Abstract
Among reptiles, morphological events involving differentiation of the gonads are similar, and their development is closely related to development of the Wolffian and Müllerian reproductive ducts and adrenal tissue. These structures have been described for some Squamata during their embryonic development. This study shows morphological characteristics of gonadal, adrenal tissue, and reproductive ducts development, during the embryonic development of oviparous lizard Sceloporus aeneus. The morphology of the urogenital complexes was examined using light microscopy, and ultrastructure of gonads was analyzed by transmission electron microscopy. The undifferentiated gonad was apparent from stage (st) 30 to st33; ovaries and testes became morphologically distinct from st34. In ovaries, the formation of the cortical and medullary region is evident, and germinal beds can be observed from st34 and synaptonemal complexes as evidence of meiosis was detected at st36. In testes, formation of testicular cords containing germ cells is observed; immature Leydig and Sertoli cells were identified from st38. Females showed evident Müllerian duct development, whereas in males, the Müllerian duct degenerated and the Wolffian duct remained. The adrenal tissue was observed as a cord of compact cells associated with the dorsal region of developing gonad from st31. In general, gonadal development of S. aeneus is similar to that observed in Sceloporus undulatus. Particularly, the presence of immature Leydig cells containing lipid droplets in testes and structures similar to germinal beds in ovaries is specific characteristics of S. aeneus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological differentiation of the gonads is very similar among reptilian sauropsids (Pieau et al. 1999; Morrish and Sinclair 2002; Raman 2002). Gonadal development begins with cell proliferation of coelomic epithelium covering the mesonephros. The genital ridges are formed from this cell proliferation, and subsequently, an undifferentiated bipotential gonad is established, essentially consisting of somatic and germ cells. These two cell types differentiate into lineages corresponding to an ovary or a testis. During male gonadal development, testicular cords form in the central region (medulla) of the gonad, whereas in ovaries, the cortex forms in the periphery, where primordial follicles are located (Pieau et al. 1999; Raman 2002; DeFalco and Capel 2009).
Among turtles, it was observed that the early development of primordial germ cells (PGCs) is similar to mammals. The PGCs are located in the posterior region of the primitive streak, from where they migrate through the gut and dorsal mesentery to the gonadal ridges (reviewed in: Bachvarova et al. 2009). In the undifferentiated gonad, PGCs are sexually bipotential and sensitive to signaling processes that induce their differentiation into male or female gametes (Adams and McLaren 2002). Among females, PGCs begin the process of meiosis and those oocytes that reach the Diplotene stage detain this process and remain in meiotic arrest (Wylie 1999; Pepling and Spradling 2001). Among males, the pre-Sertoli cells provide an environment which inhibits the onset of meiosis and germ cells remain in mitotic arrest (McLaren and Southee 1997; Adams and McLaren 2002; McLaren 2003). This general pattern of development for PGCs has been observed among reptiles, particularly in the lizards Anolis carolinensis Voigt, 1832, Calotes versicolor Daudin, 1802, and Sceloporus undulatus Bosc and Daudin, 1801 (Forbes 1956; Austin 1988; Doddamani 1994, 2006).
Among elasmobranch fishes and tetrapods (Wourms 1977), male (Wolffian) and female (Müllerian) reproductive ducts form in both sexes, during stages when gonadal sex has not been differentiated. Once the gonadal sex of the individual has been established, the development of ducts will depend on the sex that has been acquired. Among males, the Wolffian duct will develop and form the internal genitalia, while the Müllerian duct degenerates. Among females, internal genitalia differentiation takes place with the development of the Müllerian ducts, while the Wolffian ducts disappear (Hannema and Hughes 2007; Massé et al. 2009). It has been observed that the development of these ducts is similar among reptiles, including lizards A. carolinensis, Niveoscincus ocellatus Gray, 1845, and S. undulatus (Forbes 1956; Austin 1988; Neaves et al. 2006).
The adrenal gland is an endocrine organ comprised of two tissue types: steroidogenic and chromaffin (Jones et al. 1962). Among lizards, the formation of the adrenal cortex has been observed to take place during the formation of the genital ridges (Forbes 1956; Doddamani 2000). In A. carolinensis, the adrenal tissue appears during early stages as a cord of cells running dorsally to the mesonephros. Adrenal cortex cells proliferate rapidly, and during later stages, the adrenal tissue can be larger in size than the developing gonad (Forbes 1956). In C. versicolor, steroidogenic activity of the adrenal tissue is observed before gonadal differentiation, while the chromaffin tissue is evident morphologically once it has carried out the morphological differentiation of the gonads (Doddamani 2000).
