Abstract
The structural and morphometric traits of spermatozoa reflect species-specific evolutionary and functional characters. The shrew, Crocidura shantungensis, which is an indigenous species of Ulleung-do, is dominant on this island and is suspected to have unique traits different from other shrews. Based on a previous sperm ultrastructural analysis, the structural and morphometric traits of caudal epididymal spermatozoa were evaluated by light, scanning, and transmission electron microscopy. Total length was 110.0 μm. The head was paddle shaped with a globular nucleus and was 16.0 μm in length. The acrosomal cap covered four-fifths of the nucleus, and the subacrosomal space had sawtooth-shaped edges. The number of mitochondrial gyres was 84–86. Nine segmented columns in the neck consisted of 12 knobs, and each column was fused with outer dense fiber. The outer dense fibers 1, 5, and 6 were thicker than the others. Outer fiber 1 was shaped like a horseshoe, whereas 5 and 6 were fused with each other. Numerous satellite fibers were scattered on the mid-piece between outer fibers 4 and 5, and 6 and 7. Two specific strong electron-dense fibers were found uniquely near the satellite fibers. In conclusion, our results demonstrate that the caudal sperm of Ulleung-do C. shantungensis has unique ultrastructural and morphometric traits based on common sperm characters of the subfamilies, suggesting unique physiological roles in reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-meiotic male germ cells must undergo adaptive changes to migrate in viscous luminal fluid, to pass through the specialized reproductive lumen, and to penetrate the cumulus complexes (Franzén 1970; Nicander 1970). Structural integrity of the mammalian sperm head and tail occurs through extreme changes when sperm complete the development process during the final stage of spermatogenesis (Lalli and Clermont 1981; Yuan et al. 2013). In addition, sperm must undergo drastic biochemical changes at the epididymis after spermiogenesis to acquire full ability for capacitation and fertilization during travel through the female reproductive tract. The form and size of sperm are critical for fertilization. Sperm head forms are species characteristic and vary from bullet shaped to globular shaped along with the number of mitochondria in the tail region. Therefore, the sperm morphological traits, such as shape and length of the head, mid-piece, and flagellum, are believed to be associated with evolution as an independent unit (Immler et al. 2012; Beguelini et al. 2014).
After Friend (1936) reported a microscopic examination of rodent sperm, a considerable number of electron and light microscopic structural studies on mammalian sperm were conducted (Koehler 1977; Sarafis et al. 1981; Flaherty and Breed 1987; Breed 2004, 2005; Santos et al. 2013; Soares et al. 2014) and distinct structural characteristics at the level of family, subfamily, genus, and species were revealed (Friend 1936; Fawcett 1970; Uchida and Mori 1972; Mōri 1994; Immler et al. 2012). These characteristics have been recently confirmed by genome-wide analyses. Therefore, sperm ultrastructural traits are species specific and uniquely adapted for successful reproduction (Cetica et al. 1997; Beguelini et al. 2014).
Crocidura shantungensis belong to Crocidurinae. Crocidurinae and Soricinae in the family Soricidae can be classified by the following sperm morphological features (Mōri et al. 1991). The Soricinae sperm head has a small acrosome, a smooth inner acrosomal membrane, and a wavy finger-like electron-dense apical body. The neck has a solid proximal centriole filled with electron-dense material (Mōri et al. 1991; Mōri 1994). In contrast, Crocidurinae sperm have a relatively large acrosome, a serrated inner acrosomal membrane, and a common apical body. The neck has a fistulous proximal centriole with slightly electron-dense granules (Koehler 1977; Mōri et al. 1991; Mōri 1994). However, several Crocidurinae species have intermediate forms of sperm. C. suaveolens sperm have conserved characteristics of Soricinae different from C. dsinezumi and C. lasiurai (Jeong et al. 2006).
