Abstract
The study investigated the duration and peak of the daily spawning cycle of the chokka squid Loligo reynaudii, and the possibility of estimating the spawning frequency by means of classification and grouping (by age) of post-ovulatory follicles. Post-ovulatory follicles were classified into three types: new, intermediate or old, based in part on the degree of resorption of the follicles determined from histological sections. Ovulation in this species appears to be a rapid process, seen by the occurrence of both mature oocytes and post-ovulatory follicles in partially spent ovaries. Results suggest that chokka squid present a diel timing of spawning, with successive periods of egg accumulation at night, interrupted by periods of active spawning during the day. The ovarian cycle for this species appears typical of serial spawners. During the spawning phase ovaries go through ripe, partially spent and recovering stages by undergoing a process of maturation, ovulation and redeveloping, where a new batch of advanced oocytes is recruited. The cycle typically appears to last between 24 and 36 h, and may depend on a number of factors such as environmental conditions and the presence of predators. The short-lived (about 14 h) nature of the post-ovulatory follicles precludes the calculation of the exact spawning interval and does not allow us to accurately predict the spawning frequency. Together with the degree of oviduct fullness they do however give a good indication of imminent or recent spawning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective management of the South African hand jig fishery for the chokka squid, Loligo reynaudii requires a sound knowledge of their reproductive biology. Although the ecology of the chokka squid has been studied in detail (Augustyn 1989; Augustyn et al. 1994; Roberts and Sauer 1994; Roberts 2005) some aspects remain elusive. Key areas of the life cycle still requiring elucidation include age structure of the squid population, the effect of environmental perturbations on recruitment, and details of the spawning cycle. The estimation of fecundity is of particular importance as fishing concentrates on spawning aggregations, and females may be caught prior to egg laying.
A major difficulty confronting investigators studying cephalopod reproductive biology has been to establish whether egg-laying occurs in one single operation or in separates batches, and estimating the actual fecundity in cephalopods presents a number of challenges. Studies on a number of behavioural and biological aspects of the chokka squid have provided proof that this species is a serial spawner. A study on reproductive patterns, based on histological examination of the ovaries, and data on oocyte size frequency distribution from gonads at various stages of maturity, strongly suggests serial spawning (Sauer and Lipinski 1990; Melo and Sauer 1999). No mass mortality has been found on the inshore spawning grounds despite extensive diving surveys (Sauer et al. 1992). Tagging studies (Sauer et al. 2000) show individuals to move between spawning sites over a number of weeks. Finally in aquarium experiments (De Wet 1995) individual squids spawned twice within 12•36 h, depositing a total of some 7900 eggs.
Although the number of eggs produced per spawning for a single female has been estimated for chokka squid (Sauer et al. 1999), the spawning frequency (intervals between spawning) and the number of batches of eggs spawned from any particular ovary are still unknown.
To estimate spawning frequency of squid caught on the spawning grounds it is first necessary to be able to recognize a recent spawning event in an individual squid. Direct estimation of spawning frequency for multiple spawner squids was first estimated by Young and Mangold (1994), based on the frequency distribution of oviductal eggs in the mesopelagic-boundary squid, Abralia trigonura.
A histological investigation of the ovaries of chokka squid, however, showed that recent spawning seems to be linked to the presence of post-ovulatory follicles (Melo and Sauer 1999). Post-ovulatory follicles have been identified in cephalopods by Nesis et al. (1998), Melo and Sauer (1999), Laptikhovsky et al. (2002) and Macewicz et al. (2004). However no system of classification of the different stages exists and there is no knowledge of how long such stages last. Melo and Sauer (1999) conducted a preliminary classification of post-ovulatory follicles in ovaries of the chokka squid but their classification was based only on the morphological appearance of post-ovulatory follicles of a known age in the ovaries of anchovy Engraulis capensis (Melo 1994).