Among reptiles, studies on the morphological processes of sexual differentiation have focused on species with temperature-dependent sex determination (TSD), such as turtles (Rimblot et al. 1985; Merchant-Larios et al. 1989, 1997; Wibbels et al. 1991; Pieau et al. 1998) and crocodiles (Joss 1989; Smith and Joss 1993, 1994a, b). However, few studies have been performed on species with genetic sex determination (GSD; Greenbaum and Carr 2001). In the genus Sceloporus (Phrynosomatidae), GSD has been observed, and many species exhibit sex chromosomes type XX/XY (Valenzuela 2004; Pokorna and Kratochvil 2009; Rovatsos et al. 2014a, b). Particularly, Sceloporus aeneus Weigmann, 1828 is an oviparous species that manifests egg retention, has been used as an important model for studying the evolution of viviparity (Guillette 1981; Guillette and Lara-Gongora 1986), but non-sex chromosomes were found (Cole 1978), and sex determination mechanism is not known. Like other species of the same genus, S. aeneus probably presents GSD; thus, the aim of this study was to analyze the morphological characteristics of gonadal development and correlate these with the development of the adrenal cortex and reproductive ducts during the embryonic development of this Mexican oviparous lizard.
Materials and methods
Animals
Sceloporus aeneus Weigmann, 1828 is endemic to Mexico, belonging to the family Phrynosomatidae. It is in the category of least concern (LC) on the International Union for the Conservation of Nature and Natural Resources (IUCN) Red List (Canseco-Márquez et al. 2007). Fourteen gravid females from the lizard S. aeneus (Fig. 1a) were collected in Milpa Alta, a suburb southwest of Mexico City (19°11′53″ N, 99°50′49″ W), during the month of June 2012 and May 2013 (Scientific collector permit: FAUT 0186 SEMARNAT). Animals were kept in plastic boxes with natural substrate and water and food consisting of flies and crickets. All females were marked with serial numbers and monitored until oviposition. Each nest was placed in a thermal container covered with natural substrate and maintained at room temperature.
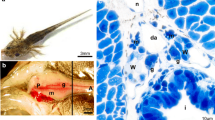
Adult female and embryonic urogenital complexes of Sceloporus aeneus. a Adult female of the lizard S. aeneus. b Caudal region of an embryo at st38, box shows the dissection zone for obtain urogenital complexes. c Dissection of the caudal region, arrows indicate the urogenital complexes. d Pair of urogenital complexes formed by the mesonephros (white arrows) and gonad (black arrows). e Histological section of an embryo at st30 indicating the location of the gonads (arrows) in relation to dorsal aorta (a), intestine (i), and mesonephros (M). The notochord (nc), neural tube (nt), and limb bud (lb) are evident. Bars b–d 1 mm; e 100 µm
Embryo collection and obtaining biological samples
All laboratory procedures were undertaken following ethical norms for animal experiments as directed by the Facultad de Ciencias, Universidad Nacional Autonoma de Mexico and in accordance with the Guidelines for the Use of Animals (2012).
Eggs were randomly extracted from each nest over periods of 5–7 days, starting on the day of the oviposition. Developmental stage of embryos was established as compared to the stage of embryonic development of the European lizard Lacerta (Zootoca) vivipara Von Jacquin, 1787 (Dufaure and Hubert 1961). Each embryo (n = 32) was killed by decapitation, and then, dissection was performed. From st34 to st40 (n = 24), urogenital complexes (mesonephros/gonad) were dissected, and from st30 to st33 (n = 8), caudal regions were obtained for microscopy analysis.
Light and electron microscopy
Each sample was fixed in Karnovsky solution (pH 7.4) for 18 h at 4 °C and stored in cacodylate buffer (Electron Microscopy Science, EMS; Hatfield, PA, USA; 0.1 mol/L, pH 7.4) at 4 °C. The tissues were post-fixed in osmium tetroxide (Sigma-Aldrich, St Louis, MO, USA) and were embedded in Epon 812 (EMS). Semi-thin histological sections were obtained (1 µm) as well as thin sections (50–70 nm) using an ultramicrotome (NOVA, Leica/LKB, Wetzlar, Germany). The semi-thin sections were stained with toluidine blue (EMS) at 0.1 % in doubly distilled water and observed under a light microscope (E200, Nikon, Melville, NY, USA). Thin sections were mounted on copper grids and contrasted using uranyl acetate and lead citrate for examination by transmission electron microscopy (TEM) using a JEOL 1010 microscope (JEM-1010, JEOL, Tokio, Japan).
Results
General anatomy and urogenital complex
In the present study, embryos of oviparous lizard S. aeneus were obtained from development st30 to st40; one to three embryos from each stage were analyzed. Urogenital complexes of S. aeneus are located in the caudal region extending from the middle region of embryo to posterior extremities (Fig. 1b). They are paired structures anchored to the dorsal body wall of the embryo and are located on each side of the dorsal spine (Fig. 1c). The mesonephros is elongated and can be distinguished by its yellowish color. Gonads are distinguished by their oval shape and whitish color (Fig. 1d), and these are attached to the mesonephros (Fig. 1d, e).
Undifferentiated sex morphology
The gonads are located in the middle region of the embryo, and they are closely associated to the aorta in the dorsal region and to the intestinal mesentery in the ventral region (Figs. 1e, 2a). At st30, the gonadal stage corresponds to gonadal stage 1 (G1, Austin 1988). At this stage, germ cells are noticeable for their round shape and their pale cytoplasm; using TEM, germ cells are conspicuous by their size and absence of granules in their cytoplasm, contain a spherical nucleus with condensed heterochromatin mainly in the periphery and a prominent nucleolus. Somatic cells are distinguished because they are irregular in shape contain intensive pigmentation, nucleus with one or two prominent nucleolus and scarce cytoplasm (Fig. 2a–d).