Crocidura shantungensis and C. suaveolens are resident shrews of Ulleung-do, Korea. C. shantungensis is the most primitive eutherian in this area (Yoon 1992; Bannikova et al. 2009). A difference in the level of cytochrome b of mtDNA and morphometrics of sperm between C. suaveolens and the other land shrew, C. lasiura, has been reported (Motokawa et al. 2000; Han et al. 2002; Ohdachi et al. 2004; Jeong et al. 2006). The electron microscopic structures of sperm from C. suaveolens and other Crocidura in Korea have been described in a few studies and have revealed both common and unique characters. Based on a previous C. shantungensis spermatogenesis study (Jeong and Lee 2007), we evaluated the morphometric and ultrastructural traits of C. shantungensis spermatozoa and comparatively determined the morphological features.
Materials and methods
Experimental animals were collected and examined under the guidelines of the Institutional Review Board at Kyungnam University. Nine C. shantungensis and three C. suaveolens were collected in Namsel-ri, Ulleung-gyun, and Gyeongsang-bukdo, Korea, from July to August 2012 using Sherman traps. Ulleung-do is an oceanic island located in the East Sea that was formed during the Cenozoic era. The animals were anesthetized immediately with ethyl ether, and caudal epididymal tissues were isolated. The extracted tissues were soaked in 3 % glutaraldehyde in 0.1 M Milloning’s phosphate buffer (pH 7.4).
The epididymal spermatozoa of the adult male shrews were flushed and smeared on glass slides after being left at room temperature for 5–10 min in preparation for light microscopy. The slides were washed in 0.1 M Milloning’s phosphate buffer (4 °C, pH 7.4). They were then fixed in 3 % glutaraldehyde (4 °C, pH 7.4, Milloning’s phosphate buffer) for 2 h. After pre-fixation, the sperm samples were washed three times with Milloning’s phosphate buffer. The samples were dehydrated through an ethanol series and immersed in hexamethyldisilazane. After desiccation, the samples were observed without staining under an optical microscope (OPTIPHOT-2, Nikon, Tokyo, Japan).
Sperm smeared on slides were fixed and dehydrated as described for light microscopy and then coated with gold using an ion coater (IB·3, Giko) for 1 min 30 s in preparation for scanning electron microscopy. The coated samples were examined by scanning electron microscopy (S-4200, Hitachi, Tokyo, Japan) at 15 kv.
The extracted epididymis was pre-fixed in a solution containing 3 % glutaraldehyde (4 °C, pH 7.4, Milloning’s phosphate buffer) for 2 h in preparation for transmission electron microscopy. The samples were cut into 1- to 1.5-mm3-thick sheets and fixed in a solution containing 3 % glutaraldehyde (4 °C, pH 7.4, Milloning’s phosphate buffer) for 2 h. After fixation, the tissue slices were washed with the same buffer (4 °C, pH 7.4, Milloning’s phosphate buffer) three times. The tissue slices underwent post-fixation in 1.33 % OsO4 (pH 7.4, Millonig’s buffer) for 2 h. After post-fixation, the tissue slices were washed with the same buffer solution three times and dehydrated in an ethanol series (60–100 %). The tissue slices were embedded in Epon 812, and 60- to 90-nm-thin sections were cut. These ultrathin sections were stained using uranyl acetate and lead citrate and examined with a H-600 (HITACHI) and a JEM 1200 EX-11 (Jeol, Tokyo, Japan) transmission electron microscopes at 75 kV.
Results
Structural and morphometric characteristics of the sperm head and general features
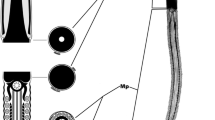
The sperm had a flattened and paddle-shaped head with a large acrosome (Figs. 1, 2). The dimensions of the sperm are summarized in Table 1. The total length of the sperm was 110.0 μm, and the head was approximately 16.0 μm. The acrosomal cap covered four-fifths of the sperm nucleus (Fig. 1). The sperm nucleus was 5.0 μm long and oval shaped with a post-nuclear cap covering one-fifth of the nucleus (Fig. 1; Table 1). The acrosome was about 10.0 μm in length, occupied most of the head region, and was also shaped like a very thin paddle (Figs. 1, 2; Table 1). The region of the sperm nucleus (excluding the post-nuclear cap) was surrounded by the subacrosomal space (Fig. 2, Text-Fig. 1b, c). The subacrosomal space was 1.0 μm in length, had sawtooth-shaped edges, and was filled with microgranules (Fig. 3).
