Abstract
Reptilian sperm morphology studies are continually increasing, and sperm biology is presently being utilized in other areas of study such as sexual selection, evolution, and phylogenetic analysis. The numbers of studies concerning sperm morphology in Squamata, however, are still limited compared to the diversity and number of lizard and snake species and the taxonomic distribution of these studies is widespread and random. Comparisons between closely related taxa are not feasible until further spermatozoal studies investigating designated comparative taxa are completed; therefore, this study aims to add a description of the mature spermatozoa of Agkistrodon contortrix (Linnaeus, 1766) and compare these data to the mature spermatid morphology of its related taxon, Agkistrodon piscivorus (Lacepede, 1789). Although the general architecture of the mature spermatozoa in A. contortrix is consistent with previous reports of mature sperm morphology in snakes, minor differences are noted including the location of the fibrous sheath starting at mitochondrial tier 4, a location that has not yet been reported for snakes. These data further suggest that minute differences in sperm ultrastructure can be observed in closely related taxa and may be beneficial in future phylogenetic and evolutionary analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The accumulating data on mature spermatozoal structures in squamates has led investigators to note variation to the general bauplan of the vertebrate spermatozoon, which consists of an apical acrosome, nucleus, and distal elongated flagellum. Ultrastructural variations have been observed in all three of these major spermatozoal structures. For example, the shape of the perforatorial base, the presence/absence of nuclear lacunae, and the most proximal origin of the fibrous sheath have all been reported as sites of plasticity within squamates (see Gribbins and Rheubert 2011, 2014 for reviews). These character modifications to the general architecture of spermatozoa within squamates are under intense selective pressures, which may be driven by post-copulatory mechanisms such as female reproductive tract evolution (Miller and Pitnick 2002; Brennan et al. 2007; Higginson et al. 2012), sperm specific protein evolution to promote sperm–egg interactions (Swanson and Vacquier 1998; Torgerson et al. 2002), and/or sperm competition (Parker 1970; Byrne et al. 2003; Tourmente et al. 2011).
Understanding ultrastructural differences among squamate spermatozoa and the potential role of spermatozoal characters in evolutionary and phylogenetic analyses have been the focus of many of the most recent works regarding sperm morphology in squamates (Jamieson and Healy 1992; Vieira et al. 2004; Tavares-Bastos et al. 2008; Rheubert et al. 2010; Gribbins and Rheubert 2011, 2014). Although taxonomic sampling in these studies has mostly been non-systematic and robust evolutionary analyses resulting in taxonomic resolution have not been feasible, phylogenetic inferences at higher taxonomic classifications (i.e., genus, family, order level) have been accomplished (Gribbins and Rheubert 2011, 2014). These studies have shown that structures in the spermatozoa may serve as synapomorphies for various taxa and may be beneficial in testing phylogenetic hypotheses. Within the Ophidia, Rheubert et al. (2010) found that a circular acrosome, dense neck collar, round midpiece mitochondria, the presence of lamellar bodies, and solid dense bodies were synapomorphies for all snakes, and fibrous sheath origin beginning at mitochondrial tier 1 was a synapomorphy for the Colubroidea. No sperm characters, however, served as synapomorphies for the Crotalidae.
Only a handful of studies dealing with male gametes have operated at taxonomic levels below the genera level. For example, Gribbins et al. (2014) investigated spermiogenesis in two closely related species, Sceloporus bicanthalis (Smith, 1937) and Sceloporus variabilis (Wiegmann, 1834), and noted that developmental aspects varied, which may lead to observed structural differences within the mature spermatozoa. Rheubert et al. (in press) investigated various populations of sister taxa, Sceloporus consobrinus (Baird and Girard, 1853) and Sceloporus undulatus (Bosc and Daudin, 1801), and noted that sperm ultrastructure did not differ between populations and/or species, which corroborated Tourmente et al. (2006) who also suggested sperm ultrastructure did not vary between populations of Boa constrictor (Linnaeus, 1758). Preliminary analysis suggests that variation does occur at the species level at least during spermiogenesis (Gribbins and Rheubert 2011, 2014). However, multiple studies utilizing closely related taxa are needed to gain a better understanding of whether this observed variation is also consistent within spermatozoal morphologies between species.