To use post-ovulatory follicles for estimation of spawning frequency it is necessary to divide the deterioration and resorption processes of the follicle into a series of distinct histological stages, each with an assigned age (time from ovulation) (Hunter and Macewicz 1980). Estimating the age of post-ovulatory follicles focuses on determining the daily peak spawning period and the duration of the different stages. This method has been used successfully to estimate spawning frequency in teleosts e.g. Hunter and Goldberg (1980), Hunter and Macewicz (1980), Alheit et al. (1984) and Melo (1994).
The objective of the current study was to determine the duration and peak of the daily spawning cycle of the chokka squid. First, to validate the preliminary classification of post-ovulatory follicles by Melo and Sauer (1999) and confirm that it is possible to group follicle by age, and second, to estimate the spawning frequency by means of classification of these follicles.
Material and methods
Samples were collected by jigging on the spawning grounds off the southern coast of South Africa, three 24 h experiments performed in November 1997, 1998 and 1999. During November 2001 the experiment was extended to 48 h to obtain more accurate information on the rate of degeneration of post-ovulatory follicles. A minimum of 25 females (170•250 mm, mantle length) were collected per hour, in order to follow the chronological sequence of degeneration of post-ovulatory follicles (POFs). Ovaries and oviducts were removed and fixed in 10% buffered formalin solution. A section of each ovary was then dehydrated in a graded ethanol series, cleared with toluene and embedded in paraplast. Sections of 5•7 um were stained with Harris tm) haematoxylin followed by eosin. Ovaries were histologically classified according to Melo and Sauer (1999).
Oviducts were removed from the formalin solution, rinsed with distilled water and soaked in a solution of 50% ethanol and glycerol to facilitate egg separation. After 24 h, the eggs were easily loosened from the oviduct by tapping the oviduct with the blunt tip of forceps. To determine oviduct fullness all the eggs in each oviduct were counted directly.
Post-ovulatory follicles were classified (by age) into three types: new, intermediate or old. The classification was based according to the stage of degeneration and resorption of the follicles visible in histological sections in relation to the time of ovulation. The histological characteristics used to assess the stage of follicle degeneration were: degree of hypertrophy of the granulosa and thecal cells, vacuolisation of the cytoplasm and nuclear pyknosis in the granulosa, vascularity, and the presence of fibroblastos and collagenous fibers in the theca. Histological evidence of ovulation was verified by observing the integrity of the surrounding follicle.
The daily spawning cycle was estimated from the following:
-
Occurrence of oocytes at each ovulation stage (To estimate peak ovulation time and calculate the approximate age of post-ovulatory follicles).
-
Oviduct fullness (Indication of recent or imminent spawning).
-
Occurrence and age of post-ovulatory follicles (To detect ovulation and a recent spawning event).
Results
Ovulation
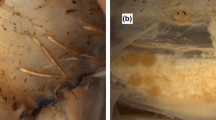
The process of ovulation in chokka squid appears seems to be similar to those in teleost fishes, with gross changes in function of the oocyte and the follicular layers prior to and during oocyte expulsion. These changes include separation of the oocyte from its surrounding follicular cells (granulosa), rupture of the follicular layer, and expulsion of the oocyte (Goetz 1983; Goetz et al. 1982). In the chokka squid these changes are clearly discernible. At the beginning of the ovulation, the oocyte is separated from its surrounding follicular cells, and the apical follicular cells change from cuboidal to columnar. This change in shape constricts the follicle to one end, and an apical rupture appears in the follicle layer opposite to the constriction (Fig.1a). At the most advanced stage of ovulation, the follicle constricts even further, pushing the mature oocyte to the area of rupture, to the opposite side of the follicle (Fig. 1b). The enlarged follicle collapses away from the mature oocyte ((Fig. 1c), and remains in the ovary as a post-ovulatory follicle, where it is quickly reabsorbed.
Oocytes at different stages of ovulation occurred most frequently (20.3%) in ovaries examined between 18:00 and 07:00 h, with a peak between 20:00•22:00 h (Fig. 2).
Histological characteristics of the post-ovulatory follicles
Each post-ovulatory follicle is characterized by an outer thecal layer of connective tissue and an inner epithelial granulose layer.