Transmission electron micrograph of caudal epididymal spermatozoa of the Ulleung-do shrew, Crocidura shantungensis. a a scanning electron micrograph of the sperm head. b a light micrograph of the entire sperm, depicting the four major segments. Sperm consists of four major segments: neck, middle tail, principal tail, and end piece (b). The shape of the sperm head was paddle formed (a), and the subacrosomal space (Ss) was covered by the acrosomal cap (Ac). The post-nuclear cap (Pnc) was not covered by the acrosomal cap (Ac) and occupied about one-fifth of the nucleus. The basal plate (Bp) was adherent to the envelope, defining the implantation fossa and forming the attachment site of the flagellum to the sperm head. Mitochondria were arranged regularly on the sides of the axoneme, and the total number of mitochondrial gyres was 84–86. A acrosome, Ep end piece, H head, Iam inner acrosomal membrane, M mitochondria, Mp middle piece, Nc neck, N nucleus, Pp principal piece
Scanning electron micrograph showing the acrosomal cap. Acrosome is stripped away to reveal the nuclear envelope. The nucleus (N) was globular shaped. The subacrosomal space (Ss) had sawtooth-shaped edges. Text-Fig. 1: diagrammatic representation, based on light, scanning, and transmission electron microscopy of horizontal and sagittal sections of the epididymal spermatozoa in Crocidura shantungensis. The acrosome occupied most of the head region and was shaped like a very thin paddle (text-Fig. 1a). The acrosomal cap covered four-fifths of the sperm nucleus, and the subacrosomal space had sawtooth-shaped edges. A subacrosomal space existed between the inner acrosomal membrane and the sperm, except the Pnc. A acrosome, Ac acrosomal cap, H head, Mp middle piece, Nc neck, N nucleus, Pnc post-nuclear cap, Ss subacrosomal space
Structural and morphometric characteristics of the sperm tail and general features
The sperm tail was composed of the neck, the mid-piece, the principle piece, and the end piece (Fig. 1, inset b). The sperm tail was approximately 94 μm long and was divided into a neck of 2.5 μm, a mid-piece of 30.0 μm, a principal piece of 52.0 μm, and an end piece of 9.5 μm (Table 1). The neck region was composed of the basal plate, the capitulum, and the proximal and distal centrioles. The latter three were located beneath the basal plate. The basal plate was composed of high-electron-dense materials at the base of the neck region. The neck also contained nine segmented columns extending from the centrosomes (Fig. 4). Each column had 12 segments (Fig. 5). Each segmented column was fused with one of nine outer dense fibers (Fig. 5a). The end of the mid-piece was characterized by a ring-like annulus. The total number of mitochondrial gyres in the mid-piece was 84–86 (Table 1).
Consecutive serial sections a–d of the Crocidura shantungensis sperm neck. The segmented columns were surrounded by the redundant membranous scroll (Ms). One of the segmented columns consisted of 12 knobs, and each segmented column in the neck region was connected with nine outer dense fibers (numbers 1–9). The nine outer dense fibers were surrounded by the mitochondrial sheath (arrows). Ax axoneme, Bp basal plate, M mitochondria, Ms redundant membranous scroll (redundant nuclear membrane), N nucleus, Ne nuclear envelope, Odf outer dense fiber, Pm plasma membrane, Asterisks electron-dense materials, Arrow head proximal centriole
Longitudinal and cross sections of the middle piece of Crocidura shantungensis sperm. Note the electron-dense material (asterisks) in the segmented columns of the neck region (a). Fiber 1 was shaped like a horseshoe. Numbers 1, 5, and 6 of the outer dense fibers (odf) were larger than the others in the middle piece (b). Uniquely thick and strong electron-dense fibers (Edf) were localized between 4 and 5, and 6 and 7 (a, b). Edf were not found in C. suaveolens (b’). Most of the satellite fibers were observed at the beginning of the mid-piece, although they were not found in the principal and end pieces (b, c). Several segmented columns (Sc) were fused in the neck region. A fibrous sheath (FS) and longitudinal column (LC) were observed in the principal piece (c). Ax axoneme, Bp basal plate, C capitulum, Edf thick and strong electron-dense fiber, M mitochondria, Ms mitochondrial sheath, N nucleus, Ne nuclear envelope, Pm plasma membrane, Sf satellite fiber, Asterisks electron-dense materials, Right Pointing Triangle proximal centriole
Among the nine outer dense fibers, fibers 1, 5, and 6 were larger than the remaining fibers (Fig. 5b, c). Fiber 1 was shaped like a horseshoe. Among them, only fibers 5 and 6 were fused with each other. Satellite fibers were observed at the beginning of the mid-piece, around fibers 5 and 6. They were not found in the principal and end pieces. A fibrous sheath and longitudinal column were observed in the principal piece (Fig. 5). Two thick and strong electron-dense fibers (Edf) uniquely developed between fibers 4 and 5, and 6 and 7, and near the satellite fibers (Fig. 5a, b). Edf was not found in C. suaveolens (Fig. 5b’).