Thus, the goal of this study was to investigate the ultrastructure of the mature spermatozoon in Agkistrodon contortrix. The ultrastructure of the Copperhead’s mature spermatozoon will be the first description of sperm morphology within the Agkistrodon genus, and this new data can then be compared to the mature spermatids in the closely related Agkistrodon piscivorus (Gribbins et al. 2010). Data obtained in this study will contribute to the growing database of spermatozoal characters in squamates and are analyzed for potential synapomorphies within ophidians, specifically the Crotalidae.
Materials and methods
Adult male Agkistrodon contortrix (Linnaeus, 1766) were collected in Arkansas under the Arkansas Game and Fish Permit #012020161 by S. E. Trauth between the months of May–October from 1993 to 2010. Snakes were transferred to the lab where they were euthanized under the authority and approval of the Arkansas State University Museum of Zoology (ASUMZ), which followed the guidelines set forth by the ASIH-HL-SSAR (Beaupre 2004). Collection sites included the following localities (decimal degrees; NAD 83): Fulton Co. (36.476497°N; 91.575072°W)—ASUMZ 30637; Fulton Co. (36.444533°N; 92.117433)—ASUMZ 25315; Marion Co. (36.157789°N; 92.459322 W)—ASUMZ 19130; Montgomery Co., 34.374864°N; 93.877778°W)—ASUMZ 18515. The reproductive tracts were removed and the ductus deferens was isolated from the reproductive tract and cut transversely distal to the epididymis and proximal to the ampulla ureter complex within the cloaca. The vas deferens was fixed and stored in 3% glutaraldehyde in 0.05 M Cacodylate buffer until histologic preparations.
After a minimal fixation period of 48 h, the ductus deferens was cut into small 1 mm sections, washed with cacodylate buffer, and post-fixed in 1% osmium tetroxide for 2 h. Following post-fixation, tissues were washed three times with cacodylate buffer (pH 7.0) and dehydrated through a gradual series of ethanol solutions from 70 to 100%. Tissues were then cleared in acetone for 20 min and gradually introduced into Mollenhauer’s Epon-Araldite #2 (Dawes 1988) using increasing solutions of acetone/resin mixtures. Tissues were placed in pure acetone/epoxy resin and agitated for 24 h. Fresh epoxy resin was prepared and tissues were embedded using rubber molds and placed under vacuum at 30 °C for 20 min. The embedded tissues were cut at 1 ųm, sections, were placed on glass slides and stained with Ladd® multiple stain (Ladd Research, Williston, VT) for tissue identification before being sectioned at 90 nm using a Reichert Ultracut S ultramicrotome (Leica, Wetzlar, Germany), and placed on 200 mesh copper grids. Grids were stained with uranyl acetate for 20 min, lead citrate for 5 min, and allowed to dry for 24 h prior to microscopic analysis.
Tissues were viewed using a Zeiss EM109 transmission electron microscope (Zeiss, Peabody, MA) at various magnifications to observe structural details. Negatives were developed and digitized using an Epson Perfection V700 Photo scanner (Epson, Long Beach, CA). Digitized images were used to build composite plates representing the various components of the mature spermatozoon using Adobe Photoshop CS6 (Adobe Systems, San Jose, CA).
Results
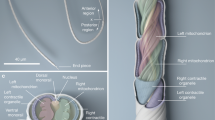
The mature spermatozoon of Agkistrodon contortrix is filiform in shape and follows the general bauplan of the typical squamate sperm. The apical portion of the spermatozoon is capped by the acrosome complex (Fig. 1, Ap), which rests on the apical portion of the nucleus (Fig. 1, Np). A portion of the acrosome envelops the tapered apical portion of the nucleus, the nuclear rostrum (Fig. 1, Nr). Caudally, the flagellum extends off of the nucleus and can be divided into the midpiece (Fig. 1, Mp), principal piece (Fig. 1, Pp), and endpiece (Fig. 1, Ep).