Undifferentiated sex morphology of Sceloporus aeneus embryos. a Gonads (g, box) at st30 associated with mesonephros (M), dorsal aorta (a), and intestine (i). Gonads and mesonephros are surrounded by a coelomic epithelium. b Amplification of box in a showing a gonad formed by germ cells (white arrows) and somatic cells (black arrows). c Transmission electron micrograph showing a germ cell (white arrow) and somatic cells (black arrows); somatic cells are conspicuous by irregular shape intensive pigmentation and scarce cytoplasm. d Amplification of a germ cell showing pale cytoplasm with scarce mitochondria and a spherical nucleus (N) with irregular nucleolus (n). e Light micrograph showing gonad (g), adjacent adrenal tissue (ad), and mesonephros (M) at st31. Gonad is attached to the mesonephros by a mesentery. f Histological section obtained at st32, the adrenal tissue (ad) is associated with cells irregularly shaped (asterisk) located in the mesonephros (M). g At st32, gonads formed by somatic (black arrows) and germ (white arrows) cells are surrounded by a cubic epithelium (black arrow heads). h Transmission electron micrograph of a germ cell showing the nucleus (N), nucleolus (n), and abundant mitochondria (asterisk) at st32. i At st33, a compact sexual cord (sc) is formed and the rete cord (rc) is evident. Bars a, b, e, f, g, i 50 µm; c, d, h 2 µm
From st31, in cross sections of the middle region of the urogenital complex, the gonad is observed as an elongated tissue attached to the adjacent mesonephros by a mesentery (Fig. 2e, f). At st31 and st32, the gonadal stage corresponds to G2 (Austin 1988). During this period, the gonads were observed as loose tissue with scarce germ cells surrounded by cubic epithelium (Fig. 2g). From this period, germ cells contain a spherical nucleus with abundant granular chromatin and fewer heterochromatin than those found at st30 and contain one or two nucleolus irregular in shape, and their cytoplasm is characterized by large quantity of mitochondria observed using TEM (Fig. 2h).
At st33, the gonadal stage corresponds to G3 (Austin 1988). At this stage, somatic cells and germ cells form a compact sexual cord surrounded by mesenchymal cells. This sexual cord occupies most of the gonad and is associated with the coelomic epithelium. The gonads are lined by cubic epithelium in the region near the mesonephros and by squamous epithelium on the opposite side. Rete cord can be observed between the mesonephros and the gonad (Fig. 2i).
At st30, the adrenal tissue has not been formed. From st31 to st33, the adrenal tissue appears as a string of cells included in the mesonephros, located in the dorsal region of the developing gonad. From st32, in some sections, the adrenal tissue is associated with a group of loose cells irregularly shaped and more stained (Fig. 2f).
Female morphology
From st34, the S. aeneus gonads are morphologically differentiated as ovaries (Fig. 3). The ovaries can be distinguished for their morphological regionalization consisting of a cortex and medulla. At st34 and st35, the gonadal stage corresponds to G4 (Austin 1988). In the medulla, somatic cells predominate with scarce germ cells, whereas germ cells together with somatic cells can be observed in the cortex. The number of cell layers in the cortex is about two to three (Fig. 3a, b). Somatic cells are irregularly shaped and are usually smaller than the germ cells. Germ cells are abundant and are in close contact, forming groups of two or more cells. They have a pale cytoplasm with abundant mitochondria and a prominent spherical nucleus with irregular nucleolus and synaptonemal complexes indicating that the onset of meiosis was not observed using TEM (Fig. 3c, d).
Female morphology of Sceloporus aeneus embryos. a Light micrograph of ovary at st34 showing the cortex (c), medulla (m) and germinal beds (gb). b Germ cells (white arrows) are located mainly in the cortex (c); somatic cells (black arrows) are located medulla (m) and cortex (c). c Transmission electron micrograph showing the compact cortical (c) region formed by developing germ cells (white arrows); lax medullary (m) region is formed by somatic cells (black arrows). d Germ cell containing spherical nucleus (N) and abundant mitochondria (asterisk). e Germinal bed (gb) is located near to the medulla (m) and is formed by somatic cells (black arrows) and scarce germ cells (white arrow). f Adrenal tissue (ad) located adjacent to the mesonephros (M) and near to the gonad (g). g Light micrograph of ovary at st40 showing the cortex (c) and medulla (m). h The cortex (c) is formed by several layers of germ (white arrows) and somatic (black arrows) cells; the medulla (m) is more compact that previous stages. i Transmission electron micrograph showing germ cells (white arrow) associated with somatic cells (black arrow). j Germ cell showing prominent spherical nucleus (N) and synaptonemal complexes (box). k Germinal bed (gb) at st40 formed by germ (white arrows) and somatic (black arrows) cells. l Light micrograph of adrenal tissue at st40; cells exhibit similar structures to lipid inclusions (box). Bars a, b, e, f, g, h, k, l 50 µm; c, d, i, j 2 µm
In the dorsal region near to the mesonephros, some germ cells are contained in the rete cord forming a structure similar to germinal bed observed in adult ovaries; this consists of germ cells surrounded by somatic cells (Fig. 3e, k). Each germinal bed was observed as a group of cells located in the anterior region of the urogenital complex; these are not found in the posterior region.
