spermatogenic and steroidogenic events, and of the intimate Sertoli–germ-cell relationship, both of which are conserved throughout the vertebrate series.
References
Atanassova NN, Walker M, McKinnell C, Fisher JS, Sharpe RM (2005) Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol 184:107–117
Blanco-Rodriguez J, Martinez-Garcia C, Porras A (2003) Correlation between DNA synthesis in the second, third and fourth generations of spermatogonia and the occurrence of apoptosis in both spermatogonia and spermatocytes. Reproduction 126:661–668
Boitani C, Politi MG, Menna T (1993) Spermatogonial cell proliferation in organ culture of immature rat testis. Biol Reprod 48:761–767
Boitani C, Stefanini M, Fragale A, Morena AR (1995) Activin stimulates Sertoli cell proliferation in a defined period of rat testis development.Endocrinology 136:5438–5444
Breukelen F van, Krumschnabel G, Podrabsky J (2010) Vertebrate cell death in energy-limited conditions and how to avoid it: what we might learn from mammalian hibernators and other stress-tolerant vertebrates. Apoptosis 15:386–399
Callard GV (1991) Spermatogenesis. In: Pang PKT, Schreibman MP (eds) Vertebrate endocrinology: fundamentals and biomedical implications, vol 4. Academic Press, New York, pp 303–342
Callard GV, Mak P (1985) Exclusive nuclear location of estrogen receptors in Squalus testis. Proc Nat Acad Sci USA 82:1336–1340
Callard GV, Pudney JA, Mak P, Canick JA (1985) Stage-dependent changes in steroidogenic enzymes and estrogen receptors during spermatogenesis in the testis of the dogfish, Squalus acanthias. Endocrinology 117:1328–1335
Callard GV, Jorgensen JC, Redding JM (1995) Biochemical analysis of programmed cell death during premeiotic stages of spermatogenesis in vivo and in vitro. Dev Genet 16:140–147
Chaves-Pozo E, Liarte S, Vargas-Chacoff L, Garcia-Lopez A, Mulero V, Meseguer J, Mancera JM, Garcia-Ayala A (2007) 17Beta-estradiol triggers postspawning in spermatogenically active gilthead seabream (Sparus aurata L.) males. Biol Reprod 76:142–148
Corriero A, Desantis S, Bridges CR, Kime DE, Megalofonou P, Santamaria N, Cirillo F, Ventriglia G, Di Summa A, Deflorio M, Campobasso F, De Metrio G (2007) Germ cell proliferation and apoptosis during different phases of swordfish (Xiphias gladius L.) spermatogenetic cycle. J Fish Biol 70:83–99
Cuevas ME, Callard G (1992a) Androgen and progesterone receptors in shark (Squalus acanthias) testis: characteristics and stage-related distribution. Endocrinology 130:2173–2182
Cuevas ME, Callard G (1992b) In vitro steroid secretion by staged spermatocysts (Sertoli/germ cell units) of dogfish (Squalus acanthias) testis. Gen Comp Endocrinol 88:151–165
Cuevas ME, Miller W, Callard G (1992) Sulfoconjugation of steroids and the vascular pathway of communication in dogfish testis. J Exp Zool 264:119–129
Dissel-Emiliani FM van, Boer-Brouwer M de, Spek ER, Donk JA van der, Rooij DG de (1993) Survival and proliferation of rat gonocytes in vitro. Cell Tissue Res 273:141–147
Dobson S, Dodd JM (1977a) Endocrine control of the testis in the dogfish Scyliorhinus canicula L. I. Effects of partial hypophysectomy on gravimetric, hormonal and biochemical aspects of testis function. Gen Comp Endocrinol 32:41–52
Dobson S, Dodd JM (1977b) Endocrine control of the testis in the dogfish Scyliorhinus canicula L. II. Histological and ultrastructural changes in the testis after partial hypophysectomy (ventral lobectomy). Gen Comp Endocrinol 32:53–71
Dobson S, Dodd JM (1977c) The roles of temperature and photoperiod in the response of the testis of the dogfish, Scyliorhinus canicula L. to partial hypophysectomy (ventral lobectomy). Gen Comp Endocrinol 32:114–115
Dodd JM (1983) Reproduction in cartilaginous fishes (Chondrichthyes). In: Hoar WS, Randal DJ, Donaldson EM (eds) Fish physiology, vol 9, part 1. Academic Press, London, pp 31–95
Dubois W, Callard G (1989) Role of the Sertoli cell in spermatogenesis: the Squalus testis model. Fish Physiol Biochem 7:221–227
Dubois W, Callard GV (1991) Culture of intact Sertoli/germ cell units and isolated Sertoli cells from Squalus testis. I. Evidence of stage-related functions in vitro. J Exp Zool 258:359–372
Dubois W, Callard GV (1993) Culture of intact Sertoli/germ cell units and isolated Sertoli cells from Squalus testis. II. Stimulatory effects of insulin and IGF-I on DNA synthesis in premeiotic stages. J Exp Zool 267:233–244
Dym M, Fawcett DW (1971) Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod 4:195–215