A schematic and an ultrastructural sagittal section of the Agkistrodon contortrix spermatozoon. The schematic shows representations of cross-sectional views of various areas within the three main regions of the spermatozoa. Ap acrosome proper, Nr nuclear rostrum, Np nucleus proper, Mp midpiece, Pp principal piece, Ep end piece
The acrosome vesicle (Fig. 1, Ap) envelops the apical region of the mature spermatozoa and drapes over the cranial portion of the nucleus with the acrosomal shoulders (Fig. 2, As) resting on the rostral shoulders of the nucleus (Fig. 2, Ns). Apically the acrosome is surrounded by membranous structures whose origins are from Sertoli cells within the seminiferous epithelium (Fig. 2, Ms). Superficially the acrosome vesicle contains an electron dense region called the acrosomal cortex (Fig. 2a, Ac), which surrounds the inner acrosomal medulla (Fig. 2a, Am). Within the basal region of the acrosome, the subacrosomal cone (Fig. 2b, Sc) is separated from the acrosomal vesicle (Fig. 2b, c, Av) by the acrosomal lucent ridge (Fig. 2b, Sl). Extending off the apical portion of the nucleus is an electron lucent region termed the epinuclear lucent zone (Fig. 2b, Ep) that abuts a dense region of the subacrosomal cone, which serves as the perforatorial basal plate (Fig. 2, Bp). The perforatorium (Fig. 2a, Pf) extends from the basal plate through the acrosomal medulla.
Cranial views of a mature spermatozoon from Agkistrodon contortrix. Left sagittal view through the apical portion of a mature spermatozoon detailing the acrosome complex: mulilaminar membranes (Ms), acrosome cortex (Ac), acrosome medulla (Am), perforatorium (Pf), perforatorial base plate (Bp), epinuclear lucent zone (Ep), subacrosomal cone (Sc), nuclear rostrum (Nr), Nuclear shoulders (Ns), and the Acrosome shoulders (As). a Cross-sectional view through the apical portion of the acrosome complex showing the subdivisions into the cortex (Ac), medulla (Am), and the perforatorium (Pf). b Cross-sectional view through the epinuclear lucent zone (Ep) detailing the acrosome vesicle (Av), subacrosomal cone (Sc), acrosomal lucent ridge (SI). c Cross-sectional view through basal portion of the acrosome complex showing the acrosome vesicle (Av), subacrosomal cone (Sc), rostrum of the nucleus (Nu), and plasma membrane (black arrow). d Cross-sectional view through the nuclear body detailing the homogenous electron density of the nucleus (Nu), the nuclear envelope (black arrowhead), the cell membrane (black arrow), and extracellular microtubules (white arrow). e Cross-sectional of the apical nuclear body revealing the nuclear lucuna (white arrow)
The nucleus is tapered apically and invades the acrosome complex as the nuclear rostrum (Fig. 2, Nr). The nucleus is surrounded by a loose cell membrane (Fig. 2b–d, black arrows) and a single row of microtubules in cross section (Fig. 2d, white arrow), which are found within the Sertoli cells remnants attached to the surface of the acrosome complex. Apically, the nucleus proper contains a nuclear lacuna (Fig. 2e, white arrow), but the lacuna does not extend through the entire nucleus and is thus absent in other cross-sectional views nuclear membrane (2d, black arrowhead). The distal end of the nucleus houses the proximal centriole (Fig. 3, Pc).