During st36 and st37, the number of cell layers in the cortex increases to about five and correspond to G5 (Austin 1988). From st38 onwards, the cortex consists of more than five layers of cells corresponding to G6 (Austin 1988). At st40, the medullar region is more compact compared to previous stages and is defined by a layer of irregularly shaped cells, which separate it from the cortex (Fig. 3g, h). Germ cells localized in the cortex are in close contact with somatic cells and synaptonemal complexes indicating the beginning of meiosis was observed from st36 (Fig. 3i, j).
Among embryos with differentiated ovaries, adrenal tissue appears as a cord of cells, which are larger than those observed during previous stages. For some sections, this cord is associated with a group of irregular, loose, and slightly more stained cells (Fig. 3f, l). At st40, cells located in the compact region of the adrenal tissue exhibit similar structures to lipid inclusions (Fig. 3l box).
Male morphology
From st34 of embryonic development onwards, the testes can be distinguished, as they present well-defined testicular cords which are located immersed in interstitial tissue. At st34 (Fig. 4a), two or three irregular shaped and large testicular cords can be observed corresponding to G4 (Austin 1988). The testicular cords contain germ cells and somatic cells that are precursors to Sertoli cells surrounded by a basal lamina (Fig. 4b). During st36 and st37, the testes correspond to G5 (Austin 1988). Somatic cells are characteristically smaller than germ cells; they have an irregularly shaped nucleus and are generally located at the periphery of the testicular cords (Fig. 4c). Some interstitial cells are oval shaped, and these are progenitors of Leydig cells, and elongated cells similar to myoid cells are attached to the periphery of the testicular cords (Fig. 4d). TEM analysis indicates that germ cells contain spherical nucleus and abundant mitochondria and are associated to somatic cells (Fig. 4e).
Male morphology of Sceloporus aeneus embryos. a Light micrograph of testis at st34 showing testicular cords (tc) surrounded by interstitial tissue (it). b Testicular cords (tc) formed by germ (white arrows) and somatic (black arrows) cells. c Transmission electron micrograph showing spherical germ cell (white arrow) associated to somatic cells (black arrows) in a testicular cord (tc) surrounded by loose interstitial tissue (it). d Myoid cell (Mc) surrounding testicular cord (tc) and progenitors of Leydig (pL) cells located in the interstitial tissue (it) are observed at st36. e Germ cell showing spherical nucleus (N) with prominent nucleolus (n) and abundant mitochondria (asterisk). f Light micrograph of adrenal tissue (ad) located adjacent to the mesonephros (M) and near to the gonad (g) at st34. g Testis at st40 showing fragmented testicular cords (tc) surrounded by interstitial tissue (it). h Round testicular cords (tc) containing germ (white arrows) and somatic (black arrows) cells. i Transmission electron micrograph showing testicular cord (tc) containing germ cell (white arrow) and Sertoli cell (Sc) morphologically differentiated at st38; Myoid cells (Mc) are located in the interstitial tissue (it). j Progenitors of Leydig (pL) cells and immature Leydig cells (iL) with lipid droplets are presented at st38. k Germ cell showing spherical nucleus (N) with prominent nucleolus (n) and abundant mitochondria (asterisk). l Light micrograph of adrenal tissue at st40 located adjacent to the mesonephros (M); adrenal cells are irregular in shape (box). Bars a, b, f, g, h, l 50 µm; c, d, e, i, j, k 2 µm
From st38 to st40, the G6 is apparent (Austin 1988). Testicular cords have been fragmented into smaller cords, and germ cells are more abundant compared to previous stages (Fig. 4g, h). Some cells within the testicular cords exhibit typical characteristics of Sertoli cells and have an irregularly or triangular-shaped nucleus and well-defined nucleolus and are generally located at the periphery of the testicular cords (Fig. 4i). Interstitial cells have morphologically differentiated into myoid cells and immature Leydig cells. The immature Leydig cells are oval or triangular in shape, and obvious lipid inclusions were observed using TEM (Fig. 4j). Germ cells contain abundant mitochondria and synaptonemal complexes or any evidence that they perform the process of meiosis was not observed using TEM (Fig. 4k).
Among embryos with differentiated testes, adrenal tissue is morphologically similar to that observed among females. Unlike females, no cells with lipid inclusions were detected during any stage that was analyzed (Fig. 4f, l).
Reproductive ducts morphology
At st30, the Wolffian duct is located adjacent to the mesonephros and appears as a tube with evident lumen (Fig. 5a), although the Müllerian duct has not been formed. During st33, the development of the Müllerian duct initiates with thickening of the coelomic epithelium cells (Fig. 5b).