Ehmcke J, Luetjens CM, Schlatt S (2005) Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix jacchus. Biol Reprod 72:293–300
Fraser EJ, Bosma PT, Trudeau VL, Docherty K (2002) The effect of water temperature on the GABAergic and reproductive systems in female and male goldfish (Carassius auratus). Gen Comp Endocrinol 125:163–175
Garnier DH, Sourdaine P, Jégou B (1999) Seasonal variations in sex steroids and male sexual characteristics in Scyliorhinus canicula. Gen Comp Endocrinol 116:281–290
Grier HJ (1992) Chordate testis: the extracellular matrix hypothesis. J Exp Zool 261:151–160
Hamer G, Roepers-Gajadien HL, Gademan IS, Kal HB, Rooij DG de (2003) Intercellular bridges and apoptosis in clones of male germ cells. Int J Androl 26:348–353
Hill CM, Anway MD, Zirkin BR, Brown TR (2004) Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol Reprod 71:1348–1358
Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ (2004) Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145:318–329
Loppion G, Crespel A, Martinez AS, Auvray P, Sourdaine P (2008) Study of the potential spermatogonial stem cell compartment in dogfish testis, Scyliorhinus canicula L. Cell Tissue Res 332:533–542
Maruska KP, Cowie EG, Tricas TC (1996) Periodic gonadal activity and protracted mating in elasmobranch fishes. J Exp Zool 276:219–323
Matova N, Cooley L (2001) Comparative aspects of animal oogenesis. Dev Biol 231:291–320
Matthiesson KL, McLachlan RI, O'Donnell L, Frydenberg M, Robertson DM, Stanton PG, Meachem SJ (2006) The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab 91:3962–3969
Mattingly C, Parton A, Dowell L, Rafferty J, Barnes D (2004) Cell and molecular biology of marine elasmobranchs: Squalus acanthias and Raja erinacea. Zebrafish 1:111–120
McClusky LM (2005) Stage and season effects on cell cycle and apoptotic activities of germ cells and Sertoli cells during spermatogenesis in the spiny dogfish (Squalus acanthias). Reproduction 129:89–102
McClusky LM (2006) Stage-dependency of apoptosis and the blood-testis barrier in the dogfish shark (Squalus acanthias): cadmium-induced changes as assessed by vital fluorescence techniques. Cell Tissue Res 325:541–553
McClusky LM (2008) Fetal bovine serum simultaneously stimulates apoptosis and DNA synthesis in premeiotic stages of spermatogenesis in spiny dogfish (Squalus acanthias) in vitro: modulation by androgen and spermatogenic activity status. Apoptosis 13:649–658
McClusky LM (2011) Testicular degeneration during spermatogenesis in the blue shark, Prionace glauca: nonconformity with expression as seen in the diametric testes of other carcharhinids. J Morphol 272:938–948
McClusky LM, Barnhoorn IE, Dyk JC van, Bornman MS (2008) Testicular apoptosis in feral Clarias gariepinus using TUNEL and cleaved caspase-3 immunohistochemistry. Ecotoxicol Environ Saf 71:41–46
McLachlan RI, Wreford NG, Meachem SJ, De Kretser DM, Robertson DM (1994) Effects of testosterone on spermatogenic cell populations in the adult rat. Biol Reprod 51:945-955
Meachem SJ, McLachlan RI, Stanton PG, Robertson DM, Wreford NG (1999) FSH immunoneutralization acutely impairs spermatogonial development in normal adult rats. J Androl 20:756–762
Meng X, Lindahl M, Hyvonen ME, Parvinen M, Rooij DG de, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493
Meistrich ML, Shetty G (2003) Inhibition of spermatogonial differentiation by testosterone. J Androl 24:135–148
Messam CA, Pittman RN (1998) Asynchrony and commitment to die during apoptosis. Exp Cell Res 238:389–398
Mull CG, Lowe CG, Young KA (2008) Photoperiod and water temperature regulation of seasonal reproduction in male round stingrays (Urobatis halleri). Comp Biochem Physiol [A] 151:717–725
Ogawa T, Dobrinski I, Avarbock MR, Brinster RL (1998) Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell 30:583–588
Parsons GR, Grier HJ (1992) Seasonal changes in shark testicular structure and spermatogenesis. J Exp Zool 261:173–184
Parvinen M, Huhtaniemi I (1990) Testosterone micromilieu in staged rat seminiferous tubules. J Steroid Biochem 36:377–381
Piferrer FC, Callard GV (1992) Paracrine regulation of premeiotic germ cell proliferation in the testis of the spiny dogfish, Squalus acanthias. Bull Mount Desert Island Biol Lab 32:49–50
Piferrer FC, Callard GV (1995) Inhibition of deoxyribonucleic acid synthesis during premeiotic stages of spermatogenesis by a factor from testis-associated lymphomyeloid tissue in the dogfish shark (Squalus acanthias). Biol Reprod 53:390–398