Posterior view of a mature spermatozoon from Agkistrodon contortrix. Left sagittal section through the posterior portion of the spermatozoon detailing the flagellum: distal centriole (Dc), midpiece (Mp), annulus (An), principal piece (Pp), and the endpiece (Ep). a Enlarged view of the neck region showing the proximal centriole (Pc) within the nuclear fossa of the nucleus (Nu), connecting piece (black arrows) extending from the proximal centriole, the distal centriole (Dc), mitochondria (Mi), and the fibrous sheath (Fs). b Cross-sectional view of the proximal centriole showing the 9 + 2 microtubule (Mt) arrangement of the axoneme (Ax), mitochondria (Mi), and the surrounding peripheral fibers (Pf). Note that the labeled peripheral fiber is grossly enlarged compared to the others around it. c Cross-sectional view of the midpiece detailing the mitochondria (Mi) and multilaminar membranes (Ms) surrounding the fibrous sheath (Fs), peripheral fibers (Pf) associated with microtubule doublets 3 and 8 of the axoneme. d Detailed sagittal view of the annulus (An) at the base of the midpiece and extension of the fibrous sheath (Fs) into the principal piece (Pp). Axoneme (Ax), mitochondria (Mi). e Cross-sectional view of the principal piece, note the distinct fibrous sheath (Fs) and the enlarged peripheral fibers 3 and 9 (Pf). Cell membrane (black arrow). f The endpiece is the terminal end of the flagellum and has no fibrous sheath but at least proximally still expresses enlarged peripheral fibers (Pf). Axoneme (Ax), cell membrane (black arrow)
The flagellum of the spermatozoon consists of proximal (Fig. 3, Pc) and distal centrioles (Fig. 3, Dc). The proximal centriole is oriented approximately 90° to that of the distal centriole. The proximal centriole is connected to the distal centriole via an electron dense connecting piece (Fig. 3a, black arrows) that is located in the neck region of the flagellum. Microtubules from the distal centriole give rise to the flagellum creating the axoneme. The axoneme of the midpiece (Fig. 3, Mp) of the flagellum is surrounded by mitochondria (Fig. 3a, Mi) and dense proteins blocks/rings that produce the helically arranged fibrous sheath (Fig. 3a, c, Fs), which begins at mitochondrial tier 4. The mitochondria surrounding the axoneme are mostly circular in both sagittal and transverse sections and there were no observations of dense bodies within the Copperhead’s elongated midpiece. The peripheral fibers (Fig. 3c, Pf) associated with microtubule doublets 3 and 8 are enlarged outside the distal centriole axoneme. The transition from the midpiece (Fig. 3, Mp) to the principal piece (Fig. 3, Pp) is demarcated by the electron dense annulus (Fig. 3d, An). The principal piece lacks mitochondria but the axoneme is surrounded by the fibrous sheath (Fig. 3e, Fs) and the peripheral fibers (Fig. 3e, Pf) associated with microtubule doublets 3 and 8 remain enlarged. The transition between the principal piece (Fig. 3, Pp) and the endpiece (Fig. 3, Ep) is marked by the loss of the fibrous sheath; however, peripheral fibers 3 and 8 are still visible within the axoneme (Fig. 3f).
Discussion
The ultrastructure of the spermatozoon in Agkistrodon contortrix follows the general ultrastructure of squamate spermatozoa. The acrosome is highly compartmentalized containing the enzymes necessary for fertilization. This high level of compartmentalization is common in squamates, and the structures found within the acrosome have served as characters in previous evolutionary analyses. Gribbins and Rheubert (2011) noted that a circular-shaped acrosome and knob-shaped perforatorial basal plate were common to the Ophidia, which was also noted in A. contortrix. To date, there have been no acrosomal characters that have been found to be unique to the Ophidia when compared to other amniotes (Gribbins and Rheubert 2011); however, an acrosomal lucent ridge is present in the acrosome complex of A. contortrix, which has not been reported or commented on in many earlier studies on spermatozoa in snakes. This character has largely been ignored in the literature but its size and development may show plasticity in squamates and must be furthered studied in snakes and lizards to deem its morphological importance and phylogenetic potential in Squamata and Crotalidae.
The nucleus in Agkistrodon contortrix is homogenous in electron density and consists of a nuclear lacuna. The presence of nuclear lacunae have been noted in Squamata and are observed in Crotalus durissus (Linnaeus, 1758), Liotyphlops beui (Amaral, 1924), and Typhlops reticulatus (Linnaeus, 1758) (Tavares-Bastos et al. 2007; Cunha et al. 2008); however, the nuclear lacunae have also been documented as absent in other species (Gribbins and Rheubert 2011; Rheubert et al. 2010). Previous authors have noted that the nuclear lacuna is typically located within the apical portion of the nucleus (Gribbins and Rheubert 2011, 2014) and, thus, its presence may have been missed in studies that suggest it is not observed within the spermatozoal nuclear body of snakes and other squamates. In all vertebrate species studied to date, the nucleus is indented caudally forming the nuclear fossa, which houses the proximal centriole.