New post-ovulatory follicles:
The structure of the follicle is well maintained. The granulosa cells are elongated with a large central nucleus. The thecal layer is distinct, very thin with abundant supply of fibroblasts and collagenous fibers and blood capillaries. The theca adheres closely to the granulosa layer (Fig. 3a).
Intermediate post-ovulatory follicles:
The size of the follicle is reduced and signs of degeneration are evident. The granulosa layer still comprises numerous cells although some cytoplasmic vacuoles and granules are present, and cells membranes are less distinct. More of the granulosa nuclei are pycnotic and vacuoles can be seen in the granulosa. The theca layer appears thicker than that in the previous stage cells (Fig. 3b).
Old post-ovulatory follicles:
The follicle is further reduced in size, the cytoplasm of the granulosa cells is heavily stained and presents a flocculent cytoplasm. The numbers of pycnotic nuclei are further increased, there are few intact cell membranes and numerous vacuoles. The theca layer remains thick and becomes more fibrous (Fig. 3c).
Trends in post-ovulatory follicles:
The percentage of ovaries with new, intermediate and old post-ovulatory follicles from the combined data for all years are presented in Fig. 4. Post-ovulatory follicles were identified in 45.8% of the total ovaries examined between 19:00 and 07:00 h. Ovaries with new post-ovulatory follicles were first detected at 21:00 h. By 02:00 h, the percentage of ovaries with new post-ovulatory follicles had reached the highest level (10.5%). Thereafter, the percentage declined and by 08:00 h none were present in the ovaries sampled. Ovaries with intermediate post-ovulatory follicles were found between 04:00 and 11:00 h, peaking (7.2%) at 08:00 h. Old post-ovulatory follicles were present between 09:00 and 14:00 h, with a peak (5.2%) at 11:00 h, after which they were no longer apparent and also difficult to distinguish from atretic oocytes. Immediately apparent is that the duration of post-ovulatory follicles is particularly short-lived, from first detection to complete resorption takes approximately 14 h.
Post-ovulatory follicles (POF) of L. reynaudii: (a) New POF, time elapsed from ovulation about 5 h; (b) Intermediate POF, time elapsed from ovulation about 10 h; (c) Old POF showing pronounced degeneration of follicles after 14 h. (G•granulose epithelial layer; T•thecal connective layer; L•lumen of follicle
Trends in oviduct fullness
Trends in oviduct fullness are presented in Fig. 5. Data for 1997,1998, and 1999 were combined to provide an adequate number of samples per hour.
Females captured between 19:00 and 23:00 h had moderately full oviducts, with a strong mode between 600 and 1200 eggs (Fig. 5a). The ovaries of these females were either at the ripe stage (85%), with oocytes at different stages of ovulation or were partially spent ovaries (15%) with new post-ovulatory follicles, indicating that some eggs had already been released to the oviduct. These females probably represent individuals that are filling or refilling their oviducts prior to spawning.
Females captured between 00:00 and 05:00 h had mostly full oviducts, indicated by a strong mode of between 1800 and 2200 eggs (Fig. 5b), A strong indication of recent ovulation was the number (67%) of females with new and intermediate post-ovulatory follicles present. The remaining females (33%) exhibited ripe ovaries.
Females captured between 06:00 and 18:00 h had either partially empty or empty oviducts. The strong mode between 0 and 200 eggs (Fig. 5c) represents at least some individuals that had spawned just prior to capture. These results agree with catch statistics which often show large catches in daylight hours on the spawning grounds with little squid caught overnight (Sauer unpublished data). The ovaries of these females were either ripe (15%) or at the partially spent stage (25%), with some intermediate and old post-ovulatory follicles or at the recovery stage (60%) with old post-ovulatory follicles present.
Females with empty oviducts were most frequent between 08:00 and 12:00 h. Particularly interesting is that a few females with empty oviducts and recovering ovaries were found between 23:00 and 04:00 h indicating that some spawning can occur at night. These results agreed with observations by divers (Sauer et al. 1992), and with acoustic experiments (O tm)Dor et al. 1996; Sauer et al. 1997).