Piferrer F, Redding M, DuBois W, Callard G (1993) Stimulatory and inhibitory regulation of DNA synthesis during spermatogenesis: studies in Squalus acanthias. Fish Physiol Biochem 11:293–298
Prisco M, Liguoro A, Comitato R, Cardone A, D’Onghia B, Ricchiari L, Angelini F, Andreucetti P (2003) Apoptosis during spermatogenesis in the spotted ray Torpedo marmorata. Mol Reprod Dev 64:341–348
Pudney J (1995) Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 32:459–497
Pudney J, Callard GV (1984a) Development of agranular reticulum in Sertoli cells of the testis of the dogfish Squalus acanthias during spermatogenesis. Anat Rec 209:311–321
Pudney J, Callard GV (1984b) Identification of Leydig-like cells in the testis of the dogfish Squalus acanthias. Anat Rec 209:323–330
Rooij DG de (2001) Proliferation and differentiation of spermatogonial stem cells. Reproduction 121:347–354
Rooij DG de, Janssen JM (1987) Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster. I. Undifferentiated spermatogonia. Anat Rec 217:124–130
Rooij DG de, Lok D (1987) Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster: II. Differentiating spermatogonia. Anat Rec 217:131–136
Rooij DG de, Russell LD (2000) All you wanted to know about spermatogonia but were afraid to ask. J Androl 21:776–798
Rooij DG de, Dissel-Emiliani FM van, Pelt AM van (1989) Regulation of spermatogonial proliferation. Ann N Y Acad Sci 564:140–153
Roosen-Runge EC (1969) Comparative aspects of spermatogenesis. Biol Reprod 1 (Suppl 1):24–31
Sariola H, Immonen T (2008) GDNF maintains mouse spermatogonial stem cells in vivo and in vitro. Methods Mol Biol 450:127–135
Sasso-Cerri E, Freymüller E, Miraglia SM (2005) Testosterone-immunopositive primordial germ cells in the testis of the bullfrog, Rana catesbeiana. J Anat 206:519–523
Sharpe RM (1994) Regulation of spermatogenesis. In: Knobil A, Neill JD (eds) The physiology of reproduction. Raven, New York, pp 1363–1434
Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784
Simpson TH, Wardle CS (1967) A seasonal cycle in the testis of the spurdog, Squalus acanthias, and the sites of 3β-hydroxysteroid dehydrogenase activity. J Mar Biol Assoc UK 47:699–708
Sourdaine P, Garnier DH (1993) Stage-dependent modulation of Sertoli cell steroid production in dogfish (Scyliorhinus canicula). J Reprod Fertil 97:133–142
Sourdaine P, Garnier DH, Jégou B (1990) The adult dogfish (Scyliorhinus canicula L.) testis: a model to study stage-dependent changes in steroid levels during spermatogenesis. J Endocrinol 127:451–460
Stehlik LL (2007) Spiny dogfish, Squalus acanthias: life history and habitat characteristics. Technical memorandum no. 203. National Oceanic and Atmospheric Administration, Woods Hole
Sumpter JP, Jenkins N, Dodd JM (1978) Gonadotrophic hormone in the pituitary gland of the dogfish (Scyliorhinus canicula L.): distribution and physiological significance. Gen Comp Endocrinol 36:275–285
Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y (2002) Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113:29–39
Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G (2005) The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146:2674–2683
Teshima K (1978) Studies on sharks. XII. Monthly change of the gonad index in male Mustelus manazo and M. griseus. Jpn J Ichthyol 24:285–289
Timmons PM, Rigby PW, Poirier F (2002) The murine seminiferous epithelial cycle is pre-figured in the Sertoli cells of the embryonic testis. Development 129:635–647
Weber JE, Russell LD (1987) A study of intercellular bridges during spermatogenesis in the rat. Am J Anat 180:1–24
Wilson MR, Close TW, Trosko JE (2000) Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp Cell Res 254:257–268
Yazawa T, Yamamoto K, Kikuyama S, Abe SI (1999) Elevation of plasma prolactin concentrations by low temperature is the cause of spermatogonial cell death in the newt, Cynops pyrrhogaster. Gen Comp Endocrinol 113:302–311
Yazawa T, Yamamoto T, Kubokawa K, Nakayama Y, Fujimoto K, Ito R, Abe S (2001) Cold suppression of follicle-stimulating hormone activity on proliferation and survival of newt spermatogonia. Gen Comp Endocrinol 122:296–303
Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD (2005) Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod 72:1010–1019
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClusky, L.M. Coordination of spermatogenic processes in the testis: lessons from cystic spermatogenesis. Cell Tissue Res 349, 703–715 (2012). https://doi.org/10.1007/s00441-011-1288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1288-1