The flagellum of the spermatozoon is the source of most variation within the sperm of squamates (Tavares-Bastos et al. 2008; Gribbins and Rheubert 2011; Rheubert et al. 2010). The only notable consistency is that dense bodies, if found in the midpiece, are solid in nature within snakes and lizards. Agkistrodon contortrix does not have conspicuous dense bodies like a few other snakes. The absence of dense bodies in A. contortrix spermatozoa is similar to what is seen within the Copperhead’s related taxon, Agkistrodon piscivorus (Gribbins et al. 2010). Interestingly, Crotalus durissus has dense bodies, while Bothrops species spermatozoa lack dense bodies (Gribbins and Rheubert 2011). The importance of the absence or presence of dense bodies needs to be worked out within the Crotalidae to merit its relevance to the evolution of the midpiece within pitvipers and other snakes. The presence of a dense collar has been reported as a synapomorphy in snakes (Gribbins and Rheubert 2011; Rheubert et al. 2010) and is also present in A. contortrix. The most variable character within the flagellum is the starting location of the fibrous sheath. In A. contortrix, the fibrous sheath begins as mitochondrial tier 4, which has not been reported within snake spermatozoa to date. In the viperids, it has previously been reported as starting at or before mitochondrial tier 1 (Furieri 1965; Cunha et al. 2008; Tourmente et al. 2008). The fibrous sheath is hypothesized to be involved in the flexibility of the flagellum and may play a role in motion dynamics (Eddy et al. 2003). The cause of variation in the size and starting point within this sheath is unknown. Peripheral fibers 3 and 8 are present in many snakes beyond the midpiece (Rheubert et al. 2010). Most squamates have enlarged peripheral fibers 3 and 8 within their principal pieces except for Eryx jayakari (Boulenger, 1888), Crotalus durissus, Nerodia Sipedon (Linnaeus, 1758), Stegonotus cucullatus (Dumeril, Bibron, Dumeril, 1854), and Iguana iguana (Linnaeus, 1758) (see Rheubert et al. 2010). Interestingly, A. contortrix has peripheral fibers 3 and 8 but Crotalus durissus does not and both are members of the family Crotalidae. This new finding could be significant phylogenetically; however, only these two species within Crotalidae show this difference within their spermatozoa. Further research within this family is required to justify this difference as important for evolutionary analysis within ophidians. A seemly unique morphological character to the flagellum of A. contortrix is the continuation of peripheral fibers 3 and 8 into at least the proximal endpiece. To our knowledge, this has not been reported for any other snake to date. Whether this anomaly is a possible autapomorphy for the Copperhead sperm or maybe a synapomorphy for Agkistrodon cannot be determined until further species are studied within Agkistrodon and within other species of Crotalidae and Viperidae.
In snakes, extracellular components are associated with the spermatozoa within the epididymis. Previous reports have shown that multilaminar structures and extracellular microtubules are present on the surfaces of epididymal spermatozoa (Jamieson and Koehler 1994; Oliver et al. 1996; Tourmente et al. 2006; Gribbins and Rheubert 2011; Rheubert et al. 2010). These structures have not been noted in other reptilian taxa and their origin and function are not known (Tavares-Bastos et al. 2008). We hypothesize that these structures are remnants from developing spermatids such as the microtubules from the manchette and membrane components of Sertoli cells that are shed during the apocrine-like release of the spermatozoa during spermiation. The multilaminar/membrane structures may be a source of energy for the spermatozoa (Bloom and Fawcett 1968) and aid in sperm motility (Oliver et al. 1996; Tourmente et al. 2006, 2011) but these hypotheses have not been directly tested.