During 2001 data was collected for 45 h after which no further females were caught. The trends (Fig. 6a•g) are particularly interesting. For the first 24 h results were similar to those obtained during the 24 h experiments. On the 16th November, during the second 24 h period, the rate of ovulation appeared to decrease, as indicated by a reduction in the modal size of full oviducts collected between 00:00 and 00:500 h (Fig. 6f). This is corroborated by the presence of females with oviducts with low number of eggs collected between 06:00 and 15:00 h (Fig. 6g). In addition 20.2% of the females collected at this time had empty stretched oviducts, but the mantle showed no signs of deterioration and the ovaries contained a wide range of oocytes at different stages of development. The most advanced oocytes were at early stages of vitellogenesis (stages VII and VIII), but oocytes at stage III and IV predominated. Thus, those females were not considered spent, but resting. The rest of the ovaries of the females collected during this period were either partially spent or recovering. As the water temperature and clarity had not changed (Sauer, pers obs through SCUBA) this likely indicated an end of a spawning cycle for the female squid in the intermediate vicinity prior to a resting phase.
Discussion
Ovarian cycle
Based on the above observations an ovarian cycle can be postulated (Fig. 7). The ovarian cycle commences with females entering the cycle on reaching sexual maturity. After the first batch of advanced yolk stage oocytes is completed, females enter an inner cycle (spawning phase) that typically characterizes serial spawners. During this spawning phase ovaries go through ripe, partially spent and recovering stages by undergoing a process of maturation, ovulation and redeveloping, where a new batch of advanced oocytes is recruited. The cycle typically appears to last between 12 and 36 h, with the histological evidence supported by results from laboratory experiments (De Wet 1995; Sauer et al. 1999). After a number of spawning bouts the ovaries undergo a resting stage, indicated by the histological characteristics of the ovaries.
The variable environmental conditions on the spawning grounds (Roberts and Sauer 1994) and the disruption from predators (Sauer and Smale 1991) point towards the possible disruption of spawning activity during a single spawning bout. Information from acoustic tags (unpublished data) revealed squid move a short distance (100 tm)s of meters) from the spawning grounds during a turbidity event and remain inactive close to or on the sea floor during daylight hours. As squid feed mostly at night (Sauer and Lipinski 1991) this may signify a forced resting phase for spawning females. It is therefore highly probable that the duration of spawning bouts may differ, dictated by the stage of the ovarian cycle, environmental conditions, and predator presence. As squid move between spawning sites (Sauer et al. 1999) a further resting phase, possibly dictated largely by the stage of the ovarian cycle, will occur during this period of movement.
Oogenesis is expensive energetically, partially in short-lived species, in which the ripe ovary accumulates a substantial quantity of vitellus. Several studies have shown that some squid appear to use energy for egg production from food rather than that from stored products (Harman et al. 1989; Moltschaniwskyj 1995; McGrath and Jackson 2002). This may well be the case for the chokka squid. Although chokka squid may feed extensively at night they will require significant energy to spawn during daylight hours and subsequently move between spawning sites over a protracted period (O tm)Dor et al. 1996). It is also possible that during periods of poor food availability a number of oocytes may be resorbed (Melo and Sauer 1998), providing another form of energy reserve. Additional information on the energy allocation between reproduction and growth in this species is needed to understand the dynamics of oocyte growth and spawning.
Squid are considered fully spent when through the process of oocyte atresia (Melo and Sauer 1998) remaining oocytes are resorbed during the regressing stage of the ovarian cycle. It is however not possible to calculate with any certainty the number of weeks or months an individual is present on the spawning grounds prior to this final stage of the life cycle, and to quantify the number of spawning periods. The highest percentage of atretic oocytes are found between December and March (Melo and Sauer 1998) signifying the end of the spawning season, but spawning squid are known to occur throughout the year with a primary peak in early summer and in some years a second less pronounced peak in winter.