Reproductive ducts morphology of Sceloporus aeneus embryos. a Light micrograph of caudal region of an embryo at st30 showing the Wolffian duct (arrow, box) adjacent to mesonephros (M) and near to dorsal aorta (a) and gonad (g). b Caudal region of an embryo at st33 showing the Wolffian duct (black arrow, box) and the primordium of Müllerian duct (white arrow, box) formed by few cells. c Müllerian (left side) and Wolffian (right side) ducts obtained from female embryos at st34. d Müllerian (left side) and Wolffian (right side) ducts obtained from male embryos at st34. Müllerian ducts are formed by two cell types: the epithelium (e) and the mesenchyme (ms). Wolffian ducts are a tubular structure formed by elongated cells. Wolffian ducts are located adjacent to the mesonephros (M) and generally associated at blood vessels (bv). e Müllerian (left side) and Wolffian (right side) ducts obtained from female embryos at st40. Wolffian duct was observed to be in regression and was smaller than the Müllerian duct. f Müllerian (left side) and Wolffian (right side) ducts obtained from male embryo at st40. Müllerian ducts enter regression and are formed by few cells. Bars 50 µm
Among females during st34 to st40, the Müllerian duct is found anchored to the mesonephros and consists of two cell types: epithelium and mesenchyme. Duct size and the number of layers of mesenchymal cells increase with advancing embryonic development. The Wolffian duct is adjacent to the Müllerian duct and is a tubular structure, comprising one or two layers of elongated cells. At st40, the Wolffian duct was observed to be in regression and was smaller than the Müllerian duct (Fig. 5c, e).
Among males during st34, morphology of the Müllerian and Wolffian ducts is similar to that observed among females. Subsequently, the Müllerian duct enters regression, losing its two layer structure; until at st40, it can be observed as a bump consisting of a few cells adjacent to the mesonephros. From st34, the Wolffian duct increases in size with advancing embryonic development and is usually found associated with blood vessels (Fig. 5d, f).
Discussion
We presented morphological characteristics of gonadal development in the oviparous lizard S. aeneus, considering embryos from st30 to st40. S. aeneus is an oviparous species that exhibits egg retention (Guillette 1981; Guillette and Lara-Gongora 1986; García-Collazo et al. 2012), permitting us to define 11 development stages, including stages when gonadal differentiation occurs.
Gonadal development has been described for several reptiles including crocodiles (Joss 1989; Smith and Joss 1993, 1994a), turtles (Merchant-Larios et al. 1989, 1997; Wibbels et al. 1991; Pieau et al. 1998; Greenbaum and Carr 2001), and lizards (Forbes 1956; Austin 1988; Doddamani 1994, 2006; Neaves et al. 2006). In S. aeneus, we observed that gonadal morphogenesis processes take place between st30 and st33. In C. versicolor, initiation of gonad formation is observed at st27, whereas in N. occellatus and S. undulatus, this occurs at st29 (Austin 1988; Doddamani 1994; Neaves et al. 2006). We observed gonadal development of the lizard S. aeneus from st30; however, morphological processes in previous stages were not analyzed, therefore unknown whether gonadal development begins before st30. Likewise, ovaries or testes differentiation in N. ocellatus take place during st30–st32, whereas in C. versicolor, gonadal differentiation is observed at st33 (Doddamani 1994; Neaves et al. 2006), and in S. undulatus and S. aeneus, ovaries and testes are detected from st34 onwards.
In S. aeneus, the presence of germ cells is evident from st30; these cells exhibit similar morphological characteristics to those described among lizards of genus Lacerta (Hubert 1974) and other vertebrates (Soto-Suazo and Zorn 2005).
Among reptilian sauropsids, the undifferentiated gonad has been described as a structure with a cortex and medulla that manifests sexual cords (Pieau et al. 1999; Raman 2002; DeFalco and Capel 2009). However, in S. aeneus, this regionalization is not morphologically well defined. Similarly, in S. undulatus, the presence of a cortex and medulla has been described, up until ovary formation (Austin 1988).
The presence of structures similar to germinal beds in S. aeneus was detected from early stages of ovary development (st34), whereas in other species such as C. versicolor, this was not observed until after hatching (Doddamani 1994). Among adult females, the number of germinal beds varies from one to six per ovary (Jones et al. 1982; Gómez and Ramírez-Pinilla 2004; Hernández-Franyutti et al. 2005; Radder and Shine 2007; Gharzi et al. 2012). Among lizards from the genus Sceloporus, particularly in S. aeneus, it has been reported that each adult ovary has two germinal beds (Jones et al. 1982). However, during the embryonic development of S. aeneus, we noticed a dorsal germinal bed in each ovary suggesting that during postnatal or adult stages, a reorganization of these germinal beds takes place. This is the first work in which similar structures to germinal beds are described during the development of the ovaries in a lizard, this being a particular feature of S. aeneus.
In S. aeneus, follicle formation was not detected in any embryo stages analyzed, so that this process occurs after hatching, as in the alligator and other lizards for example, S. undulatus, N. ocellatus, and C. versicolor (Austin 1988; Doddamani 1994; Neaves et al. 2006).
Among the marine turtles Dermochelys coriacea Vandelli, 1761 and Lepidochelys olivacea Eschscholtz, 1829, it has been reported that the process of meiosis begins during postnatal stages (Rimblot et al. 1985; Merchant-Larios et al. 1989). In C. versicolor, ovarian germ cells initiate meiosis at st35, and this process continues until after hatching, when oocytes remain in meiotic arrest during the diplotene stage (Doddamani 1994). In S. undulatus, this process has been reported to occur during st34–st35 (Austin 1988). However, in S. aeneus, synaptonemal complexes were detected at st36 indicating the onset of meiosis.