The above comparisons suggest that ultrastructure differences in the mature spermatozoa can be found between taxa. This variation has been noted in previous studies and recent work has shown that ultrastructural disparities can be common between closely related species within their spermatids during spermiogenesis (Gribbins et al. 2014; Rheubert et al. 2016). Other studies have suggested that sister taxa, which are recently diverged, do not display ultrastructural differences as far as spermatozoal architecture (Rheubert et al. in press). The significance of these antagonistic views of spermiogenesis versus the mature spermatozoa is not known. An in depth study of both spermatozoa and the spermatids within related species of the same genus may help shed light on ultrastructural uniformity of the spermatozoa versus the plasticity of morphological characters present in spermatids between species within the same genus. For example, a comparison between Agkistrodon contortrix spermatozoa and Agkistrodon piscivorus spermatids show some differences. The epinuclear lucent zone and nuclear lacuna are present in A. contortrix spermatozoa but absent in A. piscivorus spermatids. There have also been reports of post-spermiation modification (Depeiges and Dufaure 1983; Esponda and Bedford 1987; Jones 1999) of sperm and any observed differences between spermatozoa and spermatids may be a result of these modifications. As previously stated, more studies concerning post-spermiation modifications need to be completed to test hypotheses concerning the validity in comparing morphologies of the mature spermatozoa and mature spermatids.
The data in this study suggest that the overall microscopic anatomy of the mature spermatozoa is conserved, especially in snakes, but minute ultrastructural differences can be observed between taxa. The degree of variation is still not well understood and future studies concerning sperm morphology in snakes, and squamates in general, should focus on closely related species to further test the likelihood that spermiogenesis or mature spermatozoal ultrastructure can provide nontraditional morphological data robust enough to utilize during phylogenetic studies.
References
Beaupre SJ (2004) Guidelines for use of live amphibians and reptiles in field and laboratory research, 2nd edn. American Society of Ichthyologists and Herpetologists, New York
Bloom W, Fawcett DW (1968) A textbook of histology. W.B. Saunders Co, Philadelphia
Brennan PLR, Prum RO, McCracken KG, Sorenson MD, Wilson RE, Birkhead TR (2007) Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2:e418
Byrne PG, Simmons LW, Roberts JD (2003) Sperm competition and the evolution of gamete morphology in frogs. Proc R Soc Lond 270:2079–2086
Cunha LD, Tavares-Bastos L, Báo SN (2008) Ultrastructural description and cytochemical study of the spermatozoon of Crotalus durissus (Squamata, Serpentes). Micron 39:915–925
Dawes CJ (1988) Introduction to biological electron microscopy: theory and techniques. Ladd Research Industries Inc., Burlington
Depeiges A, Dufaure JP (1983) Binding to spermatozoa of a major soluble protein secreted by the epididymis of the lizard Lacerta vivipara. Gamete Res 7:401–406
Eddy EM, Toshimori K, O’Brien DA (2003) Fibrous sheath of mammalian spermatozoa. Microsc Res Tech 61:103–115
Esponda P, Bedford JM (1987) Post-testicular change in the reptile sperm surface with particular reference to the snake, Natrix fasciata. J Exp Zool 24:123–132
Furieri P (1965) Prime osservazioni al microscopio elettronico sulla ultrastruttura del spermatozoo di Vipera aspis aspis. Vollettino della Societa Italiana di Biologia Spermimentale 41:478–480
Gribbins KM, Rheubert JL (2011) The ophidian testis, spermatogenesis, and mature spermatozoa. In: Aldridge RD, Sever DM (eds) Reproductive biology and phylogeny of snakes. CRC Press, Boca Raton, pp 183–264
Gribbins KM, Rheubert JL (2014) The architecture of the testis, spermatogenesis, and mature spermatozoa. In: Rheubert JL, Siegel DS, Trauth SE (eds) Reproductive biology and phylogeny of lizards and tuatara. CRC Press, Boca Raton, pp 340–424