Although some limited spawning has been detected at night (Sauer and Smale 1993), histological analysis of the ovaries and observations of the oviduct fullness suggests that chokka squid spawn mainly during daylight hours. This has also been observed in captive females in this species (De Wet 1995). Other loliginid squids such as L. pealeii and Sepioteuthis sepiodea, whose natural behavior has been studied in the field, were found to be daytime spawners (Hanlon and Messenger 1996). Daytime spawning has also been detected in L. opalescens, in southern and northern California (Forsythe et al. 2004), although Fields (1965) and Hixon (1983) suggested indirectly that most spawning occurs at night. However, according to Forsythe et al. (2004) video observations revealed that in the absence of artificial lighting, L. opalescens does not aggregate into mating and spawning groups at night.
Post-ovulatory follicles are reliably identifiable up to 24 h after spawning in E. capensis. In chokka squid we found them to degenerate to a similar extent within 14 h. The duration of post-ovulatory follicles could have been underestimated because it becomes difficult to distinguish old post-ovulatory follicles from old atretic follicles. While there are several accounts of the occurrence of post-ovulatory follicles in other cephalopods (Nesis et al. 1998, Laptikhovsky et al. 2002; Macewicz et al. 2004) there are no reports describing their longetivity and subsequent degeneration. They probably exist for a relatively long time in “big-bang” very cold-waters spawners, like G. glacialis (Nesis et al. 1998) and in species with very large eggs, e.g. S. officinalis, in which the total number of post-ovulatory follicles is always higher than the number of ovulated eggs (Laptikhovsky et al. 2003). In serial spawners with small eggs like Alloteuthis these follicles are resorbed very quickly, and their number is usually lower than that of ovulated eggs (Laptikhovsky et al. 2002).
The short-lived nature of the post-ovulatory follicles precludes the calculation of the exact spawning interval and does not allow us to accurately predict the spawning frequency. Together with the degree of oviduct fullness they do however give a good indication of imminent or recent spawning. The available data also indicates that a single spawning bout may be protracted, and depends on a number of factors such as environmental conditions and the presence of predators. During suitable environmental periods, and with limited predators present, squid may spawn during successive daylight periods after which they go through a resting phase. The skewed sex ratio towards males shown in jig data (Sauer et al. 1992) and through visual observation over the egg bed may be as a result of a limited number of females actively spawning at any one time. A large number of females may be present in the immediate vicinity (Lipinski 1994) but not form an active part of the spawning concentration.
Other squid species have been postulated to spawn every two to three days e.g. the mesopelagic-boundary squid Abralia trigonura (Young and Mangold 1994). This species appears to spawn for the first time at ≈36 mm mantle length (≈117 d of age) and for the last time at ≈45 mm mantle length (≈189 d of age) and probably spawn a maximum of 25 times during their short life (Young and Mangold 1994). Calculation of spawning frequency for the chokka squid is complicated by the fact that spawning periods may extend over several months and that attainment of maturity is also highly variable (Augustyn 1989, Sauer and Lipinski 1991). The uncertainty of the life span and growth rate of chokka squid also affects the interpretation of the data. Acoustic tagging of specific individuals during a stable spawning period, coupled with long-term observation of mature females under laboratory conditions and a study on the dynamics of oocyte production and growth may provide some answers.