In the testes of S. aeneus, the main cell lineages (Sertoli and Leydig) are differentiated morphologically at st38. These results are similar to those observed in S. undulatus (Austin 1988) but differ from those reported in C. versicolor, where Sertoli cells are observed during st36 and st37 and immature Leydig cells at st34 (Doddamani 2006). Immature Leydig cells are characterized by steroidogenic activity and are morphologically round and contain large lipid droplets in their cytoplasm (Zirkin and Ewing 1987; Chen et al. 2009). In C. versicolor, the presence of Leydig cells similar to fibroblast has been reported during st34, and steroidogenic activity was detected from st36 (Doddamani 2006). In S. aeneus, immature Leydig cells were observed at st38, suggesting that steroid production can occur during stages close to hatching. During testicular development of S. aeneus, onset of meiosis is not observed, but proliferation of germ cells is remarkable, as occurs in A. carolinensis and C. versicolor (Forbes 1956; Doddamani 2006).
Among mammals, Sertoli cells secrete the anti-Müllerian hormone (AMH), which is responsible for inducing regression in the Müllerian ducts (Behringer 1995; Rey et al. 2003). In Alligator mississippiensis Daudin, 1802, it has been demonstrated that AMH transcripts occur in developing testes (Western et al. 1999; Urushitani et al. 2011), and it has been suggested that postnatal testes produce a factor which induces regression in the Müllerian duct, in a way similar to other vertebrates (Austin 1994). In S. aeneus, we observed that regression in the male Müllerian duct initiates during the early stages of testicular development. This suggests that factors similar to AMH induce this process and are present once the morphological differentiation of the testis has occurred. Likewise, Leydig cells in mammalian testes produce testosterone, which induces growth and differentiation in the Wolffian duct (Hannema and Hughes 2007). However, the mechanism which induces growth and differentiation of the Wolffian duct among reptiles is not known. In S. aeneus, we observed that the Wolffian duct grows during embryonic development, probably due to factors produced by the developing testis. However, no apparent morphological changes were detected, indicating that differentiation in the male genital tract occurs during postnatal stages.
Among female mammals, it is known that the development and differentiation of the Müllerian duct is induced and controlled by a complex network of Wnt and Hox genes (Massé et al. 2009). Among reptiles, the mechanisms which cause the differentiation of the Müllerian duct are unknown. At morphological level, it has been observed that in S. aeneus and other lizards, the Müllerian duct is maintained during embryonic development and is characterized by an increase in size (Forbes 1956; Austin 1988). In A. carolinensis and S. undulatus, regression in the Wolffian duct has not been observed during embryonic stages (Forbes 1956; Austin 1988). Similarly, during embryonic development of females S. aeneus, the Wolffian duct remains up to st40, and no obvious morphological changes were observed.
The development of adrenal tissue in S. aeneus is similar to that reported for the crocodile Crocodylus porosus Schneider, 1801 (Smith and Joss 1994b) and lizard C. versicolor and A. carolinensis (Forbes 1956; Doddamani 2000). In the turtle Testudo hermanni Gmelin, 1789, it has been observed that the chromaffin cells originate from a group of cells located in a ventrolateral position relative to the notochord and migrate to the mesonephros, where steroidogenic adrenal cells are located (Accordi et al. 2006). However, the process by which the chromaffin cells originate in other reptiles is unknown. Morphological evidence to indicate a process similar to that observed in T. hermanni was not observed in S. aeneus. Steroidogenic activity of adrenal tissue in early stages of development has been reported in several reptiles, including crocodiles (Joss 1989; Smith and Joss 1994b), turtles (Merchant-Larios et al. 1989; Thomas et al. 1992), and lizards (Gaitonde and Gouder 1984; Doddamani 2000); thus, the production of steroids in the adrenal tissue of S. aeneus cannot be ruled out.
Previous works have provided descriptions of gonadal development, adrenal tissue, and the reproductive tract of various species which includes one or two of these structures. Our work is the first report to have morphologically described these three tissues, and in which ultrastructural analysis of gonadal development in a species of lizard has been undertaken.
In this paper, gonadal differentiation and development were described in the lizard S. aeneus, laying the morphological basis for conducting future studies on the mechanisms of sex determination and differentiation in the genus Sceloporus. S. aeneus is an oviparous species that manifests egg retention, suggesting that this species is evolving to viviparity (Guillette et al. 1980). Therefore, S. aeneus and other species of the same genus are of particular interest for the study of the mechanisms of sex determination and its implications at physiological, ecological, and evolutionary level.
In conclusion, gonadal development of S. aeneus is similar to that observed in S. undulatus. The period of undifferentiated gonad covers st30 to st33, and ovaries or testes are evident from st34. Particularly, the presence of immature Leydig cells containing lipid droplets in testes and structures similar to germinal beds in ovaries is specific characteristics of S. aeneus.