Discussion
Sperm morphological traits are a result of adaptation of sperm to the structure and function of the female reproductive tract and are therefore associated with reproductive physiology. Sperm head shapes vary such as a paddle (Breed 2004, 2005), ladle (Breed 2004), oval (Breed 2005), and a fish hook (Lee and Mōri 2006). The rodent sperm head is typically characterized by a hook- or falciform-shaped head (Sarafis et al. 1981). The evolution of other parts of the sperm involves a long tail, although some rat subfamilies have a short tail without a head (Breed 2005). The families Soricidae and Sciuridae have neither an apical hook nor a ventral process, and they are in contrast to the families Cricetidae and Muridae. C. shantungensis sperm had a non-hook-shaped sperm head without an apical hook or a ventral process. It had a paddle-shaped sperm head, which is the same as other shrews in Korea, such as C. suareolens, C. dsinezumi, and C. lasiura (Jeong et al. 2006).
The size and shape of the sperm head and tail in various rodent species are summarized in Table 2. The size of the sperm head was the second largest next to that of Sunncus murinus (Soricidae; Mōri et al. 1991; Table 2). Sperm head size of C. shantungensis was bigger than that of other Crocidura. The length of the mid-piece of C. shantungensis was shorter compared with that of other terrestrial Crocidura, such as C. suareolens, C. dsinezumi, and C. lasiura. In addition, the total length of C. shantungensis sperm was not the same as that of other terrestrial Crocidura (Jeong et al. 2006). However, the ratio of the middle piece to the tail was smaller in C. shantungensis (Jeong et al. 2006).
The sawtooth-shaped edge observed at the subacrosomal space is a common Crocidurinae character (Koehler 1977; Mōri et al. 1991; Mōri 1994). As in Korean land shrews, C. shantungensis sperm have a large acrosome and the post-nuclear cap was surrounded by the acrosomal cap. However, the acrosome was dominant in the sperm head much more than that of other Korean land shrews, such as C. dsinezumi and C. lasiura (Jeong et al. 2006). The acrosomal cap covered four-fifths of the head. The regions of the sperm nucleus (excluding the post-nuclear cap) were surrounded by the subacrosomal space and covered third-fourths of the sperm nucleus. In contrast, the length of the post-nuclear cap was different from that of other land shrews, such as C. suaveolens (half the nuclear length), C. dsinezumi (2/5 of nuclear length), and C. lasiura (2/5 of nuclear length). It has been suggested that the length of the post-nuclear cap is a key to classifying Socriciane and Crocidurinae, including that C. shantungensis of Ulleung-do has unique sperm traits.
The neck region of the C. shantungensis sperm consisted of a high-electron-dense basal plate and nine dense fibers attached to the bottom tip of the segmented columns, which was consistent with earlier results for Korean land shrews (Jeong et al. 2006), such as Bandicota bengalensis, Bandicota indica, Paruromys dominator, Tokudaia osimensis, Apodemus agrarius (Breed 2004), Apodemus agrarius coreae, Apodemus speciosus peninsulae, and the ddY strain mouse (Lee and Mōri 2006). The morphology of the outer fibers did not match the result of Breed (2004) for A. agrarius and P. dominator, which have larger fibers 1, 5, 6, and 9 than the others. Characteristics, such as a horseshoe-shaped fiber 1 and fused 5 and 6 fibers, and the distribution of satellite fibers at the middle piece were similar to other Korean land shrews. This formation of satellite fibers likely resulted from dislodging of the cell envelope in the outer dense fibers, and these structural changes likely increase expansion of the outer dense fibers and eventually induce sperm ruffing (Oh et al. 1985). Interestingly, two specific strong electron-dense fibers uniquely developed beside the satellite fibers in C. shantungensis. This structure is not found in C. suaveolens, which resides at Jeju-do and Ulleng-do, Korea. In addition, this structure is not found in other Korean shrews (Jeong et al. 2006). Therefore, it will be interesting to determine whether the presence of strong electron-dense fibers in Ulleung-do C. Shantungensis sperm is correlated with their dominance on the island.