References
Augustyn CJ (1989) Systematics, life cycle and resource potential of the chokker squid Loligo vulgaris reynaudii. PhD Thesis, University of Port Elizabeth, 378 pp
Augustyn CJ, Lipinski MR, Sauer WHR, Roberts MJ, Mitchell-Innes BA (1994) Chokka squid on the Agulhas Bank: life, history and ecology. S Afr J Sci 90:143•154
Alheit J, Alarcon VH, Macewicz BJ (1984) Spawning frequency and sex ratio in the Peruvian anchovy, Engraulis ringens. Rep Calif Coop Oceanic Fish Invest 25:43•52
De Wet W (1995) Gonad histology, fecundity and reproductive behaviour of the chokka squid, Loligo vulgaris reynaudii. MSc Thesis, University of Port Elizabeth, 97 pp
Fields WG (1965) The structure, development, food relations, reproduction, and life history of the squid Loligo opalescens Berry. Fish Bull 131:1•108
Forsythe J, Kangas N, Hanlon R (2004) Does the California market squid (Loligo opalescens) spawn naturally during the day or night? A note on the successful use of ROVs to obtain basic fisheries biology data. Fish Bull 102:389•392
Goetz FW (1983) Hormonal control of oocyte final maturation and ovulation in fishes. In: Hoar WS, Randal DJ, Donaldson EM (eds) Fish Physiology, Vol IX B pp 117•170
Goetz FW, Smith DC, Krickl SP (1982) The effects of prostaglandins, phosphodiesterase inhibitors and cyclic AMP on ovulation of brook trout (Salvelinus fontinalis) oocytes. Gen Comp Endocrinol 48:154•160
Harman RF, Young RE, Reid SB, Mangold KM, Suzuki T, Nixon RF (1989) Evidence for multiple spawning in the tropical oceanic squid Stenoteuthis oualaniensis (Teuthoidea: Ommastrephidae). Mar Biol 101:513•519
Hanlon RT, Messenger JB (1996) Cephalopd behaviour. Cambridge University Press, Cambridge, 232 pp
Hixon RF (1983) Loligo opalescens. In: Cephalopds life cycles, V.I, species accounts, Academic Press, London, 475 pp
Hunter JR, Goldberg RS (1980) Spawning incidence and batch fecundity in the northern anchovy Engraulis mordax. Fish BullWash 77(3):641•652
Hunter JR, Macewicz BJ (1980) Sexual maturity, batch fecundity, spawning frequency and temporal patterns of spawning for the northern anchovy, Engraulis mordax, during the 1979 spawning season. Rep Calif Coop Oceanic Fish Invest 21:139•149
Laptikhovsky V, Salman A, Onsoy B, Katagan T (2002) Systematic position and reproduction of squid of the genus Alloteuthis (Cephalopda: Loliginidae) in the eastern Mediterranean. J Mar Biol Ass UK 82:983•985
Laptikhovsky V, Salman A Onsoy B, Katagan T (2003) Fecundity of the common cuttlefish, Sepia officinalis L (Cephalopoda, Sepiidae): a new look at an old problem. Sci Mar 67(3):279•284
Lipinski MR (1994) Differences among basic biological parameters in a population of chokka squid Loligo vulgaris reynaudii (Cephalopoda: Loliginidae) sampled by three methods. S Afr J Mar Sci14:281•286
McGrath BL, Jackson GD (2002) Egg production in the arrow squid Nototodarus gouldii (Cephalopoda: Ommastrephidae), fast and furious or slow and steady? Mar Biol 141:699•706
Macewicz BJ, Hunter JR, Lo CHN, LaCasella EL (2004) Fecundity, egg deposition, and mortality of market squid (Loligo opalescens). Fish Bull 102:306•327
Melo YC (1994) Spawning frequency of the anchovy Engraulis capensis. S Afr J Mar Sci 14: 321•331
Melo YC, Sauer WHH (1998) Ovarian atresia in cephalopods. S Afr J Mar Sci 20:143•151
Melo YC, Sauer WHH (1999) A histological description of post- ovulatory follicles in Loligo vulgaris reynaudii. In: Norberg B, Kjesbu OS, Taranger E, Anderson E, Stefansson SO (eds) Proceedings of the 6th International Symposium on the Reproductive Physiology of Fish, July 4•9, 1999. Bergen, Norway, 301 pp