References
Accordi F, Chimenti EC, Gallo VP, Liguori EC (2006) Differentiation of chromaffin cells in the developing adrenal gland of Testudo hermanni. Anat Embryol. doi:10.1007/s00429-006-0081-5
Adams IR, McLaren A (2002) Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 129:1155–1164
Austin HB (1988) Differentiation and development of the reproductive system in the iguanid lizard, Sceloporus undulatus. Gen Comp Endocrinol 12:351–363
Austin HB (1994) Extended production of the Müllerian duct regressor in the American alligator. Gen Comp Endocrinol 96:122–128
Bachvarova RF, Crother BI, Johnson AD (2009) Evolution of germ cell development in tetrapods: comparison of urodeles and amniotes. Evol Dev 11:603–609
Behringer RR (1995) The Müllerian inhibitor and mammalian sexual development. Phil Trans R Soc Lond B 350:285–289
Canseco-Márquez L, Mendoza-Quijano F, Ponce-Campos P (2007) Sceloporus aeneus. IUCN Red List of Threatened Species. Version 2011.2. http://www.iucnredlist.org/. Accessed 01 Aug 2012
Chen H, Geb RS, Zirkin BR (2009) Leydig cells: from stem cells to aging. Mol Cell Endocrinol 306:9–16
Cole CJ (1978) Karyotypes and systematics of the lizards in the variabilis, jalapae, and scalaris species groups of the genus Sceloporus. Am Mus Novit 2653:1–13
DeFalco T, Capel B (2009) Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol 25:457–482
Doddamani LS (1994) Histoenzymological studies on the embryonic and posthatching development of the ovary in the tropical oviparous lizard, Calotes versicolor. J Morphol 221:1–10
Doddamani LS (2000) Development of the adrenal gland in the tropical lizard Calotes versicolor. Gen Comp Endocrinol 117:89–102
Doddamani LS (2006) Differentiation and development of testis in the oviparuos lizard, Calotes versicolor (Daud). J Exp Zool 305A:299–308
Dufaure JP, Hubert J (1961) Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Arch Anat Microsc Morphol Exp 50:309–328
Forbes TR (1956) The development of the reproductive system of a lizard, Anolis carolinensis. Am J Anat 98:139–157
Gaitonde SG, Gouder BYM (1984) The structure and steroidogenic potential of the developing gonad and interrenals in the tropical oviparous lizard, Calotes versicolor (Daud.). Reprod Nutr Dév 24:915–926
García-Collazo R, Villagrán-Santa Cruz M, Morales-Guillaumin E, Meza-Lázaro RN, Méndez-de la Cruz FR (2012) Egg retention and intrauterine embryonic development in Sceloporus aeneus (Reptilia: Phrynosomatidae): Implications for the evolution of viviparity. Rev Mex Biodiv 83:802–808
Gharzi A, Yari A, Rastegar-Pouyani N (2012) Morphology and histology of ovari´s germinal beds in the lacertid lizard, Acantodactylus boskianus (Sauria; Lacertidae). Res J Anim Sci 6:8–11
Gómez D, Ramírez-Pinilla MP (2004) Ovarian histology of the placentotrophic Mabuya mabouya (Squamata, Scincidae). J Morphol 259:90–105
Greenbaum E, Carr JL (2001) Sexual differentiation in the spiny softshell turtle (Apalone spinifera), a species with genetic sex determination. J Exp Zool 290:190–200
Guidelines for the Use of Animals (2012) Guidelines for the treatment of animals in behavioral research and teaching. Anim Behav 83:301–309
Guillette LJ (1981) Reproductive strategies and evolution of the viviparity in two allopatric populations of the Mexican lizards Sceloporus aeneus. Dissertation, University of Colorado
Guillette LJ Jr, Lara-Gongora G (1986) Notes on oviposition and nesting in the high elevation lizard, Sceloporus aeneus. Copeia 1986:232–233
Guillette LJ Jr, Jones RE, Fitzgerald KT, Smith HM (1980) Evolution of viviparity in the lizard genus Sceloporus. Herpetologica 36:201–215
Hannema SE, Hughes IA (2007) Regulation of Wolffian duct development. Horm Res 67:142–151
Hernández-Franyutti A, Uribe-Aranzábal MC, Guillette LJ Jr (2005) Oogenesis in the viviparous matrotrophic lizard Mabuya brachypoda. J Morphol 265:152–164
Hubert J (1974) Ultrastructure of primordial germ cells of Lacerta muralis and Lacerta viridis. Comparison with Lacerta vivipara (autho´s transl). Arch Anat Histol Embryol 57:259–268
Jones IC, Phillips JG, Bellamy D (1962) The adrenal cortex throughout the vertebrates. Br Med Bull 18:110–113
Jones RE, Swain T, Guillette LJ Jr, Fitzgerald KT (1982) The comparative anatomy of lizard ovaries, with emphasis on the number of germinal beds. J Herpetol 16:240–252
Joss JMP (1989) Gonadal development and differentiation in Alligator mississippiensis at male and female producing incubation temperatures. J Zool 218:679–687
Massé J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I (2009) The developing female genital tract: from genetics to epigenetics. Int J Dev Biol 53:411–424
McLaren A (2003) Primordial germ cells in the mouse. Dev Biol 262:1–15