There is wide species variation between the number of gyres in the mitochondrial helix, ranging from as few as 15 to more than 300 in some rodents (Austin and Short 1982). At present, mitochondrial information on the sperm mid-piece of Crocidurinae is insufficient to evaluate the meaning of mitochondrial number in species-specific roles. Nevertheless, the number and thickness of sperm mitochondria may be related to the ability of sperm to survive for a long duration in the female reproductive tract (Son et al. 1997; Oh et al. 1985). In this study, we found that the turnover of mitochondria was 84–86 in C. shantungensis. The mitochondria were thick compared with those of rats but similar to those of mice (Breed 2004; Lee and Mōri 2006).
In summary, the characters of the sperm nuclear envelope structures of C. shantungensis are closer to those of C. suaveolens but others are closer to C. dsinezumi and C. lasiura. In contrast, C. shantungensis has its own structural characteristics such as strong electron-dense fiber, total length, and length of the post-nuclear cap. These results suggest that C. shantungensis have developed unique sperm morphometric and ultrastructural characteristics that distinguish it from other species. They also suggest that the ultrastructural and morphometric sperm traits of C. shantungensis may be unique for their physiological roles in reproduction and dominance on Ulleng-do, although further study is needed.
References
Austin CR, Short RV (1982) Reproduction in mammals. Book 1 Germ cells and fertilization. Cambridge University Press, Cambridge, pp 63–101
Bannikova AA, Sheftel BI, Lebedev VS, Aleksandrov DY, Muehlenberg M (2009) Crocidura shantungensis, a new species for Mongolia and Buratia. Dokl Biol Sci 424:68–71
Beguelini MR, Bueno LM, Caun DL, Taboga SR, Morielle-Versute E (2014) Ultrastructure of spermatogenesis in the short-tailed fruit bat, Carollia perspicillata (Chiroptera: Phyllostomidae: Carollinae). J Morphol 275:111–123
Breed WG (2004) The spermatozoon of Eurasian murine rodents: its morphological diversity and evolution. J Morphol 261:52–69
Breed WG (2005) Evolution of the spermatozoon in muroid rodents. J Morphol 265:271–290
Breed WG, Cox GA, Leigh CM, Hawkins P (1988) Sperm head structure of a murid rodent from Southern africa: the red veld rat Aethomys chrysophilus. Gamete Res 19:191–202
Cetica P, Rahn IM, Merani MS, Solari A (1997) Comparative spermatology in Dasypodidae II (Chaetophractus vellerosus, Zaedyus pichiy, Euphractus sexcinctus, Tolypeutes matacus, Dasypus septemcinctus and Dasypus novemcinctus). Biocell 21:195–204
Fawcett DW (1970) A comparative view of sperm ultrastructure. Biol Reprod Suppl 2:90–129
Flaherty SP, Breed WG (1987) Formation of the ventral hooks on the sperm head of the plains mouse, Pseudomys australis. Gamete Res 17:115–129
Franzén A (1970) Phylogenetic aspects of the morphology of spermatozoa and spermatogenesis. In: Baccetti B (ed) Comparative spermatology. Acccademia Nazionale dei Lincel, Rome, pp 29–46
Friend GF (1936) The sperms of the British Muridae. Q J Microsc Sci 78:419–443
Han SH, Iwasa MA, Ohdachi SD, Oh HS, Suzuki H, Tsuchiya K, Abe H (2002) Molecular phylogeny of Crocidura shrews in northeastern Asia: a special reference to specimens on Cheju Island, South Korea. Acta Theriol 47:369–379
Immler S, Gonzalez-Voyer A, Birkhead TR (2012) Distinct evolutionary patterns of morphometric sperm traits in passerine birds. Proc R Soc B 279:4174–4182