Melo YC, Sauer WHH (1999) Confirmation of serial spawning in the chokka squid Loligo vulgaris reynaudii off the coast of South Africa. Mar Biol 135(2):307•313
Moltschaniwskyj NA (1995) Multiple spawning in the tropical squid Photololigo sp.: what is the cost in somatic growth? Mar Biol 124:127•135
Nesis KN, Nigmatullin ChM, Nikitina IV (1998) Spent females of deepwater squid Galiteuthis glacialis under the ice at the surface of the Weddell Sea (Antartic). J Zool Lond 277:185•200
O tm)Dor RK, Webber DM, Sauer WHH, Roberts MJ, Smale MJ (1996) High-resolution, 3D tracking of squid on spawning grounds. In: Cristalli C, Amlaner CJ, Neuman MR (eds) Procedings of the 13th International Symposium on Biotelemetry, Williamsburg, Virginia, March 1995. University of Arkansas Press, Fayetteville pp 193•198
Roberts MJ (2005) Chokka squid (Loligo vulgaris reynaudii) abundance linked to changes in South Africa tm)s Agulhas Bank ecosystem during spawning and early life cycle. ICES J Mar Sci 62:33•55
Roberts MJ, Sauer WHH (1994) Environment: the key to understanding the South African chokka squid (Loligo vulgaris reynaudii) life cycle and fishery? Antart Sci 6:249•258
Sauer WHH, Lipinski MR (1990) Histological validation of morphological stages of sexual maturity in chokker squid Loligo vulgaris reynaudii D tm)Orb (Cephalopoda:Loliginidae). S Afr J Mar Sci 9:189•200
Sauer WHH, Lipinski MR (1991) Food of squid Loligo vulgaris reynaudii (Cephalopoda: Loliginidae) on their spawning grounds off the eastern Cape, South Africa. S Afr J Mar Sci 10:193•201
Sauer WHH, Smale MJ (1991) Predation patterns on the inshore spawning grounds of the squid Loligo vulgaris reynaudii (Cephalopoda: Loliginidae) off the south-eastern Cape, South Africa. S Afr J Mar Sci 11:513•523
Sauer WHH, Smale MJ, Lipinski MR (1992) The location of spawning grounds, spawning and schooling behaviour of the squid Loligo vulgaris reynaudii (Cephalopoda: Myopsida ) off the eastern Cape coast, South Africa. Mar Biol 114:97•107
Sauer WHH, Smale MJ (1993) Spawning behaviour of Loligo vulgaris reynaudii in shallow coastal waters of the south-eastern Cape, South Africa. In: Okutani T, O tm)Dor RK, Kubodera T (eds) Recent Advances in Cephalopods Fisheries Biology. Tokyo, Tokai University Press, pp 469•498
Sauer WHH, Roberts MJ, Lipinski MR, Smale MJ, Hanlon RT, O tm)Dor RK (1997) Choreography of the squid tm)s “nuptial dance”. Biological Bulletin 192:203•207
Sauer WHH, Melo YC, De Wet W (1999) Fecundity of the chokka squid Loligo vulgaris reynaudii on the southeastern coast of South Africa. Mar Biol 135(2):315•319
Sauer WHH, Lipinski MR, Augustyn CJ (2000) Tag recapture studies of the chokka squid Loligo vulgaris reynaudii d tm)Orbigny, 1845 on inshore spawning grounds on the south-east coast of South Africa. Fish Res 45:283•289
Young RE, Mangold KM. (1994) Growth and reproduction in the mesopelagic-boundary squid Abralia trigonura. Mar Biol 119:413•421
Acknowledgements
We appreciate the constructive comments made by Drs. V. Laptikhovsky (Falkland Islands Government Fisheries Department) G. Pecl (Tasmania Aquaculture and Fisheries Institute) and also to the anonymous reviewer, Special thanks are due to Ms. S. Du Plessis (Marine and Coastal Management) for assistance in collecting the samples and to the South African Squid Management Association (SASMIA) for the funding of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melo, Y., Sauer, W.H.H. Determining the daily spawning cycle of the chokka squid, Loligo reynaudii off the South African Coast. Rev Fish Biol Fisheries 17, 247–257 (2007). https://doi.org/10.1007/s11160-006-9034-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-006-9034-6