McLaren A, Southee D (1997) Entry of mouse embryonic germ cells into meiosis. Dev Biol 187:107–113
Merchant-Larios H, Villalpando-Fierro I, Centeno-Urruiza B (1989) Gonadal morphogenesis under controlled temperature in the sea turtle Lepidochelys olivacea. Herpetol Monogr 3:43–61
Merchant-Larios H, Ruiz-Ramírez S, Moreno-Mendoza N, Marmolejo-Valencia A (1997) Correlation among thermosensitive period, estradiol response and gonadal differentiation in the sea turtle Lepidochelys olivacea. Gen Comp Endocrinol 107:373–385
Morrish BC, Sinclair AH (2002) Vertebrate sex determination: many means to an end. Reproduction 124:447–457
Neaves L, Wapstra E, Birch D, Girling JE, Joss JMP (2006) Embryonic gonadal and sexual organ development in a small viviparous skink, Niveoscincus ocellatus. J Exp Zool 305A:74–82
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351
Pieau C, Dorizzi M, Richard-Mercier N, Desvages G (1998) Sexual differentiation of gonads as a function of temperature in the turtle Emys orbicularis: endocrine function, intersexuality and growth. J Exp Zool 281:400–408
Pieau C, Dorizzi M, Richard-Mercier N (1999) Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci 55:887–900
Pokorna M, Kratochvil L (2009) Phylogeny of sex-determining mechanisms in squamate reptiles: Are sex chromosomes an evolutionary trap? Zool J Linn Soc 156:168–183
Radder R, Shine R (2007) Germinal bed condition in a polyautochronic single-clutched lizard, Bassiana duperreyi (Scincidae). Amphib-Reptil 28:159–162
Raman R (2002) Sex determination and gonadal differentiation in vertebrates: a case for unity in diversity. Proc Indian Natl Sci Acad (PINSA) B 6:529–546
Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P (2003) AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 211:21–31
Rimblot F, Fretey J, Mrosovsky N, Lescure J, Pieau C (1985) Sexual differentiation as a function of the incubation temperature of eggs in the sea-turtle Dermochelys coriacea (Vandelli, 1761). Amphib-Reptil 6:83–92
Rovatsos M, Altmanová M, Pokorná M, Kratochvíl L (2014a) Conserved sex chromosomes across adaptively radiated Anolis lizards. Evolution 68:2079–2085
Rovatsos M, Pokorná M, Altmanová M, Kratochvíl L (2014b) Cretaceous park of sex determination: sex chromosomes are conserved across iguanas. Biol Lett 10:20131093
Smith CA, Joss JMP (1993) Gonadal sex differentiation in Alligator mississippiensis, a species with temperature dependent sex determination. Cell Tissue Res 273:149–162
Smith CA, Joss JMP (1994a) Sertoli cell differentiation and gonadogenesis in Alligator mississippiensis. J Exp Zool 270:57–70
Smith CA, Joss JMP (1994b) Steroidogenic enzyme activity and ovarian differentiation in the saltwater crocodile, Crocodylus porosus. Gen Comp Endocrinol 93:232–245
Soto-Suazo M, Zorn TM (2005) Primordial germ cells migration: morphological and molecular aspects. Anim Reprod 2:147–160
Thomas EO, Licht P, Wibbels T, Crews D (1992) Hydroxysteroid dehydrogenase activity associated with sexual differentiation in embryos of the turtle Trachemys scripta. Biol Reprod 46:140–145
Urushitani H, Katsu Y, Miyagawa S, Kohno S, Ohta Y, Guillette LJ Jr, Iguchi T (2011) Molecular cloning of anti-Müllerian hormone from the American alligator, Alligator mississippiensis. Mol Cell Endocrinol 333:190–199
Valenzuela N (2004) Temperature-dependent sex determination. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 211–227
Western PS, Harry JL, Marshall-Graves JA, Sinclair AH (1999) Temperature-dependent sex determination in the American alligator: AMH precedes SOX9 expression. Dev Dyn 216:411–419
Wibbels T, Bull JJ, Crews D (1991) Chronology and morphology of temperature-dependent sex determination. J Exp Zool 260:371–381
Wourms JP (1977) Reproduction and development in Chondrichthyan fishes. Am Zool 17:379–410
Wylie C (1999) Germ Cells. Cell 96:165–174
Zirkin BR, Ewing LL (1987) Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec 219:157–163
Acknowledgments
We thank Oswaldo Hernández-Gallegos and Gisela Granados-González for help in capturing the pregnant animals used in this study. We would also like to thank Pedro Medina-Granados and Alejandro Marmolejo-Valencia for technical assistance. Antonio-Rubio NR was granted a postdoctoral fellowship from DGAPA-UNAM for this study. This work was supported by DGAPA-UNAM (IN205011).
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Antonio-Rubio, N.R., Villagrán-SantaCruz, M., Santos-Vázquez, A. et al. Gonadal morphogenesis and sex differentiation in the oviparous lizard, Sceloporus aeneus (Squamata: Phrynosomatidae). Zoomorphology 134, 279–289 (2015). https://doi.org/10.1007/s00435-015-0259-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-015-0259-6