Jeong SD, Lee JH (2007) Spermiogenesis in the Crocidura shantungensis. Dev Reprod 11:31–41
Jeong SJ, Lark JC, Kim HJ, Bae CS, Yoon MH, Lim DS, Jeong MJ (2006) Comparative fine structure of the epididymal spermatozoa from three Korean shrews with considerations on their phylogenetic relationships. Biocell 30:279–286
Koehler JK (1977) Fine structure of the spermatozoa of the Asiatic musk shrew, Suncus murinus. J Anat 149:135–152
Lalli M, Clermont Y (1981) Structural changes of the head components of the rat spermatid during late spermiogenesis. Am J Anat 160:419–434
Lee JH, Mōri T (2006) Ultrastructural observations on the sperm of two Apodemus species, Apodemus agrarius coreae and Apodemus speciosus peninsulae, in Korea. J Fac Agr Kyushu Univ 51:125–133
Mōri T (1994) Phylogenetic implications of sperm ultrastructure in Japanese Insectivores. Sci Mamm Jpn 34:51–57
Mōri T, Arai S, Shiraishi S, Uchida TA (1991) Ultrastructural observations on spermatozoa of the Soricidae, with special attention to a subfamily revision of the Japanese water shrew Chimarrogale himalayica. J Mamm Soc Jpn 16:1–12
Motokawa M, Suzuki H, Harada M, Lin LK, Koyasu K, Oda S (2000) Phylogenetic relationships among east asian species of Crocidura (Mammalia, Insectivora) inferred from mitochondrial cytochrome b gene sequences. Zool Sci 17:497–504
Nicander L (1970) Comparative studies on the fine structure of vertebrate spermatozoa. In: Baccetti B (ed) Comparative spermatology. Acccademia Nazionale dei Lincel, Rome, pp 47–56
Oh YK, Mōri T, Uchida TA (1985) Spermiogenesis in the Japanese greater horseshoe bat, Rhinolophus ferrumequnium nippon. J Fac Agr Kyushu Univ 29:203–209
Ohdachi SD, Iwasa MA, Nesterenko VA, Abe H, Masuda R, Haberl W (2004) Molecular phylogenetics of Crocidura shrews (Insectivora) in east and central Asia. J Mamm 85:396–403
Santos PR, Oliveira MF, Arroyo MA, Silva AR, Rici RE, Miglino MA, Assis Neto AC (2013) Ultrastructure of spermatogenesis in Spix’s yellow-toothed cavy (Galea spixii). Reproduction 147:13–19
Sarafis V, Lambert RW, Breed WG (1981) Sperm head morphology of the plains mouse Pseudomys australis. J Reprod Fertil 61:399–401
Soares AT, Silva SV, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MM (2014) Ultrastructure evaluation of goat spermatozoa after freezing in a skim milk-based extender with trolox supplementation. Andrologia. doi:10.1111/and.12279
Son SW, Lee JH, Chon HM (1997) Spermiogenesis in the Korean daubenton’s bat (Myotis daubentonii ussuriensis). Dev Reprod 1:9–24
Uchida TA, Mōri T (1972) Electron microscope studies on the fine structure of germ cells in Chiroptera I. Spermiogenesis in some bats and notes on its phylogenetic significance. Sci Bull Fac Agr Kyushu 26:399–418
Yoon MH (1992) Wild animals, Korea. Daewonsa Publishing Co Ltd, Seoul, pp 17–27
Yuan S, Zheng H, Zheng Z, Yan W (2013) Proteomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PLoS ONE 8:e77466. doi:10.1371/journal.pone.0077466
Acknowledgments
We thank the Society of Developmental Biology in Korea.
Conflict of interest
The authors reported no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Andreas Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Cheon, YP., Lee, SH. & Lee, JH. Fine structural traits of Crocidura shantungensis caudal epididymal spermatozoa. Zoomorphology 134, 317–324 (2015). https://doi.org/10.1007/s00435-014-0249-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-014-0249-0