Gribbins KM, Rheubert JL, Anzalone M, Siegel DS, Sever DM (2010) The ultrastructure of spermiogenesis in the Western Cottonmouth, Agkistrodon piscivorus. J Morph 271:2294–2304
Gribbins KM, Matchett CL, DelBello KA, Rheubert JL, Villagrán-SantaCruz M, Granados-González G, Hernández-Gallegos O (2014) The ultrastructure of spermatid development during spermiogenesis within the Rosebelly Lizard, Sceloporus variabilis (Reptilia, Squamata, Phrynosomatidae). J Morph 275:258–268
Higginson DM, Miller KB, Segrave KA, Pitnick S (2012) Female reproductive tract form drives the evolution of complex sperm morphology. Proc Natl Acad Sci 109:4538–4543
Jamieson BGM, Healy JM (1992) The phylogenetic position of the Tuatara, Sphenodon (Sphenodontida, Amniota), as indicated by cladistic analysis of the ultrastructure of spermatozoa. Philos Trans R Soc B 335:207–219
Jamieson BGM, Koehler L (1994) The ultrastructure of the spermatozoon of the Northern Water Snake, Nerodia sipedon (Colubridae, Serpentes), with phylogenetic considerations. Can J Zool 72:1648–1652
Jones RC (1999) To store or mature spermatozoa? The primary role of the epididymis. Int J Androl 22:57–66
Miller GT, Pitnick S (2002) Sperm-female coevolution in Drosophila. Science 298:1230–1233
Oliver SC, Jamieson BGM, Scheltinga DM (1996) The ultrastructure of spermatozoa of Squamta, II. Agamidae, Varanidae, Colubridae, Elapidae, and Boideae (Reptilia). Herpetologica 1996:216–241
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Rheubert JL, McMahann CD, Sever DM, Bundy MR, Siegel DS, Gribbins KM (2010) Ultrastructure of the reproductive system of the Black Swamp Snake (Seminatrix pygaea), VII. Spermatozoon morphology and evolutionary trends of sperm characters in snakes. J Zool Syst Evol Res 48:366–375
Rheubert JL, Sever DM, Siegel DS, Gribbins KM (2016) Ultrastructural analysis of spermiogenesis in the Eastern Fence Lizards, Sceloporus undulatus (Squamata: Phrynosomatidae). Micron 81:16–22
Rheubert JL, Mesak J Siegel DS, Gribbins KM, Sever DM, Trauth SE (in press) Inter and intra specific variation in sperm morphology between populations of Sceloporus consobrinus and Sceloporus undulatus. Biol J Lin Soc
Swanson WJ, Vacquier VD (1998) Concerted evolution in an egg receptor for a rapidly evolving abalone sperm protein. Science 28:710–712
Tavares-Bastos L, Cunha LDE, Colli GR, Báo SN (2007) Ultrastructure of spermatozoa of scolecophidian snakes (Lepidosauria, Squamata). Acta Zool 88:189–197
Tavares-Bastos L, Colli GR, Báo SN (2008) The evolution of sperm ultrastructure among Boidae (Serpentes). Zoomorphology 127:189–202
Torgerson DG, Kulathinal RJ, Singh RS (2002) Mammalian sperm proteins are rapidly evolving: evidence of positive selection in functionally diverse genes. Mol Biol Evol 19:1973–1980
Tourmente M, Cardoza G, Bertona M, Guidobaldi A, Giojalas L, Chiaraviglio M (2006) The ultrastructure of the spermatozoa of Boa constrictor occidentalis, with considerations on its mating system and sperm competition theories. Acta Zool 87:25–32
Tourmente M, Giojalas L, Chiaraviglio M (2008) Sperm ultrastructure of Bothrops alternatus and Bothhrops diporus (Viperidae, Serpentes) and its possible relation to the reproductive features of the species. Zoomorphology 127:241–248
Tourmente M, Gomendio M, Roldan ER (2011) Sperm competition and the evolution of sperm design in mammals. BMC Evol Biol 11:1
Vieira GHC, Colli GR, Báo SN (2004) The ultrastructure of the spermatozoon of the lizard Iguana iguana (Reptilia, Squamata, Iguanidae) and the variability of sperm morphology among iguanian lizards. J Anat 204:451–464
Acknowledgements
The authors would like to thank the University of Indianapolis and the University of Findlay for their continuous support and providing funding for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rheubert, J.L., Khan, A., Vollmer, E. et al. Ultrastructural analysis of the mature spermatozoon in the copperhead, Agkistrodon contortrix (Linnaeus, 1766). Zoomorphology 136, 379–386 (2017). https://doi.org/10.1007/s00435-017-0353-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-017-0353-z